Abstract
Biodiesel is one of the renewable energy (RE) sources that has received much interest due to its promising properties. Recently, the use of coconut oil as biodiesel has caught the attention of many researchers. As a result, this paper presents a comprehensive overview of the current catalysts used to produce coconut oil biodiesel via the transesterification method.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Worldwide primary energy demand has been growing by 1.6% on an annual basis, and its escalation is bound to increase through the next decade. Most of the primary energy used at this present time is comprised of crude oil (35%), coal (29%), and natural gas (24%), all generated from fossil fuel resources. Meanwhile, renewable energy (RE) and nuclear resources contribute about 5% and 7% of global energy consumption, respectively [1, 2]. The continuous increment in energy demand due to global industrialization and modernization has led to a non-RE crisis. The use of non-RE sources is deleterious to the environment. Upon burning, fossil fuels emit particles that pollute the air, water, and land, which have been linked to the devastating effects of climate change, global warming, and even worse, the emergence of incurable diseases. Developing countries have begun to realise the importance of biofuels such as alcohol, vegetable oil, biomass, biogas, and synthetic fuels. Therefore, biodiesel has piqued the interest of many researchers as a potential substitute for petroleum-based fossil fuel.
Biodiesel offers multiple advantages, including high flash point, high cetane number, high lubricity, lower carbon monoxide emission profile, and most importantly, it is biodegradable [3]. Notably, biodiesel has a significantly higher flash point than that of petroleum diesel or gasoline, thus contributing to the exceptional safety properties offered by biodiesel. It is also nontoxic, eco-friendlier than fossil fuels, and contains no sulphur. Besides, biodiesel is an affordable, good lubricant with comparable quality to diesel fuel, hence its potential to be an alternative lubricant in compression ignition engines [4]. Biodiesel can be produced from renewable biological sources such as vegetable oils and fats. Therefore, it is a potential RE source and a carbon-neutral alternative to petroleum fuels, as depicted in numerous reviews [2, 4,5,6]. Vegetable oil-based fuel emits fewer harmful gases (e.g., sulphur oxide, carbon dioxide, carbon monoxide, and unburned hydrocarbons) into the environment when compared to petroleum-based diesel fuels [7, 8].
Due to current industrial levels of demand, edible oils with food value, such as corn, soybean, coconut, and palm, have been placed under huge pressure [9]. Vegetable fats and oils (edible and non-edible) and animal fat feedstock contain monoglycerides (MGs), diglycerides (DGs), and triglycerides (TGs), which are long-chain fatty acid groups attached via ester linkages to a glycerol backbone. These three chemicals have similar physicochemical properties to petrol and diesel, but are unsuitable for direct injection engines due to the combustion residue of the oil. However, this problem may be addressed by modifying both feedstocks via transesterification reactions to form alkyl esters, which refers to biodiesel. The triglycerides derived from vegetable oils or animal fats are combined with short-chain alcohols such as methanol, ethanol, and propanol in order to obtain alkyl esters or biodiesel via transesterification reaction with glycerol as the by-product [10, 11].
Coconut oil can be extracted from the meat or kernel of a matured coconut and is a type of edible oil. Coconut oil can be used for biodiesel production due to its availability and lower free fatty acid (FFA) content when compared to non-edible oils. Besides, coconut biodiesel has better lubricity and a similar flash point to that of diesel fuel [4]. Typically, coconut contains 10%–15% of extractable oil content, with a fatty acid methyl ester (FAME) composition of C8 to C12 [5]. According to Nakpong and Wootthikanokkhan [12], in order to produce biodiesel via a two-step process, coconut oil with 12.8% FFA content can be used as feedstock. First, through acid-catalyzed esterification, the FFA content of coconut oil is reduced to 0.6%. Second, with the use of methanol and the presence of an alkaline catalyst, triglyceride is transesterified to produce methyl esters and glycerol as by-products. The coconut biodiesel viscosity is similar to that of Thai petroleum diesel. The other measured properties of coconut biodiesel also meet Thai biodiesel (B100) specifications.
Conventionally, biodiesel is synthesized using homogeneous catalysts, such as NaOH [13], KOH [14], and H2SO4 [15], to yield high conversion values. . However, there are several disadvantages to homogeneous catalysts, such as failure to reuse the catalyst, as well as high production of potentially hazardous and corrosive waste and effluents. Therefore, solid heterogeneous catalysts are used as an alternative to traditional homogenous catalysts. Heterogeneous catalysts offer numerous advantages that can minimise environmental damage and enhance the efficacy of the biodiesel process. The solid catalysts can easily be detached from the reaction medium and are reusable several times without deterioration [16]. Besides, solid catalysts can reduce the volume of wash water and organic solvent required to purify the biodiesel, thus saving on the cost of biodiesel production.
Therefore, this paper looked into the potential of coconut oil as a biodiesel feedstock as part of the RE source to lower the current dependence on conventional fossil fuel sources. This paper reviews the recent catalysis methods for biodiesel production using transesterification and highlights several recommendations for future research in biodiesel production.
2 Transesterification Method
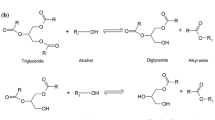
In general, biodiesel is produced via the transesterification process, where the oil or fat obtained from specific sources is reacted with alcohol in the presence of a catalyst to form esters (biodiesel) and glycerol. The by-product of the transesterification reaction is glycerol. Once treated and subjected to further processing, useful products may also be produced. Fig. 1 illustrates the process of transesterification and the range of products that can be manufactured using glycerol.
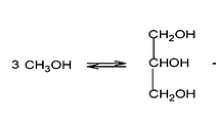
Transesterification is a reversible reaction that involves the exchange of alkyl groups from triglyceride ester with alkyl groups of alcohol, which is also known as alcoholysis (see Equation 1). The three moles of triglyceride from vegetable oil will react with alcohol to produce an alkyl ester and 3 moles of glycerol. The step of the direct alcoholysis of tri-acyl glycerol (TAG) contained in vegetable oils into fatty acid alkyl ester involves the conversion of a reversible reaction of ester in TAG to di-acyl glycerol (DAG) and mono-acyl glycerol (MAG) as the intermediate products before the final product of biodiesel is produced [17].

The removal of TAG to form three molecules of fatty acid alkyl ester and one molecule of glycerol is shown in Equation 2.

Transesterification is a crucial process for producing biodiesel, as it can improve some properties of the oil, such as viscosity, cetane number, flash point, and other characteristics, to a level nearer to that of conventional fossil-based diesel oil [15, 16]. Methanol and ethanol are the common alcohols used in transesterification due to their availability, low cost, as well as short carbonic chains and polarity, making them chemically and physically advantageous. Other alcohols that are less commonly used include 1-propanol, 1-butanol, and amyl alcohol [18].
The parameters of the transesterification reaction are essential to optimising biodiesel production. The parameters with the most effect on biodiesel yield are reaction temperature, catalyst type and amount, alcohol-to-oil molar ratio, and reaction time. The yield of biodiesel is calculated using Eq. 3 below.
A catalyst improves the reaction rate and the biodiesel yield. Catalysts can be alkaline, acidic, or enzymatic. Usually, an alkaline catalyst is preferred and has been applied commercially because it induces a faster reaction rate when compared to an acidic catalyst. Besides, it is also preferred for oil with a lower FFA content [18]. Hence, this paper describes the transesterification of coconut biodiesel using different catalysed reactions applied in various studies.
2.1 Chemical Catalyzed Reaction
The chemical-catalysed reaction works in the presence of alcohol and a catalyst to chemically breakdown triglyceride molecules into oil. The chemical-catalysed reaction is divided into two categories: homogeneous (alkali or acid) and heterogeneous (solid acid or solid alkali). Generally, homogeneous catalysis is more important on an industrial scale because it is more active and demands less time for transesterification, while heterogeneous catalysis is more useful for oil containing higher FFA content [19]. Homogeneous catalysts require purification steps as well as extensive biodiesel and glycerol conditioning to separate the catalysts [20]. The transesterification process for biodiesel production favours heterogeneous catalysts instead of homogeneous catalysts. Heterogeneous catalysts cause easy separation from the reaction medium due to their immiscibility with both the reaction products [21]. This process can produce high-quality biodiesel and glycerol that are easily separable, thus preventing the costly refining process and reducing the drawbacks of transesterification for biodiesel production [22].
2.1.1 Homogeneous Catalysis Reaction
Homogenous catalysts are widely accepted, with almost all biodiesel production using homogenous base catalysts due to their fast reaction rate, high catalytic activity, moderate operating conditions, and ready availability. Homogeneous alkaline catalysts, such as NaOH, KOH, CH3ONa, and CH3OK, are suitable for virgin oil containing less FFA content because high FFA content can lead to saponification (soap formation) and complex separation, while acidic catalysts (e.g., HCl, H2SO4, and H3PO4) are commonly used for high-FFA-content oil, such as waste cooking oil (WCO) [19]. The acid catalyst in moderate-FFA-content oil might negatively affect the production of biodiesel if water in the reaction mixture is above a certain threshold value [23, 24]. In comparison, the acidic catalyst requires a longer time and a higher temperature to complete the transesterification reaction when compared to an alkaline catalyst. Alkaline catalysts are preferred over acid catalysts since the former induces faster transesterification at lower temperatures and pressures, apart from incurring lower capital and operating costs for the biodiesel plant [18].
Alkaline metal alkoxides (NaOCH3 and KOCH3) are better catalysts when compared to hydroxides (NaOH and KOH) because the former does not generate water during the reaction. When water is generated, the hydrolysis reaction is enhanced to form FFA, thus increasing the acid value [19, 25]. Upon mixing NaOH with methanol, a small amount of water will be produced; this hydrolysis reaction will affect the biodiesel yield [19]. Ester saponification may occur when water is formed under alkaline conditions. Moreover, an alkali catalyst is sensitive to FFAs as it can react with the FFA to produce soap and water. Therefore, to make the base catalyst system commercially viable, dehydrated oil with less than 2 wt% FFA, anhydrous alcohol, and a catalyst are necessary for the transesterification process [26]. Furthermore, when the catalyst (NaOH) concentration reaches 1.5 wt%, the biodiesel yield reaches an optimal value. However, as the catalyst concentration is increased further, the yield decreases due to the formation of soap.
Equation 4 presents the mechanism for base-catalyzed reaction transesterification. The alkoxide ion attacks the carbonyl carbon of the triglyceride molecule to form a tetrahedral intermediate that will react with alcohol to regenerate the alkoxide ion. The last step refers to the rearrangement of a tetrahedral intermediate to form an alkyl ester and a diglyceride. Even a small amount of water being generated during the transesterification process may result in soap formation and lower ester yields [25].

The homogeneous acid catalyst is not as popular as the homogeneous base catalyst. However, the former still holds an important advantage over the latter, i.e., the FFA content in oil neither affects the transesterification process nor produces soap and water. It can catalyse both esterification and transesterification processes simultaneously, thus allowing the ability to directly produce biodiesel from low-cost lipid feedstock with high FFA content [19]. However, it is crucial to maintain the water content in biodiesel below 0.5 wt%. This is because water can surround protons (H+) and form water-rich methanol proton complexes, that are less hydrophobic than methanol. The presence of these complexes may cause difficulties for the catalytic species (H+) in approaching hydrophobic triglyceride molecules [25].
As for the mechanism in the acid-catalysed transesterification reaction, as shown in Equation 5, protonation of the carbonyl group molecule leads to carbocation. Next, the alcohol attack will generate a tetrahedral intermediate. The glycerol backbone is removed from the intermediate to regenerate the catalyst, resulting in ester production [25].

2.1.2 Heterogeneous Catalyst Reaction
The heterogeneous catalyst reaction is classified by its Bronsted or Lewis acidity, the number of active sites, and the structural characteristics of the support. The stronger the basicity of the catalyst, the greater the presence of active sites and the catalyst performs better. The heterogeneous catalyst can bring out grafting and entrapment of the active molecule on the surface and inside the pores of a solid support, such as silica, alumina, and ceria [2]. In heterogeneous transesterification, alkali metal oxide [27], mixed metal oxide [28], and alkali metal compounds supported on polymer [29] have been used to produce biodiesel from coconut oil.
The heterogeneous catalyst generates a higher yield, as it does not form soap or fatty acids. It also has a manageable design because it dismisses purification and separation steps. Besides, it contains no salt contaminants, has high glycerin purity with low water content [30]; and can be reused. Heterogeneous basic catalysts that are commonly used in the transesterification reaction are CaO-La2O3 [5], Ca-Al hydrocalumite [18], CaO [19, 24], BaO [23], Li-CaO [25], CaO-ZnO [26], CaO/Al2O3[31], calcium/chitosan spheres [32], natural calcium [33], and MgO [34]. Of these, CaO shows exceptional catalyst properties under mild reaction conditions, along with a longer lifetime at a low cost [35]. Some natural sources, such as quail eggshell waste, are also viewed as potential catalytic precursors for biodiesel production due to their low cost and highly efficient active phase in the transesterification reaction after being subjected to thermal treatment to form the CaO phase. The catalyst of CaO/PVA [29], zirconium tungstate [36] and activated carbon [37] can be reused up to 3 times and still produce a yield of above 80%. Another study tested the reusability of a zeolite catalyst and reported a maximum biodiesel yield that exceeded 75% after 5 runs [38].
The mechanism of heterogeneous catalyst reaction involves the Bronsted basic and Lewis basic activity centers, where they can provide electrons (or accept protons) for (or from) the reactants [39]. As for the solid-acid catalyst, it provides a positively-charged acid site for fatty acid content in oil to get adsorbed, whereas the solid-base catalyst provides a negatively-charged base site for methanol to be adsorbed [40]. The heterogeneous base catalyst mechanism involves the following: (1) physical adsorption of methanol to the basic site of the catalyst; (2) reaction between fatty acid TAG and methanol; (3) formation of methyl ester and side product of glycerol; and (4) the re-availability of the basic site in the catalyst [40]. Equations 6 and 7 display the mechanisms of solid-acid and solid-base catalysts, respectively.


Heterogeneous catalyst has several drawbacks, including higher energy consumption, being costly [30], and slower reaction rate [24]. It can also lead to mass transfer resistance in the presence of three phases (oil/alcohol/catalyst) within the reaction mixture.
2.2 Enzymatic Process
Enzyme, oil, and an acyl acceptor (usually an alcohol) are the main components in the enzymatic process of producing biodiesel. Enzymes, also known as lipase, can be extracted from various sources, such as fungi, bacteria, and yeast. It can catalyse a range of substrates, including FFA [41] and can be performed at lower temperature (up to 323 K) [42]. Lipases are categorised as hydrolases since they carry the hydrolysis of triglycerides in oil to glycerol and fatty acids, in which case they are well defined as carboxylesterases that catalyse both hydrolysis and synthesis of long-chain acylglycerols [43]. Different lipases have different specificity toward oil and alcohol. For example, the type and length of fatty acid, as well as the presence of double bonds and branching in triglycerides, should be considered in choosing lipase [41]; while for alcohol, high or branched alcohol can affect lipase [44].
The yield of biodiesel may be affected by the specificity, efficiency, and immobilisation of lipase, the fatty acid composition of the substrate, and the type of acyl acceptor used. Free enzymes are more suitable for biodiesel production as they are low-cost biocatalysts with high activity [41]. However, the use of immobilised lipase can be beneficial in terms of stabilisation and reusability of lipase [45]. Lipases, such as Candida rugosa lipase (CRL) [44, 46] and Candida antarctica [44], have been used commercially in various companies and industries. Methanol and ethanol are widely used as acyl acceptors, and it is highly necessary to control the molar ratio of oil in order to avoid enzyme deactivation [41, 46]. In an enzymatic reaction, the FFA content in the substrate can be lower or higher than 1%, thus providing an advantage to the enzymatic process over chemical catalysis.
Enzymatic catalysed transesterification adheres to the mechanism of Ping-Pong Bi-Bi with the following four steps: 1) the addition of nucleophilic (oxygen in the O-H group in the enzyme) to form an enzyme-substrate complex; 2) proton transfer to the alkyl oxygen atom of a substrate from the conjugate acid of the amine that forms the glycerol moiety [47]. The initial substrate of TAG will be transformed into DAG and MAG.; 3) the oxygen atom from the alcohol molecule will be added into the carbon atom of C=O acyl enzyme intermediate to form the acylated enzyme-alcohol complex; and finally, 4) the elimination of the acylated enzyme-alcohol complex and the transfer of proton from the conjugated acid of amine to produce fatty acid alkyl ester. Equation 8 expresses the mechanism of Ping-Pong Bi-Bi, where A and B are substrates, E is an enzyme, P and Q are products, EAc and EAcb are intermediate complexes of enzyme and substrate, while EB is an inactive complex enzyme-substrate B.

The transesterification process is mostly carried out below the boiling point of alcohol to avoid alcohol evaporation. The transesterification process is commonly conducted at 30–60 °C, which is below the boiling point of alcohol (in most cases, methanol and ethanol are used as their boiling points are 65 °C and 78 °C, respectively) and the range refers to the optimum temperature range for most lipases [43]. The reaction time of the enzymatic process is generally longer than that of the chemical catalysed process. The enzymatic reaction usually depends on the reaction temperature, reactant, and catalyst concentration.
Some studies applied the ultrasonic-assisted enzymatic method because it can offer a high methyl ester yield in a shorter time at a lower reaction temperature simply with a low amount of methanol and catalysts [48,49,50]. This is because the ultrasonic-assisted method can maximise the interfacial surface area between the immiscible reactants at a lower energy input in comparison to the conventional stirred rectors that can improve the mass transfer between immiscible reactants [48].
The enzymatic method, however, has several drawbacks, including a slower reaction rate, enzyme inhibition, and a high enzyme cost [43, 44]. Overall, the enzymatic method is not widely used as the chemical catalysed method, but still has the potential to become the most promising method to generate biodiesel, where the good enzyme reaction should be designed to gain an optimum amount of biodiesel yield, apart from reducing the cost of production [43].
3 Alternative of Biodiesel Feedstock
The feedstock of biodiesel can be obtained from various sources, particularly from three generations: 1st generation (food crops, i.e., corn, wheat, palm oil, soybean oil, and sunflower oil); 2nd generation (energy crops and waste, i.e., miscanthus, jatropha, food waste, and municipal solid waste); and 3rd generation (microalgae). To date, biodiesel production mostly uses edible oils as the feedstock, such as soybean oil in the US and Argentina, rapeseed oil in the European Union countries, as well as palm oil in tropical countries such as Malaysia, Indonesia, Nigeria, and Columbia [51]. The high demand and cost of edible oil for various purposes have led to the promotion of alternative feedstocks, including non-edible oil and waste oil. For example, jatropha, castorbean, and karanja oils are used in India and Brazil, whereas WCO is used in Japan to produce biodiesel [51].
The high amount of waste generated due to the rapid increase in the world population and industrial development has caused a range of socio-economic issues and environmental problems [52]. The consumption of waste materials is highly advantageous for biodiesel production since there are existing financial or environmental costs associated with their disposal, as these may be alleviated or negated by their diversion to a valuable product like biodiesel.
Different types of oil contain different major fatty acid contents. Table 1 presents the comparison of fatty acids between non-edible oil and waste oil. These oils contain mainly long-chain fatty acids (palmitic acid and oleic acid) and unsaturated fatty acids (i.e., linoleic acid). Typically, biodiesel from vegetable oil sources consists of FAME with a number of carbon atoms ranging from 14 to 22, as well as various levels of unsaturation that give the biodiesel variable thermo-physical properties [53].
The WCO and waste frying oil (WFO) have been considered as alternative biodiesel feedstock since they provide non-competitive biodiesel (with food) in a sustainable and reasonably-priced manner, as well as utilisation of non-edible oil [54]. In the US, about 18 billion litres of biodiesel can be produced from WCO and fat, with McDonald's fast food restaurants being one of the largest sources [51]. However, the main challenge of using WCO is that the high FFA can result in some undesirable side reactions, thus requiring some pre-treatment steps using acid catalyst prior to the transesterification process [54, 55].
Coffee waste and food waste can also be used for biodiesel production. Generally, coffee beans are one of the largest agricultural products in the world, with about 7.2 billion tonnes of coffee being produced annually [56]. On an average, 15% of oil can be extracted from spent coffee grounds that can be used for biodiesel production through transesterification process [56]. Food wasted by a person per year accumulates to about 65 kg, whereby 25% of the wasted foods are vegetables, 24% are cereals, and 12% are fruits [57]. As for biodiesel production, the lipid extraction process from food waste must be executed first before the transesterification process. Table 2 shows some examples of FAME conversion from prior studies using waste sources and various types of catalysts. Notably, some studies proved excellent biodiesel conversions with up to 90% biodiesel yield using waste feedstock.
3.1 Overview of Coconut Oil as the Biodiesel Feedstock
Unlike other waste and non-edible oils, coconut oil is mainly composed of saturated fatty acids (SFA) and medium-chain fatty acids (MCFAs), such as caproic acid (C6:0), caprylic acid (C8:0), capric acid (C10:0), and the highest fatty acid content of lauric acid (C12:0) (~ 50–55% of the fatty acids present) [58,59,60,61]. The coconut oil waste in coconut milk manufacturing contained 40.55% of lauric acid, 17.43% of myristic acid, 15.14% of palmitic acid, and 11.73% of oleic acid [62].
Despite the varying fatty acid profiles, coconut oil biodiesel has demonstrated physicochemical properties that are comparable with standards and other sources of biodiesel. The coconut oil biodiesel has a low viscosity and acid value when compared to the American Society for Testing and Materials (ASTM) standard, thus preventing operational issues and corrosion [63]. Coconut oil biodiesel also has cloud point and pour points within the ASTM standard and a high flash point [59]; indicating that the biodiesel is indeed suitable to be used across cold and hot regions. Biodiesel production using waste coconut oil has several properties that are in the range of the biodiesel standards (ASTM D6751-07 and EN 14214) and some of the properties (i.e. density, acid value, viscosity, and cloud point) are comparable to biodiesel produced from palm oil and virgin coconut biodiesel [32, 64].
Recently, a study assessed the production of biodiesel from coconut waste using sulfuric acid as the homogeneous catalyst to observe the properties, the performance, and the addition value of the coconut waste biodiesel [65]. The B10, B20, and B25 of blending coconut oil biodiesel with diesel were compared with mineral diesel. Apparently, B25 biodiesel may be used and standardised due to its higher volumetric efficiency at a large range of loads when compared to diesel, besides being almost equivalent to diesel in terms of brake power performance.
Next, Zareh et al. [31] assessed the use of coconut, castor, and WCO to produce biodiesel by using potassium hydroxide as the homogeneous catalyst. The part of the coconut waste used was copra (dried meat of coconut), with the oil content ranging from 65 to 72%. The characteristic results showed that the coconut oil properties did fall within the range of the ASTM standard and the coconut biodiesel had the lowest particulate matter content, NOx percentage, as well as CO and CO2 emissions when compared to castor and WCO biodiesels and diesel.
Tables 3, 4, 5 depict previous studies that looked into the coconut oil conversion into biodiesel using homogeneous, heterogeneous, and enzyme catalysts, respectively. Several studies reported excellent conversion with up to 90% biodiesel yield from coconut oil as a source. Hence, the use of coconut oil, especially its waste, is encouraged for biodiesel production.
4 Environmental Impact on Energy Policy and Usage in Malaysia
Generally, all energy sources have an impact on the environment, including energy from non-RE and RE sources. However, energy from non-renewable sources, such as fossil fuels, coal, and natural gas, is more harmful to the environment than energy from renewable sources, because the former emits more CO2, nitrous oxide, methane, and other harmful gases and particles upon combustion. In Malaysia, the dependency on fossil fuels as the main source of electricity generation is high as the country is rich in fossil fuels. The excessive demand for fossil fuels has led to climate change and global warming, along with an alarming depletion of fossil fuels faster than they are replenished. In 2001, the government of Malaysia initiated a cleaner alternative solution, which is RE. However, the RE only managed to raise 1–2% of the total energy mix even after more than a decade, despite the initiation of several RE relevant programmes [66].
In 2011, 35% of industrial, 32% of transportation, 19% of commercial, 12% of residential, and 2% of agriculture were the end-use sectors of energy consumed by Malaysians, whereby the total of this energy consumed reached 62.7 Mtoe [67]. Electricity is the major energy form consumed in the end-use sectors, which accounted for about 45.6% of the total end-use energy usage. Besides, after deducting the transportation sector from the end-use, this share of electricity would increase to 67.1%.
Based on a two-decade duration, the final rate of annual growth of energy consumption in Malaysia stood at 6%, with 13 million tonnes of energy being used in 1990, whereas 41 million tonnes in 2010, with 9% and 6% annual growth rates for the same period for electricity generation and gross domestic product (GDP), respectively [68]. According to Suruhanjaya Tenaga, Malaysia is one of the Asian countries with the highest energy usage and magnitude of energy per capita [68].
The Malaysian government has highlighted energy generation, supply, and usage as part of the national energy policy [69]. In the early 1980s, the Four-Fuel Diversification Policy was introduced to generate electricity from four main energy resources: natural gas, coal, hydropower, and oil/distillate. After the government identified RE sources, the Five-Fuel Diversification Policy was introduced in 2001, with RE set as the fifth energy source in the energy generation mix [66, 69]. Based on the 2001–2005 mandated policy, RE was set to contribute 5% of the electricity generated by 2010. However, only 1.8% was achieved in that period. Since the take-up of RE in Malaysia was rather slow when compared to other countries, the Malaysian government launched the National Renewable Energy Policy in 2011 [67].
Tables 6 and 7 show the policy targets for RE installation capacity and RE electricity generation in Malaysia [67]. Other policies that have affected energy and power in Malaysia are the National Biofuel Policy of 2006 and the National Green Technology Policy of 2009. Both policies have targets to reduce the dependency on fossil fuel reserves. More importantly, the National Biofuel Policy emphasises producing a biodiesel fuel blend of 5% processed palm oil and 95% petroleum diesel. The National Green Technology Policy was introduced, underlining energy, environmental, economic, and social requirements with the overall objective of motivating Malaysians to continuously enjoy a high quality of life in a better environment.
By 2030, reducing greenhouse gas (GHG) emissions intensity of GDP by 45% was designed in Malaysia. In 2014, the GHG intensity level rose by 27%, or equivalent to 317.63 metric tonnes (MT) of CO2 and 50.48 MT of net emissions, in comparison to those in 2005 [70]. Finally, the government targets achieving 20% of the RE mix by 2025. Hence, the RE capacity penetration target must be aggressively pursued by introducing government-provided incentives and establishing proper alignment in terms of government policies and implementation.
The geographic location of Malaysia, with a total landmass of 329,845 km2, offers advantages for maximising renewable resources [71]. Hydroelectric, municipal waste, biomass, solar, and biogas are the renewable resources available in Malaysia. The Ministry of Energy, Green Technology, and Water highlighted that biomass and solar energy have the most potential as renewable resources [71]. Malaysia has active agriculture activities, so palm oil residue, wood residue, coconut waste, and rice husks can be easily obtained for biomass sources. Besides, the climatic conditions in Malaysia encourage the use of solar energy. About 168 million tonnes of biomass were produced in Malaysia, with an approximate deployment of 17% when compared to other technologies. Moreover, biomass can generate up to 2400 MW of energy [70].
Coconut is also one of the agricultural products cultivated in Malaysia. As one of the oldest agro-based industries, coconut has been the fourth most important industrial crop in Malaysia after oil palm, rubber, and paddy based on total planted area [72]. Most of the coconut parts are beneficial for multiple purposes, such as coconut milk, derived from squeezed coconut meat, in which the latter would turn into waste. The waste of coconut meat has been mostly used as animal feed and fertilizer. Regardless of the usage of coconut waste, the amount of waste produced is still excessive, whether it comes from a factory, grocery store, restaurant, or home. About 3960 MT of coconut waste is generated in Malaysia on a yearly basis [5]. Apart from being used as animal feed and fertiliser, coconut waste is normally left to decay in the field. The waste of coconuts should be reduced by making them a useful resource for end products.
5 Conclusion and Future Recommendations
Biodiesel is an excellent alternative fuel that is more environmentally friendly when compared to the diesel fuel used nowadays. Biodiesel lowers pollution and reduces GHG emissions into the atmosphere. The use of coconut waste as a source of biodiesel will reduce the country's demand for fossil fuel and aid the country in addressing the rising issue of energy deficit. Moreover, it helps in the containment of abundant coconut waste in Malaysia. Producing biodiesel using coconut waste benefits waste management companies. This is because the coconut pulp can be recovered and used to reduce the amount of waste disposal, save space in landfills, and conserve natural resources.
As prescribed, by using a heterogeneous or acid homogeneous catalyst, the biodiesel production using coconut waste may provide a higher FAME yield. For further studies, the microbial technology method may be deployed to produce biodiesel from coconut oil to observe the efficacy of this method, the transesterification reaction of coconut oil, and the FAME yield, as these aspects are still untapped.
Some studies have used various coconut parts (e.g., coconut waste, coconut oil, and coconut cooking oil) as the source of biodiesel. However, in general, the biodiesel price will be determined based on the price of the sources; a high source price leads to a high biodiesel price. The high demand for the sources will also lead to a high price for the feedstock. The use of coconut cooking oil should be prevented as it might lead to high demand while coconut waste is more viable to be converted into something valuable, such as biodiesel. Besides, the use of biodiesel can help to reduce the alarming rate of pollution that is affecting both the environment and the use of fossil fuels.
More studies are sought to look into the production of biodiesel from coconut waste in order to achieve the Five-Fuel Diversification Policy, as well as other policies and planning that have targeted the increase of RE in energy usage. Such technologies are at their infancy stage in Malaysia, especially biodiesel. Hence, more research projects should be carried out to make biodiesel commercially available in the future. High investment values are required for Malaysia as RE denotes new energy sources, thus incentives from the government are important at the early stage of development. In conclusion, biodiesel generated from coconut oil can be an environmentally friendly alternative to diesel derived from fossil fuels.
References
Jahirul MI, Brown RJ, Senadeera W, O’Hara IM, Ristovski ZD (2013) Energies 6(8):3764
Thangaraj B, Solomon PR, Muniyandi B, Ranganathan S, Lin L (2019) Clean Energy 3(1):2
Wong YC, Tan YP, Taufiq-Yap YH, Ramli I (2014) SainsMalaysiana 43(5):783
Habibullah M, Masjuki HH, Kalam MA, Rahman SA, Mofijur M, Mobarak HM, Ashraful AM (2015) Renew Sustain Energy Rev 50:819
Sulaiman S, Raman AAA, Aroua MK (2010) 2nd International Conference on Chemical, Biological and Environmental Engineering, p. 254
Aransiola EF, Ojumu TV, Oyekola OO, Madzimbamuto TF, Ikhu-Omoregbe DIO (2014) Biomass Bioenerg 61:276
Altın R, Cetinkaya S, Yücesu HS (2001) Energy Convers Manage 42(5):529
Borges ME, Díaz L (2012) Renew Sustain Energy Rev 16(5):2839
Adeyemi NA, Mohiuddin AKM, Jameel AT (2012) Int Energy J 12(1):15
Ma F, Hanna MA (1999) Biores Technol 70(1):1
Zexue DU, Zhong TANG, Haijing WANG, Jianli ZENG, Yanfeng CHEN, Enze MIN (2013) Chin J Catal 34(1):101
Nakpong P, Wootthikanokkhan S (2010) Renewable Energy 35(8):1682
Leung DYC, Guo Y (2006) Fuel Process Technol 87(10):883
Darunde Dhiraj S, Deshmukh Mangesh M (2012) Int J Emer Technol Adv Eng 2(10):179
Mumtaz MW, Adnan A, Mukhtar H, Rashid U, Danish M (2017) Clean Energy for Sustainable Development, p. 465
Karki S, Sanjel N, Poudel J, Choi JH, Oh SC (2017) Appl Sci 7(6):632
Firdaus MY, Guo Z, Fedosov SN (2016) Biochem Eng J 105:52
Sánchez-Arreola E, Bach H, Hernández LR (2019) Biores Technol Rep 7:100220
Tariq M, Ali S, Khalid N (2012) Renew Sustain Energy Rev 16(8):6303
Alonso DM, Mariscal R, Granados ML, Maireles-Torres P (2009) Catal Today 143(1–2):167
Mohadesi M, Hojabri Z, Moradi G (2014) Biofuel Res J 1(1):30
Chen CL, Huang CC, Tran DT, Chang JS (2012) Biores Technol 113:8
Demirbas A (2010) Energy Convers Manage 51(12):2595
Avhad MR, Marchetti JM (2015) Renew Sustain Energy Rev 50:696
Dalai AK, Issariyakul T, Baroi C (2012) Biodiesel production using homogeneous and heterogeneous catalysts a review. In: Guczi L, Erdôhelyi A (eds) Catalysis for Alternative Energy Generation. Springer, NY
Rachimoellah HM, Resti DA, Zibbeni A, Susila IW (2009) Jurnal Teknik Mesin 11(2):85
Mahfud M, Suryanto A, Qadariyah L, Suprapto S, Kusuma HS (2018) Korean Chem Eng Res 56(2):275
Jaggernauth-Ali P, John E, Bridgemohan P (2015) Fuel 158(143):372
Talha NS, Sulaiman S (2018) Waste Manage 78:929
Kiss FE, Jovanović M, Bošković GC (2010) Fuel Process Technol 91(10):1316
Zareh P, Zare AA, Ghobadian B (2017) Energy 139:883
Sulaiman S, Aziz AA, Aroua K (2014) Adv Environ Biol 781
Sulaiman S, Aziz AA, Aroua MK (2013) J Taiwan Inst Chem Eng 44(2):233
Baskar G, Kalavathy G, Aiswarya R, Selvakumari IA (2019) Advances in bio-oil extraction from nonedible oil seeds and algal biomass. Advances in Eco-Fuels for a Sustainable Environment, p. 187
Khang DS, Razon LF, Madrazo CF, Tan RR (2014) Chem Eng Res Des 92(8):1512
Guldhe A, Singh P, Ansari FA, Singh B, Bux F (2017) Fuel 187:180
Narowska B, Kułażyński M, Łukaszewicz M, Burchacka E (2019) Renew Energy 135:176
Du L, Ding S, Li Z, Lv E, Lu J, Ding J (2018) Energy Convers Manage 173:728
Faruque MO, Razzak SA, Hossain MM (2020) Catalysts 10(9):1025
Sharma S, Saxena V, Baranwal A, Chandra P, Pandey LM (2018) Mater Sci Energy Technol 1(1):11
Norjannah B, Ong HC, Masjuki HH, Juan JC, Chong WT (2016) RSC Adv 6(65):60034
Lopresto CG, Naccarato S, Albo L, De Paola MG, Chakraborty S, Curcio S, Calabro V (2015) Ecotoxicol Environ Saf 121:229
Christopher LP, Kumar H, Zambare VP (2014) Appl Energy 119:497
Budžaki S, Miljić G, Sundaram S, Tišma M, Hessel V (2018) Appl Energy 210:268
Malani RS, Umriwad SB, Kumar K, Goyal A, Moholkar VS (2019) Energy Convers Manage 188:142
Aghababaie M, Beheshti M, Razmjou A, Bordbar AK (2019) Renew Energy 140:104
Sandoval G, Casas-Godoy L, Bonet-Ragel K, Rodrigues J, Ferreira-Dias S, Valero F (2017) Curr Biochem Eng 4(2):109
Veljković VB, Avramović JM, Stamenković OS (2012) Renew Sustain Energy Rev 16(2):1193
Zhang X, Yan S, Tyagi RD, Surampalli RY, Valéro JR (2014) Biores Technol 169:175
Subhedar PB, Gogate PR (2016) Ultrason Sonochem 29:67
Rincon LE, Jaramillo JJ, Cardona CA (2014) Renewable Energy 69:479
Ali J, Rasheed T, Afreen M, Anwar MT, Nawaz Z, Anwar H, Rizwan K (2020) Sci Total Environ 727:138610
Santori G, Nicola GD, Moglie M, Polonara F (2012) Appl Energy 92:109
Tabatabaei M, Karimi K, Horvath IS, Kumar R (2015) Biofuel Res J 7:258
Sahar, Sadaf S, Iqbal J, Ullah I, Bhatti HN, Nouren S, Habib-ur-Rehman, Nisar J, Iqbal M (2018) Sustain Cities Soc 41:220
Uddin MN, Techato K, Rasul MG, Hassan NMS, Mofijur M (2019) Energy Procedia 160:677
Chen C, Chaudhary A, Mathys A (2020) Resour Conserv Recycling 160:104912
Appaiah P, Sunil L, Prasanth Kumar PK, Gopala Krishna AG (2014) J Am Oil Chem Soc 91(6):917
Bello EI, Adekanbi IT, Akinbode FO (2015) European J Eng Technol 3(3):25
Ghani NAA, Channip AA, Phoebe CHH, Ja’afar F, Yasin HM, Usman A (2018) Food Sci Nutr 1
Prasanth Kumar PK, Gopala Krishna AG (2015) Grasas Aceites 66(1):e062
Karnasuta S, Punsuvon V, Nokkaew R (2015) Walailak J Sci Technol 12(3):291
Maitera ON, Louis H, Dass PM, Akakuru UO, Joshua Y (2017) World News Nat Sci 9:62
Sulaiman S, Abdul Aziz AR, Aroua MK (2013) Optimization and modeling of extraction of solid coconut waste oil. J Food Eng 114:228
Krupa R, Gowda RVS, Shreyas R, Srijan AV, Subash S (2018) Int J Res Appl Sci Eng Technol 6(4):2135
Oh TH, Hasanuzzaman M, Selvaraj J, Teo SC, Chua SC (2018) Renew Sustain Energy Rev 81:3021
Chong C, Ni W, Ma L, Liu P, Li Z (2015) Energies 8(4):2828
Abd Rahman NA, Kamaruzzaman SN, Akashah FW (2019) Scenario and strategy towards energy efficiency in Malaysia: a review. MATEC Web Conf 266:02012
Hannan MA, Begum RA, Abdolrasol MG, Lipu MH, Mohamed A, Rashid MM (2018) Renew Sustain Energy Rev 94:551
Abdullah WSW, Osman M, Ab Kadir MZA, Verayiah R (2019) The potential and status of renewable energy development in Malaysia. Energies 12(12):2437
Shafie SM, Mahlia TMI, Masjuki HH, Andriyana A (2011) Renew Sustain Energy Rev 15(9):4370
Alyaqoubi S, Abdullah A, Samudi M, Abdullah N, Addai ZR, Musa KH (2015) J Chem Pharm Res 7(4):967
Chhetri AB, Chris Watts K, Rafiqul Islam M (2008) Energies 1:3
Bautista LF, Vicente G, Rodriguez R, Pacheco M (2009) Biomass Bioenerg 33:862
Koshima Y, Kitamura Y, Islam MZ, Kokawa M (2020) Food Science and Technology 26(4):545
Muangrat R, Pongsirikul I (2019) CyTa Journal of Food 17(1):334
Carmona-Cabello M, Saez-Bastante J, Pinzi S, Dorado MP (2019) Fuel 255:115817
Aransiola EF, Daramola MO, Ojumu TV, Aremu MO, Layokun SK, Solomon BO (2012) Int J Renew Energy Res 2(2):317
Akbar E, Yaakob Z, Kamarudin SK, Ismail M, Salimon J (2009) Eur J Sci Res 29(3):396
Azeez AM, Fasakin AO, Orege JI (2019) Green Sustain Chem 9(1):1–10
Salimon J, Mohd Noor DA, Nazrizawati AT, Mohd Firdaus MY, Noraishah A (2010) Sains Malays 35(9):761
Patel VR, Dumancas GG, Kasi Vismanath LC, Maples R, Subong BJJ (2016) Lipid Insights 9:1
Naik M, Meher LC, Naik SN, Das LM (2008) Biomass Bioenerg 32:354
Nayak SK, Mishra PC, Kumar A, Behera GR, Nayak (2017) Energy Sources A: Recovery Util Environ Eff 39(3):306
Alves CT, Oliveira A, Carneiro SAV, Silva AG, Andrade HMC, Viera de Melo SAB, Torres EA (2013) Fuel Process Technol 106:102
Borah MJ, Das A, Das V, Bhuyan N, Deka D (2019) Fuel 242:345
Zango ZU, Kadir HA, Imam SS, Ibrahim Muhammad A, Abu IG (2019) Am J Chem 9(2):27
Widayat W, Putra DA, Nursafitri I (2019) Materials Today: Proceeding 13:97
Sulaiman NF, Nor Hashim AN, Toeman S, Mat Rosid SJ, Wan Mokhtar WNA, Nadarajan R, Wan Abu Bakar WA (2020) Renew Energy 153:1
Rabie AM, Shaban M, Abukhadra MR, Hosny R, Ahmed SA, Negm NA (2019) J Mol Liq 279:224
Mohd Shohaimi NA, Marodzi FNS (2018) Malaysian J Anal Sci 22(1):157
Tarigan JB, Ginting M, Mubarokah SN, Sebayang F, Karo-karo J, Nguyen TT, Ginting J, Sitepu EK (2019) RSC Adv 9:35109–35116
Caetano NS, Silva VFM, Mata TM (2012) Chem Eng Trans 26:267
Karmee SK (2020) Biofuels 11(2):155
Park J, Kim B, Lee JW (2016) Bioresour Technol 221:55
Barik S, Paul KK, Priyadarshi D (2018) IOP Conf Series Earth Environ Sci 167:012031
Redzwan G, Mohd Amin M, Zulkarnain NN, Abu Mansor MR, Mohamad Annuar MS, Ilham Z (2015) J Mater Cycles Waste Manag 19:676. https://doi.org/10.1007/s10163-015-0463-y
Karmee SK, Linardi D, Lee J, Lin CSK (2015) Waste Manage 41:169
Nahadi JJ, Atadashi MI (2018) Int J Res-Granthaalayah 6(9):487
Ribeiro LMO, Santos BCS, Almeida RMRG (2012) Biomass Bioenergy 47:498
Bouaid A, Acherki H, Garcia A, Martinez M, Aracil J (2017) Fuel 209:141
Lan TTB, Hoa PN (2015) Biol Chem Res 258
Acknowledgements
The authors would like to thank Ministry of Higher Education, Malaysia under FRGS/1/2018/STG01/UNISZA/02/4/RR281 and Universiti Sultan Zainal Abidin for human capital.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, A.F., Zulkurnain, N., Rosid, S.J.M. et al. Catalytic Transesterification of Coconut Oil in Biodiesel Production: A Review. Catal Surv Asia 26, 129–143 (2022). https://doi.org/10.1007/s10563-022-09358-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10563-022-09358-8