Abstract
The direct CO2 oxidative dehydrogenation of ethyl benzene (ODH-EB) is a great potential for the production of valuable styrene monomer. In contrast, past/present styrene (ST) synthesis is mainly obtained from oxidative dehydrogenation of ethyl benzene (EB) and being transformed into pilot scale under CO2 atmosphere. It was due to few unresolved restrictions existed in the synthesis of styrene monomer using the steam assisted process and commercial ST production technology. These problems are being rectified by ODH-EB process using CO2 as a soft oxidant. Therefore, ODH of EB is well-known high temperature process to convert the EB (petroleum by product) into valuable ST monomer through the utilizing of CO2. Present study clearly explains the concise history of dehydrogenation process used to convert EB to ST monomer, which is essential feedstock in the wide range of industrial commodities production. In this discussion we majorly devoted to design, development and synthesis of different Co based catalysts by applying different support materials such as SiO2, MgO, MgAl2O4 and γ-Al2O3 respectively. Moreover, this study extensively deals with chemical behavior of oxidants, utilization of viable active promoters and its characteristics features in the oxidative dehydrogenation process. Different reaction mechanisms in the ODH of EB process to describe CO2 utilization as well as surface styrene monomer formation and evaluation of other by products were discussed widely in this review paper. The surface acidic and basic chemistry of various support materials, its preparation, utilization and its catalytic activity applications have been discussed. Acidic–basic textural properties of different solid oxide support materials have been extensively illustrated through incorporation of variety active metallic oxide and promoters. The catalyst activity evaluation in ODH of EB process as well as plausible reaction mechanism of styrene monomer formation has been explained.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Styrene (ST) manufacture is one of the economical importance methods of oxidative dehydrogenation of ethyl benzene (EB). It was supposed to be due to no other process cannot produce desired ST yields for wide range of industrial requirements. Therefore, desired ST monomer obtained from EB dehydrogenation using the existed and new developed commercial methods [1]. Typically, ST synthesis was developed in the 1930 s by BASF and Dow Chemical companies. Over 25 × 106 tons/year of ST chemical is being produced globally [2]. The annual production of ST in the U.S.A. approximately exceeds 6 × 106 tons and emerged as one of the largest ST producers [3]. Oxidative dehydrogenation of EB is one of most important commercial process for production of ST, which is promised for ~ 80–90% of the commercial production [4]. The K-promoted Fe2O3 solid oxides have been widely functional for ST generation [5]. The average potential of ST production plants is beyond 100 thousand metric tons per year and plants which have an ability of 4 lakh metric ton per year is not remarkable [6]. Evidently, a little development in the plant operation results in considerable increase in outcome of useful products. However, the research towards the basic kinetic modeling depends on Hougen–Watson theory which is not much taking into consideration by several ST synthesized and research groups. They depend on the empirical polynomial correlations for the unit optimization [7,8,9]. The reaction rates were investigated by most of the reviews were not important but efficient [10, 11]. The fundamental kinetic behavior depends on basic ideas those were typically needed for regulation of different reactor configurations with numerous reaction operation conditions. It was essential to build up the precise kinetic representation for the large-scale ST production and to explore the influence of operating procedure on the fixed bed industrial reactors. Moreover, competent side reactions are being emerged in EB dehydrogenation like ST as major product along with side products of benzene and toluene respectively.
The exploration of the kinetic action of minor by-products, such as phenyl acetylene and α-methyl styrene etc., is also important in terms of the styrene chemical quality and separation cost of the outcome products. During the decades, there have been several developments in ST manufacture, because of essential requirement in wide range of industrial applications. Hence, few scientific groups were devoted to ST synthesis through different possible routes with low cost production and energy requirements. Thereby, one of the steam assisted process was evaluated in ST manufacture, but it considerably needs high temperature and an elevated energy requirements [12]. Moreover, this process has few other disadvantages like easy catalyst deactivation, therefore presently not being widely used in ST manufacture. Later, it has been modified to variety of commercial methods such as adiabatic and isothermal reactors which were applied in manufacture of high ST yields through viable reaction conditions. All these processes are being operated under high temperature conditions and much expensive.
In these circumstances, several research groups are exploring new alternative methods to develop unique ST produced methods and applications in ST manufacture with better yields. Thus, present trend majorly deals with CO2 as soft oxidant in ST synthesis using EB as raw material at viable reaction conditions. Moreover, this process mitigates some extent global warming by utilization of CO2 as oxidant in significant levels. Present, this process operating at small scale levels and being transformed into pilot scale due to huge ST monomer requirement in production of different useful commodities. Therefore, this process emerged as an attractive method and alternative route in synthesis of desired ST yields. Till date this method has not been commercialized, but nowadays widely being tested for laboratory scale ST production and applying towards pilot scale level at Samsung General Chemicals Co., Ltd. (SGC) [13]. Hence, it needs to be commercialized by applying CO2 as soft oxidant in ST production compared to other oxidants. In this context, several solid oxides and metal-based materials are applied and displayed better activity results, but present review merely committed to variety of active cobalt oxide phase on different support materials. This is supposed to be due to unusual catalytic properties and high thermal stability of cobalt oxide species in Fischer–Tropsch synthesis (FTS) and several other useful catalytic transformations [14,15,16,17,18,19,20].
In recent years, several cobalt based materials emerged as active catalysts for oxidative dehydrogenation process under influence of CO2 environment along with other oxidants. As a result, beneficial activity results were afforded in terms of high ST yields as well as prolonged activity measurements. Amongst few cobalt based materials such as Co/Al pillared catalysts [21], Co/natural pillard and Co/Al pillard catalysts [22], CoFeSi, NiFeSi and FeSi [23], Co3Mo3N [24], Co/COK-12 [25], Co and Ni carbon nanotube catalysts [26] were displayed a promised activity result in EB dehydrogenation. In the present review, we have focused merely on various supported cobalt oxide catalysts, which are promoting potential catalytic activity for dehydrogenation process to obtain sustained activity accomplishment in terms of high ST yields. Moreover, active role of different promoter species in selective product distribution and catalyst preparation methods aspects have been thoroughly investigated. The product selectivity varied by varying the support materials acidic–basic atmosphere and some extent depends on surface area, these textural properties noticeably regulates the evaluation of desired product outcome along with other possible side products. A dramatic role of CO2 dissociation on acidic and basic oxide moieties are comprehensively investigated in the oxidative dehydrogenation process. Amongst all catalysts, thus Co doped MA catalysts were synthesized by in-situ co-precipitation method and displayed a remarkable role in EB dehydrogenation process. Furthermore, these catalysts were obviously better than Co/MgAl2O4 catalysts prepared by wetness-impregnated method and few reported Co based catalysts, with respect to prolonged product selectivity. This type of spinel oxide materials not yet extensively employed for styrene production in the presence of different oxidants (CO2 and O2, He, N2O, Ar and N2).
In addition, different calcination temperatures of MA materials were synthesized by wetness-impregnation method and applied for oxidative dehydrogenation reactions with Co oxide as active species. In this discussion neither high heating temperature of MA spinels nor mild temperature cobalt supported MA spinel based catalysts were suitable in dehydrogenation reaction. Herein, La doped Co oxide/γ-Al2O3 materials also applied in styrene monomer manufacture, which were apparently better than bare Co/γ-Al2O3 species. On the other words, Co/MgO and Co/SiO2 mixed oxide catalysts were employed for dehydrogenation of EB, although activity mostly depends on surface acidic–basic atmosphere and few other textural properties of the catalysts. All these oxide materials were exhibited different catalytic active species in the reaction environment, meanwhile evaluated a crucial role in getting sustained activity results.
1.1 Types of Dehydrogenation Reactions and Other Methods in Styrene Synthesis
There are different types of styrene production paths amongst major reactions were, commercial methods, oxidative dehydrogenation and normal dehydrogenation except steam assisted synthesis as described in Fig. 1. The active metals like Cr, Fe, Mn, Ce, Pd, Ru, and V on different supported materials were typically related to EB dehydrogenation reaction [27,28,29,30,31,32,33]. Generally, ODH reaction being performed under influence of several oxidants such as O2, SO2, N2O, and CO2, while normal dehydrogenation investigated by using N2, He and Ar as inert atmosphere over several transitions and other metal doped solid oxide catalysts [34,35,36]. Amongst all oxidants CO2 displayed a dramatic role in getting high ST production, which is based on removal of H2 via RWGSR phenomenon. Hence present and reported reviews majorly related with CO2 as suitable diluent for high EB conversion than other normal oxidants and other several oxidants role. The different surface textural properties of the catalysts, such as acidic–basic character, oxidation properties and extent of metal-support interaction determines vital role in larger fraction of ST production. Synthesis of solid oxide catalysts possessed suitable acidic–basic support materials and sustained catalytic properties are interesting and challenging job. It is useful to explore the nature of active metallic species present on variety of support materials in terms of oxidation characteristics, active metal ions dispersion and metal-support interactions.
1.2 Role of Steaming in the Ethyl Benzene Dehydrogenation
Steam is appearing in excess quantity in the ST production process. Over the years, immense hard work had done to control the steam/hydrocarbon ratio to molar values lower than 6, basically through significant changes in active species composition. As increase in styrene/hydrocarbon ration increase in styrene synthesis along with efficiency of the catalyst. There are few advantages using steam are: (1) steam mostly facilitate a desired heat for reaction operation conditions, (2) steam acts as beneficial medium for high equilibrium forward reaction, by decreasing the partial pressure of reactants like EB and H2, (3) steam significantly removes the deposited carbon through considerable gasification reaction. Remarkable influence of steam on the catalytic potential was widely investigated by Coulter et al. [37]. They have determined that decrease in carbon deposition with raising H2O/EB molar ratio and that H2O/EB molar ratio of three was suitable to regulate the accumulated carbon on catalyst surface, at the same time significant enhancement investigated in the catalytic activity presentation. Even though, few drawbacks are existed in steam assisted process such as high reaction temperature, facile catalyst deactivation and lesser styrene production yields. There must be several requirements in replacement of steam as diluents in prolonged ST manufacture. Herein, some of the representative commercial catalytic active materials are illustrated in Table 1.
1.3 Deactivation Phenomenon in Ethyl Benzene Dehydrogenation
Both the catalytic surface texture and dehydrogenation method have been improving over the years. Though, the relocation of active K promoter and its elimination from the catalytic atmosphere catalyst still remain as major problems [50]. In adiabatic method active K species are mostly volatile and moves in the stream of liquid flow during the course of catalytic test. In case of small scale, K migrates from the reactor external surface into core of each pellet in the catalyst owing to temperature gradient results in endothermic nature of the reaction [51]. This relocation and disappearance of active K species leads to adverse change in catalytic activity potential and mechanical stability. Muhler et al. extensively investigated that the H2 as co-product in the reaction which can reduces the active species of the catalyst into magnetite phase. If these types of phases are appeared in the catalyst obviously leads to instant formation of segregated phases in the catalytic atmosphere. As a result, it was investigated that the accumulation of an excessive K-core and lesser K-shell in the reaction atmosphere. Moreover, noteworthy leaching of basic K-promoter in the catalytic environment leads to increase in surface acidic nature. Thereby, afforded mild ST chemical selectivity, it was considerably due to larger fraction of EB molecules dissociation into particularly BZ and TOL along with oxidative side-products. With increased K-content increase in catalyst deactivation due to noticeable moisture content emerged in the catalytic atmosphere [52]. Therefore, catalyst can easily transform into different unusual phases which are typically decreases the catalytic activity staidness. The significant decrease in pressure drop of the reactor atmosphere leads to smooth deterioration of the catalytic activity efficiency. Moreover, considerable reduction of Fe2O3 to Fe3O4 phase results in severe changes in internal textural properties of the active species, thus leads to adverse physical strength and vulnerability to deprivation by association with H2O at low temperature conditions. Dellinger et al. [52], investigated that the doping of Na and Ca fraction to Fe oxide increase the stability of the active species. In this circumstance, several active solid oxides, zeolite materials, reactor developments like palladium membrane reactor, onion-type carbon materials, iron-zeolite species, role of reverse water gas shift reaction phenomenon and mesoporous catalysts are existed and employed for EB dehydrogenation reaction [88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105]. For that reason, several research groups are exploring for sustained catalytic active species in beneficial ST yields. As a result, present review focused on highly active cobalt oxide based catalytic active species design, development, preparation and application in the oxidative dehydrogenation reaction at viable reaction conditions.
1.3.1 Significance of Oxidative Dehydrogenation Process
The ODH of EB is unique route for ST manufacture in comparison to steam assisted and dehydrogenation process in small scale methods. In case of EB dehydrogenation reaction CO2 played a promising role in getting beneficial ST yields owing to elevated equilibrium conditions. Moreover, decreases the partial pressure of input reactants and energy requirements using CO2 as active diluents. In contrast, steam assisted processes extensively needs an elevated operation temperature with noticeable catalyst deactivation through leaching of active metallic species and significant coke accumulation properties. In similar way, normal dehydrogenation procedure merely operated under influence of numerous mild oxidants with marginal catalytic activity accomplishment; owing to absence of RWGSR phenomenon. As we know, styrene yields are obviously higher under CO2 atmospheric reaction conditions compared to steam assisted and normal dehydrogenation process. Other than, oxidative dehydrogenation procedure there are several other methods widely applied and employed in ST monomer manufacture. Amongst, 1-phenylethanol dehydration in liquid and vapor phase conditions afforded a mild styrene yields, even though, this method has not been preferable than oxidative dehydrogenation method. Moreover, 1-phenylethanol dehydration process is obviously expensive and cannot sustain a desired ST monomer synthesis for prolonged activity measurements even though reaction operated at mild temperatures [53,54,55,56,57].
2 Characteristics of Dehydrogenation Reactions
2.1 Steam Assisted Process
Steam assisted process is not much using mostly in nowadays because of existence of several drawbacks which cannot be controlled in significant levels. The regulation of this process is also very difficult than those of normal dehydrogenation and oxidative dehydrogenation process. During the reaction active species deterioration was also much considerable due to moisture fraction emerged in the reaction environment. Thus, apparently leads to instant catalyst deactivation at elevated reaction temperatures. Moreover, it has been investigated that the significant leaching of active K-promoter from the surface of the catalyst during steam assisted method [58].
2.2 Dehydrogenation
In normal dehydrogenation process ST monomer synthesis mostly obtained by utilization of different mild oxidants like N2, He, N2O and Ar respectively [36]. The ST manufacture reaction is endothermic in nature and typically needed an elevated temperature due to high partial pressure of reactants (N2 and EB). While using mild oxidants a marginal activity results are investigated owing to inferior latent heat during the course of reaction. Higher fraction of oxidative products (benzene, toluene, and few gaseous products) is also afforded under influence of mild oxidants and decreases the desired ST selectivity. The normal ST production method could not sustain a beneficial activity results with mild oxidants in the reaction operating conditions.
2.3 Oxidative Dehydrogenation
The ODH is a unique procedure for outstanding styrene production in presence of inferior temperature reaction conditions. In present discussion, this method emerged as beneficial styrene production in laboratory scale when compared to conventionally existed process. It was due to maximum CO2 molecules utilization which major contribution in severe environmental hazardous during the decades and presently too [59]. This, ODH process some extent diminish the environmental hazardous by utilizing CO2 as active diluent in several catalytic conversions. In addition, this process appreciably minimizes the energy requirements for ODH with beneficial products yields in synthesis of numerous useful commodities. Thereby, many scientific people are much interested in exploitation of carbon dioxide as beneficial diluent than those of several mild oxidants over variety of active solid oxide catalysts. Till date there are different oxidants are extensively applied for dehydrogenation method with marginal desired product yields. Amongst all oxidants, O2 majorly facilitated an adverse dissociation of EB molecules into unwanted COX products. Thereby, oxidant like O2 did not attain remarkable activity steadiness in production of ST monomer, even though reaction operated at inferior reaction conditions (< 600 °C) [34, 60]. While using N2O as oxidant, no significant ST yields are achieved owing to steep oxidation of EB molecules into different by products like benzene and toluene respectively [61]. On the other hand, SO2 atmosphere facilitated an adverse environment on active surface of solid oxide catalysts subsequently stayed at mild ST yields, as well as existed few difficulties in operation conditions [62].
3 Types of Commercial Reactor Technologies in Dehydrogenation Process
More than 75–85% of styrene manufacture has been achieved by applying the multiple adiabatic reactor and single reactor with separate beds. The heat needed for isothermal dehydrogenation was supplied by fired heater at 760 °C, and steam to EB weight ratio probably about 1 and steam temperatures are high compared to adiabatic process. Both isothermal processes have advantages in desired styrene fraction and minimize of steam cost [1, 63,64,65, 101].
4 Plausible Reaction Mechanism for Synthesis of Styrene Monomer
Styrene production mechanism varied by utilization of several acidic and basic textures of the catalytic materials, which are crucial requirement in regulation of ST product selectivity. Based on surface acidity of the catalyst, thus noticeable ST production obviously contrasts to basic properties of the active oxides and supported species. Strong acidic oxide catalysts are majorly willing to excessive dissociation/and more activation of EB’s C–C bonds rather than C–H bonds, leads to requirement of elevated activation energy [60]. Therefore, strong acidic behavior of applied catalytic materials is competently achieving ST, Bz and TOL as possible products during the activity measurements. Moreover, unwanted gaseous molecules are also existed because of over oxidation or disorder cracking of EB molecules. In contrast, basic nature of the catalysts are mostly favor towards dissociation of C–H bonds rather than C–C bonds due to low activation energy requirements but EB conversion was very low as depicted in Fig. 2. The basic textural properties of the catalytic active materials are some extent enhances the efficient interface between stream of EB molecules and nascent O species which are generated from prompt dissociation of CO2 molecules. Hence, elevated ST selectivity along with marginal by-products contribution was achieved on basic behavior of the catalytic species [13].
4.1 Types of Plausible Mechanisms on Acidic Sites of γ-Al2O3 in EB Dehydrogenation
Herein, γ-Al2O3 is an essential acidic support for ST manufacture in presence of variety of mild and soft oxidants like N2, N2O, Ar, He and CO2, which are clearly discussed in the above section. Therefore, the activity result on mild oxidants leads to mild ST yields which quite opposite to soft oxidant CO2 molecules. The mild oxidants merely facilitate the undesired products yields along with noticeable carbon deposition during the catalytic activity test. This is because of absence of RWGSR phenomenon when mild oxidants used as active medium in EB dehydrogenation reaction, meanwhile high partial pressures of reactant molecules have been observed (EB and mild oxidant species, N2, N2O, Ar, and He). Moreover, mild oxidants and other oxidants (except CO2) majorly offer C–C bond activation rather than C–H bond activation leads to mild ST selectivity. Herein, γ-Al2O3 used as acidic support which results in competent formation of ST, BZ and Toluene as outcome products during oxidative dehydrogenation. Based on inferior ST selectivity we speculated that there might be strong interactions between stream of EB molecules and Lewis acidic sites possessed γ-Al2O3 species. According to different products evaluation in the EB dehydrogenation process, we tentatively proposed three possible reaction path ways on surface of γ-Al2O3 material as depicted in Figs. 3, 4 and 5.
4.2 General Reaction Mechanism of EB Oxidative Dehydrogenation Through the CO2 Flow
ST synthesis is a surface dehydrogenation reaction which is association between EB stream and CO2 molecules on applied catalytic active materials. First, desired styrene monomer from ethyl benzene obtained through removal of molecular H2 molecules. This styrene monomer was subsequently transformed to yield plausible side products like Bz and TOL as described above. It was due to consecutive oxidative or non-oxidative chemical transformations are emerged in dehydrogenation system. Thereby, producing desired products along with unwanted products as mentioned above. Reactions (1) to (3) summarize the typical forms of the transformations are observed and investigated in dehydrogenation process. Styrene is one of the most important products in dehydrogenation reaction than those of BZ and TOL. The produced styrene from Eq. (1) is mainly aromatic unsaturated hydrocarbons; while Eqs. (2) and (3) are represent the plausible reaction products which are oxygenated products obtained from excessive cracking of EB as illustrated in Figs. 3, 4 and 5.
Regardless of the reaction’s parameters, mostly dehydrogenation mechanisms are based on ability of active metallic elements of the applied materials to dissociative chemisorbed carbon dioxide molecules. The mechanisms of dehydrogenation method had encouraged several chemists in the past [13]. The description of the complex reaction networks could describe the existence of carbon–carbon (C=C) double bonds, in catalytic reaction of EB and how subsequently the styrene synthesis proceeds. These investigations explain a comprehensive surface dehydrogenation reaction which could improve the design of active catalytic materials in the present and future experiments. Different mechanisms based on different intermediates were proposed to govern the consecutive dehydrogenation reactions. Different mechanisms are promised based on outcome of the products in normal dehydrogenation, oxidative dehydrogenation and steam assisted process. Herein, we are clearly illustrated that the dehydrogenation mechanisms in presence of CO2 flow through three different steps (initiation, propagation and chain termination steps in Figs. 6, 7 and 8). The mechanisms differ with the evaluation of desired chemicals and paths that the surface reactions govern which is converted to the proposed products. In oxidative dehydrogenation mechanism, adsorption of CO2 on active catalyst surface initiates the ST production. The first step results in the active metal species (M) being absorbed by stream of reactant and gaseous carbon dioxide molecules. The CO2 gaseous stream was chemisorbed initially in a bridge mode involving two surface acid or base texture of the active catalytic material and also equilibrated in the form of linear mode linking only one site of the active catalytic species. The O=C=O bond is subsequently dissociated into CO and nascent O species. Hydrogen as another co-product generated from EB dehydrogenation via activation of acid or base sites of the applied catalytic materials, then H2 is promptly associated with nascent O species generated from decompose of carbon dioxide molecules in the propagation step. While it is the situation that CO2 majorly chemical adsorbed on acid or base moieties of catalytic materials for maximum dissociation into CO and nascent O species. At end of the reaction ST was major product, meanwhile CO and H2O emerged as by-products then reaction terminated by forward equilibrium phenomenon as mentioned in above Figs. 6, 7 and 8.
4.3 Product Distribution in EB Dehydrogenation in Presence of O2 and N2O Flow
The dehydrogenation reaction under influence of O2 and N2O flow significantly results in formation of variety of possible products like toluene, benzene, and few gaseous specious as shown in Fig. 9. It has been investigated that oxidant O2 mostly promised for excessive dissociation of EB molecules at elevated energy requirements. Hence, oxidant O2 and N2O cannot regulate the desired product formation rather than undesired products yields. Moreover, high cost requirements and special reaction condition needed for O2 and N2O oxidants for EB dehydrogenation reaction as illustrated in Fig. 9. Based on few above undesired catalytic representation and severe reaction operational conditions, many of scientific people are not much willing to perform dehydrogenation process using O2 and N2O as oxidants. A research group investigated that mild ST fraction afforded under influence of O2 oxidant over ceria HT catalysts [36]. Therefore, till date few EB dehydrogenation reports are existed using the O2 oxidant thus leads to mild desired product yield.
In this discussion two types of reaction paths are majorly emerged in laboratory scale in the presence of mild oxidants like N2, O2, He, Ar, N2O and soft oxidant CO2 flow [34,35,36]. Under influence of N2 atmosphere merely normal EB dehydrogenation reaction has been investigated, due to absence of nascent oxygen species which are great requirement in RWGSR phenomenon. Moreover, N2 environment facilitates significant fraction of EB’s C–C bond dissociation phenomenon rather than scissoring of C–H bond. As s result, N2 flow promptly responsible for higher cracking of EB molecules subsequently yielded considerable fraction of undesired side products like BZ and TOL molecules when compared to desired ST product. By contrast CO2 atmosphere diminish the excessive cracking of EB molecules by supplying nascent O species and explained in Figs. 6, 7 and 8. Moreover, under influence of CO2 environment the dehydrogenation process considerably better in small scale methods than existed conventional methods in view of the energy saving and experimental conditions. It was apparently due to evaluation of RWGSR phenomenon thus noticeably enhances EB conversion and ST selectivity. In contrast dehydrogenation reaction under influence of N2, He, Ar, and O2 atmosphere no considerable enhancement investigated in high ST production. While using these oxidants, it was observed that an excessive dissociation of EB molecules into various possible oxygenated products, if catalytic materials possessed an excessive surface acidic texture. In conclusion CO2 environment mostly diminishes an adverse dissociation of EB molecules due to selective styrene production through release of nascent O species with selective activation of C–H bonds on EB molecules.
5 Co Based Catalysts and Its Significance in Dehydrogenation of EB Reaction
5.1 Co/Al Pillared Catalysts
Moronta et al. studied the dehydrogenation process over Co-Mo assisted oxide and metallic mono and bimetallic active moieties on natural and Al-pillared clays. The undesired surface area textural properties were observed in all catalysts, consequently adverse distribution of beneficial Co-Mo species. Moreover, undesired dehydrogenation was afforded by using calcined Co oxides on pillared catalytic active species. The monometallic Co oxide stayed at mild EB conversions while Mo samples are more preferable for superior catalytic activity efficiency. The inferior catalytic activity accomplishment obtained from cobalt species by utilization of cobalt chloride as raw material that generates residual Cl−1 ions and masking the active surface Co sites. On the other words, analogous activity representations are obtained for Co active species in metallic and oxide forms along with weakening of the catalytic efficiency. In case of Mo catalysts, the reduced form displayed a superior catalytic activity behavior than its oxide counterpart catalysts. In the latter case, the oxygen species from the solid metal oxide could favor towards activation of EB molecules to give molecular H2 abstraction, which combine with nascent O species towards production of H2O molecules along with higher fraction of ST monomer. While in the presence of reduced catalysts, EB interacts with active metallic sites to form metal-hydride interactions during reaction, subsequently production of molecular H2 and ST monomer. Regarding the type of clay used, the Mo embedded Al-pillared clay claimed higher conversion in comparison with Mo oxide possessed natural clay. It was due to some extent accessible surface area generated by Al-pillared materials which highly responsible for an improved Mo species distribution. The mild activity efficiency has been achieved on Co active species because of disorder alignment of Co oxide particles. In case of bimetallic catalysts, beneficial activity measurements are afforded using Al-clay as support material; however metallic phases are more advantage than its oxide ones. With increased cobalt content in the catalysts, significant decrease observed in catalytic activity for styrene production, it was supposed to be due to Co species might be blocked by Mo sites; which are in the form of isolated manner. The Co catalysts were prepared by using Co(NO3)2 precursors were displayed noticeable positive results than utilization of Co(CH3COO)2 and Co moieties doped natural clays are better useful than that of Al clay. The catalysts are synthesized from CoCl2 were less active for EB dehydrogenation, which either in metallic or oxide nature. For that reason, it was speculated that the residual Cl−1 anions adversely masking the active Co-Mo species higher than catalyst obtained from cobalt nitrate as precursors [21].
5.2 Co/Natural Pillard and Co/Al Pillard Based Catalysts
Gonzalez and Moronta et al. investigated that the catalytic behavior of different pillared clays materials for EB dehydrogenation. Moreover, they have found that the different catalytic conversions such as dissociation of EB into benzene and ethylene as co-products. In other words, transalkylation of EB generates diethyl benzene and benzene as side products, and dissociation of ethyl group leads to evaluation of toluene and styrene are major products. In this study better ST selectivity afforded on natural Co-impregnated clays compared to Co doped Al-pillared clay catalysts. Hence, natural Co-doped clays are more active for ST production than those of Co doped Al-pillared clay catalysts. Regarding the impregnation with cobalt nitrate or cobalt acetate, the active materials are obtained by acetate salts are represented the mild ST selectivity in both natural and pillared forms. Based on reported one, CH3COO− ions are mostly hidden the active acid sites through high coke accumulation over the surface of active species [22].
5.3 CoFeSi, NiFeSi and FeSi Catalysts
A research group studied the CoFeSi catalysts for high ST selectivity; because of remarkable chemical steadiness of active phase along with existence of basic sites in reaction atmosphere. In contrast, NiFeSi catalyst afforded high carbon deposition leads to mild catalytic efficiency, which could be due to an inferior CO2 utilization efficiency and significant phase change in active species of the catalysts. Moreover, adverse catalytic activity strength has been emerged in FeSi catalyst that implies the formation of Fe3O4 oxide phase during the EB dehydrogenation reaction. For that reason, despite of high coke deposition on surface of the catalysts, ferrites of cobalt catalysts are mostly suitable for high ST production. As a function of difference in activity measurements, thus Co possessed ferrite catalysts are prompt for sustained catalytic activity. According to better ST selectivity, the balanced acidic–basic sites are needed more than merely high acidic catalysts like NiFeSi and FeSi species [23].
5.4 Co3Mo3N/γ-Al2O3 Catalysts
Co3Mo3N catalysts have been emerging as active materials for dehydrogenation procedure and other catalytic conversion process in this decade. In this discussion, Co3Mo3N catalysts are displayed a superior activity for styrene synthesis under influence of CO2 environment because of RWGSR phenomenon. In contrast, N2 atmosphere afforded an inferior catalytic presentation through evolution of excessive quantity of NH3. However, dramatic activity measurements are accomplished in the presence of CO2 + N2 gaseous mixture, because of simultaneous evaluation of RWGSR phenomenon and NH3 as co-product. Moreover, using CO2 + N2 gaseous atmosphere there could be noticeable diminish in surface acid nature of γ-Al2O3 catalyst via clear production of basic properties of NH3 molecules, which is played crucial role in getting desired ST monomer production [24].
5.5 Co/COK-12 (Centrum voor Oppervlaktechemie & Katalyse) Catalyst
Our group earlier studied the oxidative dehydrogenation reaction over Co doped COK-12 materials. The silica source of mesoporous COK-12 material utilized as active support for uniform distribution of Co oxide particles. Therefore, COK-12 support was most favorable for the production of desired EB conversion and ST selectivity [25].
5.6 Co and Ni Carbon Nanotube Catalysts
Guo et al. thoroughly investigated the ODH of EB reaction over Ni and Co doped carbon nanotubes (CNT) in CO2 and O2 flow. In this reaction, Co-CNT catalysts are emerged as unique catalytic active materials for the production of high EB conversion and ST selectivity. It was found that the existence of Co2–C phases is prominent contribution in getting sustained catalytic activity efficiency [26].
6 Present Reviews Majorly Focused on the Following Investigations
6.1 Introduction of γ-Al2O3, SiO2, MgO and MgAl2O4 Spinel as Supporting Materials and Its Significance in Dehydrogenation Reaction
Herein, γ-Al2O3 is a versatile material for several catalytic transformations, which is possessed sufficient Bronsted and Lewis acidic sites for effective interaction with EB and CO2 molecules. The alumina material synthesis is varied by varying the different parameters like calcination temperature, active material synthesis and usage of nitrate precursors. There are varieties of Al2O3 phases obtained by applying different calcination temperatures with variety of surface catalytic active properties. Amongst, γ-Al2O3 obviously provides sufficient Lewis acidic sites for active conversion of EB dehydrogenation under influence of CO2 flow. In contrast, N2 flow facilitated undesirable activity potential due to prompt EB molecules dissociation into coke deposition. In the presence of CO2 flow, the oxidative dehydrogenation process decreases the partial pressure of reactant molecules as a result achieved beneficial equilibrium reaction [66]. Therefore, CO2 environment mostly displayed selective dehydrogenation product like styrene, compared to N2 atmosphere. There are different phases of Al2O3 and being utilized for oxidative dehydrogenation reactions, but significant variation investigated in catalytic activity during the reaction [67]. Amongst all alumina forms, γ-Al2O3 phase was highly suitable for achieving selective production of ST synthesis. Thus because of γ-Al2O3 possess a favorable thermal stability with accessible surface atmosphere and adequate pore size to maintain highly ordered metallic and other metal oxide species. For this reason, several research groups were made positive affords to utilize γ-Al2O3 as promising support material in dehydrogenation and other catalytic activity conversions. Thereby, varieties of chemical experiments like steam reforming of ethanol, Fischer–Tropsch synthesis, and partial oxidation of methane reactions are investigated over γ-Al2O3 incorporated catalysts [68,69,70,71,72,73,74,75]. In addition, MgAl2O4 (MA) spinels, SiO2 and MgO solid oxide catalysts are emerged as remarkable supporting materials in numerous catalytic active transformations. These type materials can be synthesized by conventional preparation methods like, hydrothermal methods, sol–gel, co-precipitation method etc. Therefore, MA spinel, SiO2 and MgO solid oxide materials possessed unusual thermal stability, surface acidic–basic textural properties, beneficial chemical homogeneity and performed as active support materials in different catalytic conversions [76,77,78].
In this point of view, present review focused on Co/MA spinels, Co/SiO2, Co/MgO and Co/γ-Al2O3 materials and employed for dehydrogenation method in the presence of variety of oxidants like O2, N2, CO2, N2O, He and Ar respectively.
6.2 Role of Co on γ-Al2O3 Oxides in Dehydrogenation
In the present review we have investigated that optimal Co/γ-Al2O3 (CA) oxide in N2 flow which could achieve an inferior catalytic activity presentation. Because of, adverse cracking of EB molecules have been observed on active surface of Co/γ-Al2O3 oxide materials. Optimized Co/γ-Al2O3 oxide afforded superior EB conversion along with ST selectivity for prolonged activity using CO2 as oxidant, thus owing to more number of isolated Co particles possessed high surface properties along with inferior crystallite size. In case of low cobalt content, the lesser number of Co particles available on active catalytic atmosphere evidenced from CO2 pulse chemisorption technique, consequently yielded mild EB conversion but no severe changes are observed about ST selectivity and mechanism illustrated in Fig. 6, 7 and 8. While, beyond optimized Co content loaded on γ-Al2O3 (20 wt%) support stayed at some extent mild EB conversion because of significant increase in crystallite size subsequently decrease in surface acidic texture. In presence of CO2 flow EB conversion was increased to high level on 15CA catalyst (shown in Fig. 10), thus because of significant RWGSR phenomenon when compared to N2 gas flow. Moreover, O2 flow applied in present EB dehydrogenation process and showed low ST selectivity due to formation of oxygenated products, further evidenced from reported studies [36, 60, 89]. On the other words, He, Ar and N2O oxidants are also employed in EB dehydrogenation reaction, however, did not achieve beneficial ST yields as well as EB conversion. In addition, all these oxidants are cost effective than CO2 as soft oxidant in this process. Several research groups were expressed this oxidative dehydrogenation method as described in below equations.
In Eq. (4) one indicates the dehydrogenation of EB, and Eq. (5) indicates the RWGSR phenomenon. Moreover, Eq. (6) dictates the simultaneous existence of dehydrogenation and RWGS reaction. ST selectivity was low while using N2 as reaction medium, due to adverse cracking of EB molecules on catalyst surface. Based on activity measurements in CO2 flow, thus suitable acidic–basic properties are majorly favorable along with synergistic effect in optimized CA catalyst. Thereby, larger number of CO2 molecules conversion during on line gas analysis, and endorsed with high CO yield liberated via RWGSR phenomenon [59, 66, 79, 89]. Hence, optimized CA catalyst has been stayed at remarkable EB conversion, when using CO2 as oxidant because of activated cobalt oxide ions along with synergistic Co-Al species. In that way, available more number of CO2 molecules on optimized CA catalyst subsequently transformation into CO species which are vital involvement in the existence of more number of CO2 molecules via RWGSR phenomenon (CO2 + H2 = CO + H2O).
Therefore, significant fractions of carbon deposition transformed into CO2 molecules on surface of CA catalysts, it was close association with reported study [59, 66]. Thereby, one speculation emerged that the generation of more number of CO molecules compared to H2 evolution during the catalytic activity. High ST selectivity has been afforded using CO2 atmosphere rather than O2, He, N2O and N2 oxidants. These unusual results are obviously due to remarkable RWGSR phenomenon with soft oxidant CO2 than those of O2, He, N2O and N2 as diluents. By contrast, an adverse EB molecules decomposition investigated using O2, He, N2O and N2 atmosphere with high coke deposition. While mild carbon deposition accumulation and better activity results were investigated under influence of carbon dioxide atmosphere. Hence, CO2 is emerged as remarkable oxidant in terms of achieving high EB conversion along with ST selectivity. The spent CA catalyst has been subjected to regeneration in air flow, for overnight at 450 °C, and then CA catalyst achieved similar activity results as first-time usage. In this context γ-Al2O3 exhibited a remarkable synergistic property in CA catalyst with vital resistance of instant catalytic deactivation through high surface area properties, steady mechanical and thermal stability.
6.3 La as Unique Promoter on Co/γ-Al2O3 Oxide Material
The endothermic pattern and thermodynamic limitations of dehydrogenation needs an elevated reaction atmosphere which play a promised role in increase of activity results. Oxidative dehydrogenation of CO2 is helpful for the enhancement of desired outcome products thus because CO2 reacts with H2 produced in dehydrogenation of EB to generate larger CO molecules along with water as co-product via RWGSR phenomenon. Temperature effect (600 °C) on catalysts dehydrogenation (with O2, He, N2O and N2 flow) and oxidative dehydrogenation (with CO2 flow) over different LCA samples as illustrated in Fig. 11. With increased reaction temperature, increase in EB conversion because of endothermic nature of dehydrogenation. Hence, sudden increase in EB conversion has been investigated at reaction temperature of > 600 °C. Compared to simple dehydrogenation (in N2 flow), thus dehydrogenation with carbon dioxide yields desire EB conversion because of remarkable RWGSR. As per superior catalytic activity, it is understandable that the reaction about at 600 °C and soft oxidant CO2 flow are the optimum conditions to get high EB conversion. While performing the catalytic activity test, Park et al. found that the noticeable dissociation of carbon dioxide molecules by way of releasing lattice oxygen is helpful in yielding high EB conversion [80, 81]. Under optimized experimental conditions, CA, LCA and LA catalysts were screened for EB dehydrogenation and activity results were discussed in the present review. Thus, La2O3-γ-Al2O3 (LA) has displayed an adverse dehydrogenation results; even though displayed high ST selectivity due to diminish of active acidic sites. Surprisingly bare γ-Al2O3 yielded some extent higher EB dehydrogenation efficiency with noticeable decrease in ST product selectivity which was quite opposite to LA catalyst. After that high EB conversion achieved over CA catalyst because of Co3O4 as active component. The mild dehydrogenation results achieved over bare Co3O4/γ-Al2O3 oxides due to high coke deposition and agglomeration properties. After, incorporation of La2O3 content on Co3O4/γ-Al2O3 catalysts yielded greater catalytic activity during the reaction because of high thermal stability. In the present discussion both Co3O4 and La2O3 contents can be regarded as active species in generation of synergistic catalytic activity. To know the efficiency of the catalysts, La2O3 embedded Co3O4/γ-Al2O3 catalysts were tested for EB dehydrogenation using CO2 as soft oxidant [30, 59, 66]. Initially, La content on Co3O4/γ-Al2O3 increased from 2 to 4 wt%, consequently increase in catalytic efficiency with respect to maximum EB conversion and ST selectivity. After that, incorporation of 6 wt% La2O3 content on Co3O4/γ-Al2O3 catalyst has displayed high crystallite size species due to severe agglomeration properties of Co ions. In addition, high lanthanum content reduces surface acidic atmosphere of γ-Al2O3 in significant fraction, which played a negative role in promised catalytic activity. Herein, HR-TEM images of cobalt oxide incorporated lanthanum/γ-Al2O3 catalysts are clearly reveals the foam type morphology of cobalt oxide species and some of the agglomerated species can be seen in Fig. 12a and b.
Hence high lanthanum content on CA exhibited mild EB conversion when compared to bare CA. It was observed that the neither higher lanthanum oxide (6 wt% La2O3), nor mild La fraction (2 wt% La2O3) in CA catalysts are suitable to achieve desired product yields. On the other hand, EB dehydrogenation reaction performed on Co3O4/γ-Al2O3 and La2O3/Co3O4/γ-Al2O3 oxides in the presence of N2 flow. Even though, initial catalytic activity over LCA catalyst is significantly high compared to CA catalyst however both catalysts undergo activity loss during the reaction. The significant decline in activity under N2 environment with time is due to formation of high carbon material deposition. Moreover, oxygen releasing capability of oxide materials are not playing any role to release nascent O species because of the absence of oxidants [82, 83]. During the catalytic activity, LCA catalyst exhibited an improved EB dehydrogenation compared to bare CA with noticeable changes in activity results. Thus, dehydrogenation activity of CA and LCA oxides are majorly depends on CO2 adsorption capability and thermal stability. The optimal lanthanum embedded CA catalyst displayed a promised catalytic activity because of mild coke deposition as explained in present and reported ones [35, 59]. Therefore, carbonaceous material deposition growth is noticeably higher on bare CA rather than lanthanum doped CA catalyst. Herein, La species greatly retards high coke deposition on surface of the catalyst during the reaction. In addition, CO2 emerged as suitable oxidant along with promotional effect of La2O3 species played a dramatic role on enhancement of the dehydrogenation activity. The beneficial interface between La2O3 and CO2 molecules are remarkable involvement in formation of La2O2CO3 like moieties, which are crucial requirement in promoting the quick RWGSR phenomenon [35]. Hence, noticeable CO fraction investigated on surface of LCA catalyst during the dehydrogenation of EB. Therefore, superb catalytic activity obtained in the presence of CO2 flow with dramatic CO evolution over CA and LCA catalysts. In conclusion, high CO yields achieved on LCA catalysts when compared to bare CA.
6.4 Unique Advantage of La2O3 on γ-Al2O3 in Presence of CO2 Flow
The synergistic effect of La2O3/γ-Al2O3 species promotes the fine distribution of cobalt oxide species into octahedral vacancies of γ-Al2O3 which yielded enhanced catalytic activity. By contrast, CA catalyst could not achieve high catalytic activity compared to lanthanum doped CA oxide because of occupation of cobalt oxide ions into tetrahedral vacancies. After, incorporation of La2O3 on Co3O4/γ-Al2O3 catalyst showed an advantageous influence on catalytic activity by inhibiting the adverse interactions between Co3O4 and γ-Al2O3 species [72]. In presence of La ions, Co oxide species would appear as highly isolated manner rather than disorder bulky Co oxide ions on bare CA catalyst surface as depicted in Fig. 13. Moreover, La2O3 has played a dramatic role in better adsorption of CO2 molecules during EB dehydrogenation as described in Fig. 11.
6.5 Remarkable Influence of Active Co Phase on MgO, SiO2, γ-Al2O3, MgAl2O4 Spinels for Oxidative Dehydrogenation and Product Distribution
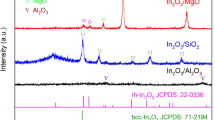
MgAl2O4 spinel exhibited lesser oxidative dehydrogenation activity results with inferior products yields which were not prompt in terms of enhanced catalytic activity presentation. The major by-products formation on MgAl2O4 spinel is toluene and benzene, which are promoted by adverse hydrogenolysis of EB molecules during the reaction. It was majorly due to, significant fraction of acidic properties emerged by Al species in MA spinel. However, ST yields are increased to maximum on MA spinel compared to bare γ-Al2O3 as supported material. After incorporation of Co ions (different atomic ratios of cobalt) on MA catalyst by in-situ co-precipitation method leads to generation of efficient synergistic effect with respect to insertion of Co ions into MA spinel. Therefore, it was investigated that the formation of active MgCo2O4 and MgxCo(1−x)Al2O4 type particles most responsible for getting desired ST product yields. In case of Co/MgAl2O4 catalysts, increased cobalt content increases acidic nature on MgAl2O4 phase, which is crucial requirement in gaining high EB conversion and ST selectivity as illustrated in Fig. 14. By contrast, high basic nature possessed MgO catalyst was favor towards achieving mild EB dehydrogenation with high ST selectivity. On the other hand, an efficient interface needed between Co3O4 and high surface area possessed γ-Al2O3 particles which results in formation of uniform Co oxide particles on catalyst surface. Therefore, desired activity results (high EB conversion) were observed on series of Co3O4/γ-Al2O3 catalysts. While agglomerated Co oxide particles investigated on MgO support, because of lowest surface area of MgO materials. On the other hand, basic properties of MgO and MgCoO4 species in Co3O4/MgO catalyst may helpful in getting beneficial ST yield compared to Co3O4/γ-Al2O3. As reported by [84], MgO can be easily transformed into Mg(OH)2 phase through evaluation of H2O as side product in the reaction atmosphere. Herein, we retard the existence of MgO phase through complete formation of MgAl2O4 species along with active solid solution species in CMA catalysts, Therefore, desired activity results are investigated on CMA catalysts compared to bare Co3O4/MgO catalyst. Herein, Co/SiO2 catalyst also tested for EB dehydrogenation, even though did not yield high EB conversion due to lesser surface acidic texture. Even with high surface area possessed Co/SiO2 catalyst did not get better catalytic activity, because of all catalytic reactions would not depend on high surface area of catalysts. According to the literature, catalytic activity of EB oxidative dehydrogenation reactions are typically depends on some extent acidic–basic properties of the catalysts. To overcome adverse catalytic active properties on CA and CM catalysts, we made an alternative and stable cobalt oxide embedded MgAl2O4 catalysts with adequate acidic–basic atmosphere and thermal stability consequently afforded an excellent catalytic activity presentation. It was supposed to due to, existence of more number of active Co oxide particles distributions through facile reducible patterns and appeared as active solid solution species. Therefore, Co incorporated MA catalyst in which formation of uniform synergistic MgCo2O4 or MgxCo(1−x)Al2O4 moieties are responsible for achieving superior catalytic activity. From HR-TEM images (Fig. 17a, b) we have found different springs which are majorly described the formation of isolated Co oxide phase and solid solution species along with bulk Co oxide species, further evidenced from H2-TPR analysis [66]. Moreover, formation of active solid solution species investigated from XRD and H2-TPR patterns in our previous report [66]. In contrast, agglomerated disorder MgCo2O4 or MgxCo(1−x)Al2O4 species and larger size Co3O4 particles are investigated at higher atomic ratio of cobalt in CMA catalyst as explained in reported study [66]. Hence, 1.25CMA catalyst had displayed mild EB conversion, which was apparent, evidenced from marginal surface area, high crystallite size properties and complex reduction texture. According to present study investigations, activity results Co supported catalysts follows order like MgAl2O4 > γ-Al2O3 > SiO2 > MgO.
6.6 Influence of Reaction Conditions in Activity Results
The styrene manufacture on 1CMA and 1.25CMA catalysts were performed at various temperatures in the range of 450 to 650 °C and results were shown in Fig. 18. Increase in dehydrogenation activity results with increasing temperature owing to endothermic properties of ODH reactions and showed that temperature is prominent requirement in these reactions. Maximum styrene monomer selectivity investigated at reaction temperatures of 450 and 550 °C respectively then smooth decrease investigated in ST selectivity at reaction temperature of 600 °C. Then, high reaction temperature (650 °C) favors an excessive cracking of EB’s C–C bonds over applied catalytic active materials. Therefore, elevated temperature like > 600 °C of ODH leads to smooth cracking of EB into carbonaceous deposits (COX) and evaluation of other byproducts, consequently achieved mild ST selectivity.
But, better ST selectivity afforded on 1CMA oxide catalyst at 600 °C (better thermal and mechanical stability) compared to 1.25CMA catalyst, due to mutual interaction of cobalt, magnesium oxide and γ-Al2O3 species as solid solution texture like MgCo2O4 and MgxCo(1−x)Al2O4. Therefore, 1CMA catalyst had afforded a remarkable catalytic property through favorable acidic–basic texture and formation of active MgCo2O4 or MgxCo(1−x)Al2O4 particles as shown in Fig. 14. In other words, during online analysis high CO yield achieved on 1CMA rather than 1.25CMA catalyst, because of mutual interaction between active Co oxide ions and CO2 molecules. Hence, larger fraction of CO2 molecules availability and efficiently utilized on active surface of 1CMA catalyst which would be transformed into CO species with great involvement in ST production via RWGSR phenomenon (CO2 + H2 = CO + H2O) [89]. Thus, results in higher fraction of deposited carbon material considerably oxidized into CO2 molecules on surface of 1CMA rather than 1.25CMA catalyst; it was close correlation with reported study [29, 30, 59]. As a result, high CO yield investigated when compared to H2 evolution on surface of the 1CMA catalyst; hence this scenario concludes that great involvement of H2 in RWGSR. While, 1.25CMA catalyst achieved mild ODH-EB activity at 1 h owing to lesser quantity of CO2 molecules exploitation consequently investigated mild CO yields evidenced from online gas analysis. Considerable diminish in carbon material accumulation investigated in the presence of CO2 flow which was significantly lower than N2 atmosphere. Generally reaction scheme of the oxidative dehydrogenation of EB in the presence of carbon dioxide will be observed as described in the Fig. 19.
6.7 Influence of Calcination Temperature in the Formation of MgAl2O4 Spinels (MA)
An extensive study made on preparation of MgAl2O4 spinels, which is playing a prominent role on regulation of Co particles dispersion for sustained catalytic activity accomplishment. In 600MA spinel, it has been investigated that the competent formation of γ-Al2O3 and MA spinel phase results in accessible surface area. Even though, high acidity in 600MA spinel did not facilitate a preferred ST selectivity and EB conversion. After Co oxide impregnation on 600MA spinel, ST selectivity has not been attractive owing to slight increase in surface acidic properties of Co/600MA catalyst. Based on beneficial catalytic activity potential, thus balanced acidic–basic atmosphere is typically required for an elevated catalytic activity. Then, calcination temperature of 700 °C is majorly needed for complete MA spinel formation. Thereby, surface acidic nature was decreased with increase in crystallite size which is negative role on advanced catalytic activity as explained in Fig. 20. Moreover, considerable decrease of 700MA spinel surface acidic density compared to 600MA spinel. While increasing calcination temperature of MA spinels from 600 to 700 °C significant increase in crystallite size have been investigated. By contrast, 800MA spinel displayed an unusual surface area with irrespective of high calcination temperature and emerged as unique support material for uniform Co particles dispersion. Moreover, slight decrease in surface acidic behavior leads to beneficial role in getting high catalytic activity. By investigation of Nasser et al. [78, 85] the calcination temperature of 800 °C was beneficial for a desirable surface area with marginal crystallite nature. On the other hand, formation of 900MA spinel significantly changes catalyst surface chemical and acidic textural properties, consequently decrease in surface acidic contribution when compared to low temperature MA spinels.
6.8 Co3O4 doped MgAl2O4 (CMA) Catalysts
In this context, MgAl2O4 spinels were obtained by co-precipitation method then calcined at different calcination temperatures (600–900 °C). After that active cobalt oxide (wt%) loaded on MgAl2O4 spinels prepared by wetness-impregnation method and evaluated for EB dehydrogenation reactions. Initially, EB dehydrogenation reaction was performed on different MA spinels and activity results were described in Fig. 20. The different calcination temperatures of MA spinels are significantly regulating surface textural properties as well as Co particles dispersion, which lead to dissimilar catalytic activity results. After, Co loaded 600MA spinel afforded a marginal ST selectivity thus because of increase in surface acidic nature. Furthermore, Co oxide doped 700MA spinel catalyst, displayed a slight decrease in acidic nature with an improved ST yields as depicted in Fig. 21. Typically, high calcination temperature of 700MA spinel results in decrease of surface acidic nature through high crystalline size of the catalyst. Even though, CO2 molecules exploitation as well as dissociation obviously high compared to Co/600MA catalyst. Hence, dissociation of CO2 species and interaction with EB molecules were prominent influence on Co/700MA catalyst, due to formation of active single-phase MA spinel. But, Co doped 800MA spinel catalyst provided remarkable activity results in the view of better EB conversion and ST yield compared to Co/700MA catalyst. For that reason, we assumed that the noteworthy involvement of RWGSR on surface of Co/800MA catalyst. Moreover, 800MA spinel noticeably facilitates the desirable surface area platform for fine dispersion of Co oxide particles on surface of the catalyst (seen in Fig. 15) consequently afforded higher CO2 molecules utilization and dissociation [78]. By contrast, low surface area possessed 900MA spinel has not been provided any high surface area towards more number of Co oxide species distribution, subsequently achieved marginal catalytic activity. In addition, agglomerated Co oxide particles investigated on surface of 900MA spinels as shown in Fig. 16.
6.9 Effect of Catalyst Preparation Procedure in EB Dehydrogenation
There are several factors to influence catalytic activity on EB dehydrogenation and ST production such as, catalyst preparation procedure and nature of the support materials. In the present discussion, we are simply discussing the wetness-impregnation and co-precipitation methods to prepare cobalt based and promoter doped catalysts. In wetness-impregnation method, the active cobalt oxide species are dispersed simply on outer surface of the catalysts. This could results in either formation of fine cobalt species if support material possesses accessible surface area, or growth of bulk Co species with mild surface area of support materials. All these factors majorly depend on some extent specific surface area of support materials to be impregnated with active metallic nitrate precursors. As a function of catalytic activity, few support materials are sustained up to certain fraction of metallic loading during the catalyst’s preparation, furthermore significant growth in size of cobalt ions has been observed. Based on reported and present review findings the mild catalytic activity has been observed on wetness-impregnation method catalysts [21, 72]. Moreover, wetness-impregnation method catalysts are facilitating agglomerated particles while adding the excessive cobalt oxide content on support material during the catalysts preparation. Thereby, noteworthy decrease in surface area of active species, as well as of ST yields and EB conversion. By contrast, co-precipitation method catalysts are advantageous for EB dehydrogenation compared to wetness-impregnation method. In co-precipitation method catalysts, the active metal species are distributed either on outer space of support material or diffusion into inner core of the active catalytic materials. This type of catalytic chemical interactions generates the dramatic textural properties for sustained dehydrogenation process and several other catalytic transformations. In addition, efficient support-metal interactions are emerged as active catalytic solid solution phases which are supporting significant contribution in getting wonderful catalytic activity [72]. In co-precipitation catalysts, there may be high diffusion properties of EB molecules into active core of the catalyst leads to promised ST yields. On the other way, agglomeration properties are diminished to significant level than that of wetness-impregnated catalysts, hence displayed prolonged activity achievement in EB dehydrogenation. According to crystallite pattern, more number of smaller size Co species are obtained from co-precipitation method when compared to of wetness-impregnation catalysts [66, 72, 88, 90, 92].
6.9.1 Influence of Metallic Precursors on Catalytic Activity
Based on reported catalyst preparation methods, there were variety of metallic salts utilized to get cobalt based catalysts and applied for oxidative dehydrogenation process. Amongst, Co(NO3)2 materials are much preferable for beneficial cobalt particles distribution on surface of the support materials. In case of cobalt nitrate precursors, a marginal crystallite pattern has also been observed, which played a key role in dramatic catalytic activity efficiency. But cobalt acetate precursors are not much advantage for formation of active cobalt species towards desired products yields. Thereby, appearance of larger crystallite size when using the metal acetate as active precursors. In similar manner, cobalt chloride salts are also not advantage for fine cobalt species formation, which mostly regulate the activity efficiency of the reaction. It was clearly investigated that cobalt chloride salts cannot be decomposed in to active cobalt species during the low and high calcination temperature conditions. As function of superior catalytic activity, numerous cobalt precursors were used in active cobalt-based catalysts preparation, thus order follows as cobalt nitrate, >Co(CH3COO)2 and >cobalt chloride respectively [21]. According to the aforementioned results, Co nitrate precursors emerged as active precursor’s materials and utilized in preparation of CA, LCA, Co/800MA and 1CMA catalysts in the present review article. Therefore, generated unusual catalytic activity compared with reported cobalt-based catalysts. Moreover, we achieved a marginal cobalt oxide crystallite size by using the cobalt nitrate as precursors in all synthesized catalysts.
6.9.2 Types of Promoters Tested for Oxidative Dehydrogenation Reactions
During the decades, several active promoters are being investigated and still promoter-based catalysts are testing continuously for dehydrogenation process. Herein, active promoters are being provided outstanding activity and textural properties, subsequently existence of unusual catalytic efficiency. In this perspective, active potassium promoter was employed along with Fe3O4 species for steam assisted EB dehydrogenation process. However, steam assisted process did not provide beneficial atmosphere for prolonged interaction between potassium and iron oxide phase. Because of leaching of active potassium promoter from surface of active species and some extent surface potassium diffusion into core of the catalytic moieties leads to marginal catalytic activity performance [12]. Braga et al. investigated that the active role of ferrites in CoFeSi and NiFeSi catalysts, which are exhibited high ST yield. Herein, Fe behaves like active promoter in both the catalysts, but significant dissimilarity investigated in their catalytic activity efficiency [23]. In present trend, COK-12 emerged as one of the mesoporous support material for styrene manufacture and several other catalytic transformations. It was mostly due to efficient diffusion of Co species on high surface area possessed COK-12 material [25]. After that, molybdenum nitride incorporated Co catalysts were existed as useful species in ST production, by facilitating a basic nature in the catalytic atmosphere. In these catalysts’ molybdenum nitride acts as active promoter in superior activity [24]. In other words, Co2-C moieties are more advantage than Ni2-C species, due to basic nature of cobalt based carbon catalyst [26]. The Co ions doped HT materials are afforded an improved EB conversion compared to bare HT support material, because of active role of Co species [86]. Fe-Co/Mg(Al)O derived from hydrotalcites has been afforded an improved catalytic activity owing to beneficial role of Co content [87]. In the present review also associated with different active promoters in the catalyst’s preparation amongst La is significantly better than Mg as active promoter it was close correlation with reported ones [35, 59]. After incorporation of La on CA oxide, results in significant increase in catalytic properties with respect to prolonged activity. In case of Mg promoted CA spinel oxide, there has been noticeable growth in carbon deposition on surface of the active species, because of existence of MgO species in the catalytic atmosphere. Present review majorly focused on formation of active MgAl2O4 spinel rather than inactive MgO phase for better dispersion of Co ions and high catalytic activity. Herein, we included reported and present catalysts activity results in Table 2.
6.9.3 Economical Importance of Styrene Production
During the decades there has been growing much interest in ST production owing to global economical importance as well as high capacity and demand as described in Fig. 22. Based on diagram ST production capacity has gradually been increasing since 2011 to till date, which leads to development of new commercial pilot plants in across the world. Presently, USA, china and Japan countries are account for the largest 40% to 50% of global ST production.
In 2015 the total annual production of styrene approximately 30 to 40 metric tons, thus expected to rise until 2020 and assumed to be above 40 metric tons. Therefore, ST production and demand almost equal during the decades with growth rate of 3.2%. All these ST productions mostly obtained from commercial pilot plants in the world wide. In this perspective, if we apply pilot scale EB dehydrogenation in the presence of CO2 environment, leads to noticeable increase in global economical growth and decrease in cost requirements compared to operational commercial methods [13]. Moreover, this process beneficial in terms of some extent mitigation of global warming issues by efficient utilization of CO2 as soft oxidant.
7 Conclusions
Approximately, in worldwide 27,180.000 metric tons of styrene and styrene derivative compounds are being needed for essential commodities production. The major contribution of styrene chemical manufacture is obtaining via existed industrial process such as commercial dehydrogenation and steam assisted process. Styrene is not a sustainable source of energy; it has been producing since several decades through viable industrial dehydrogenation methods. Moreover, it is estimated that worldwide cumulative styrene demand and supply gradually increasing as mentioned in previous section because of numerous industrial necessities. This study systematically explains a broad literature review on dehydrogenation reaction including the production of liquid styrene monomer through several existed commercial systems. In the present trend several research groups have been focusing on extensive utilization of CO2 as oxidant in styrene manufacture owing to beneficial properties than other oxidants and commercial methods.
This process needs to be commercialized for EB dehydrogenation in view of the worldwide huge ST supply and demand. In addition, this review broadly illustrates the various surface catalytic active species role in remarkable EB dehydrogenation. The complete dehydration of 1-phenylethanol and hydrogenolysis path employed for synthesis of higher fraction of styrene molecules and its derivative chemicals. Even though, these methods are cannot mitigate few unresolved drawbacks in terms of low-cost requirements and desired ST yields. Hence, we mostly focused on preparation of Lewis and Bronsted acidic sites possessed γ-Al2O3 material which was incorporated in CA, LCA, 1CMA and Co/800MA catalytic materials and exhibited desired activity efficiency in EB dehydrogenation process. Amongst, active solid solution species (MgCo2O4 or MgxCo(1−x)Al2O4) promoted 1CMA catalysts played a major role in prolonged activity measurements. These types of catalysts noticeably retard the huge carbon material accumulation on surface of the active species owing to high thermal stability. Thereby, 1CMA catalytic species are emerged as promised and active materials in EB oxidative dehydrogenation process. On the other words, different calcination temperatures of MA spinels with cobalt oxide material obviously regulate the possible product formation in EB dehydrogenation. In this context, neither high acidic Co/600MA material nor inferior acidic nature of Co/900MA catalysts are beneficial in high ST production. By contrast, Co/800MA catalysts are suitable and selective for better activity measurements than its competent catalysts in the present discussion. In case of LCA catalysts, active synergistic Co-La moieties are significant influence on advanced activity measurements in terms of high EB conversion and ST yields. Till date this type of catalytic active materials did not broadly applied in ST manufacture, so this type of cobalt based materials are preferable solid oxide catalysts compared to few reported cobalt based catalysts as listed in Table 2.
Additionally, the development of several cobalt based catalysts and different preparation methods and past dehydrogenation process have been investigated. As a result, in the present investigation catalysts obtained from co-precipitation methods are remarkable than wetness-impregnation method. Intensive investigations require in terms of technical development of EB dehydrogenation to ensure the success in deployment. The contribution of ST production via several methods such as steam assisted process, liquid phase dehydration of 1-phenyl ethanol dehydration and benzene hydro peroxide dehydration process are being less important. Therefore, styrene monomer is one of the consequences of global economy growth, which makes the exploration about the EB dehydrogenation in CO2 atmosphere; it is an extremely essential method. Hence, green research has continued to transformation of EB into ST monomer through utilizing CO2 as an oxidant under optimized conditions. The huge ST chemical synthesis noticeably enhances the global economic growth as well as wide range of useful commodities production.
Abbreviations
- ODH:
-
Oxidative dehydrogenation
- EB:
-
Ethyl benzene
- ST:
-
Styrene
- TOL:
-
Toluene
- BZ:
-
Benzene
- RWGSR:
-
Reverse water gas shift reaction
- Dehydrogenation oxidants:
-
N2, He and Ar
- Soft oxidants:
-
CO2, O2, N2O and SO2
- Steam:
-
Water vapor
- CA:
-
Co3O4/γ-Al2O3
- CMA:
-
Co3O4/MgAl2O4
- LA:
-
La3O3/γ-Al2O3
- LCA:
-
La3O3/Co3O4/Al2O3
- MA:
-
MgAl2O4
- CM:
-
Co3O4/MgO
- CS:
-
Co3O4/SiO2
- COK:
-
12-Centrum voor Oppervlaktechemie & Katalyse
- XMA:
-
X denotes the calcination temperature of MgAl2O4 spinel like 600MA, 700MA, 800MA and 900MA respectively
References
Cavani F, Trifiro F (1995) Appl Catal A Gen 133:219–239
Product Focus: Styrene (2002) Chem Week 15:36
Profile Chemical (2001) Propylene glycol. Chem Mark Rep 249:37
James DH, Castor WM (1994) Styrene. In: Campbell FT, Pfefferkorn R, Rounsaville JF (eds) Ullmann’s encyclopedia of industrial chemistry, vol 25. Wiley-VCH, Weinheim, p 329
Kerby KK (1945) US Patent 2,370,797
Sundaram KM, Sardina H, Fernandez-Baujin JM, Hildreth JM (1991) Styrene Plant Simul Optim Hydrocarbon Process 70:93
Kolios G, Eigenberger G (1999) Chem Eng Sci 54:2637–2646
Savoretti AA, Borio DO, Bucala V, Porras JA (1999) Chem Eng Sci 54:205–213
Yee AKY, Ray AK, Rangaiah GP (2003) Comput Chem Eng 27:111–130
Sheel JGP, Crowe CM (1969) Can J Chem Eng 47:183–196
Clough DE, Ramirez WF (1976) AIChE J 22:1097
Lee EH (1973) Catal Rev 8:285–305
Mohd Bismillah A, Park SE (2012) Energy Environ Sci 5:9419–9437
Yasuo O, Takashi A, Satoshi T, Naoto T (2003) Energy Fuels 17:804–809
Abdullah I, All H, Ebrahim Vasheghani F, Kambiz S (2007) J Nat Gas Chem 16:115–120
Márton K, AdrianaDe S, Hanna ES, Magdolna RM, József V, Anthony AGT (2010) J Mol Catal A: Chem 333:37–45
David V, Freek K, John N (2012) Ruud van Ommen. J Catal Sci Technol 2:1221–1233
Mohsen M, Hossein A, Ali AM, Masoud K (2012) J Thermodyn Catal 3(2):1–6. https://doi.org/10.4172/2157-7544.1000113
Eddie M, John MV (2012) J Catal 291:79–86
Hyuntae S, Umit SO (2016) Energy Fuels 30:5309–5322
Moronta A, Troconis ME, Gonzalez E, Moran C, Sanchez J, Gonzalez A, Quinonez J (2006) Appl Catal A Gen 310:199–204
Gonzáleza E, Moronta A (2004) Appl Catal A Gen 258:99–105
Braga TP, Campos Sales BM, Pinheiro AN, Herrera WT, Saitovitchb B, Valentini A (2011) Catal Sci Technol 1:1383–1392
Madhavi J, Suresh M, Ramesh Babu GV, Sai Prasad PS, David Raju B, Rama-Rao KS (2014) J CO2 Util 8:21–26
Ramudu P, Anand N, Mohan V, Muralidhar G, Sai Prasad PS, David Raju B, Rama Rao KS (2015) J Chem Sci 127:701–709
Xiao FG, Joong HK, Geon JK (2011) Catal Today 164:336–340
Xingnan Y, Yinghong Y, Changxi M, Zaiku X, Weiming H, Zi G (2005) Green Chem 7:524–528
Mimuraa N, Takaharaa I, Saitoa M, Hattorib T, Ohkumac K, Andod M (1998) Stud Surf Sci Catal 114:415–418
David Raju B, Kwang Min C, Dae-Soo H, Jeong-Boon K, Park SE (2006) Catal Today 115:242–247
Li Z, Zili W, Nicholas CN, Aaron DS, Igor IS, Steven HO (2015) ACS Catal 5:6426–6435
Engaldas H, Vanama Pavan K, Kuna R, Komandur VRC, Vattikonda VR (2015) Appl Petrochem Res 5:71–80
Toshimitsu S, Kiyoharu N (2011) J Jpn Pet Inst 54:66–79
Rao KN, Reddy BM, Abhishek B, Yeong-Hui S, Nanzhe J, Sang-Eon P (2009) Appl Catal B Environ 91:649–656
Reddy BM, Rao KN, Reddy GK, Khan A, Park SE (2007) J Phys Chem C 111:18751–18758
Liu BS, Chang RZ, Jiang L, Liu W, Au CT (2008) J Phy Chem C 112:15490–15501
Venugopal AK, Venugopalan AT, Kaliyappan P, Swamy Raja T (2013) Green Chem 15:3259–3267
Coulter K, Goodman DW, Moore RG (1995) Catal Lett 31:1–8
Sionnest PG (2007) Mater Matters 2:10
Chestnoy N, Hull R, Brus LE (1986) J Phys Chem 85:2237
Somorjai GA, Rioux RM (2005) Catal Today 100:201
Anastas PT, Williamson TC (eds) (1998) Green chemistry: frontiers in chemical synthesis and processes. Oxford University Press, Oxford
Zalesskiy S, Ananikov V (2012) Organometallics 31:2302–2309
Astruc D (ed) Nanoparticles and catalysis. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, p 621
Anastas PT, Kirchhoff MM (2002) Acc Chem Res 35:686–694
Shaikhutdinov SK, Joseph Y, Kuhrs C, Ranke W, Weiss W (1999) Faraday Discuss 114:363
Anastas PT, Heine LG, Williamson TC (eds) (2000) Green chemical syntheses and processes. American Chemical Society, Washington DC
Kuhrs C, Swoboda M, Weiss W (2001) Top Catal 15:13
Anastas PT, Farris CA (eds) (1994) Benign by design: alternative synthetic design for pollution prevention. ACS symposium series, vol 577. American Chemical Society, Washington DC
Emig G, Hofmann H (1983) J Catal 84:15–26
Mross WD (1983) Catal Rev Sci Eng 25:17
Dellinger PW, Moore RG, Sherrod FA, Smith AR (1996) US Patent 5,510,552
Rase HF (2000) Handbook of commercial catalysts: heterogeneous catalysts. CRC Press, Boca Raton
Nicolás MB, Carlos RA, Alberto JM (2008) Catal Commun 10:261–265
Nazmul AK, Jin-Soo H, Sung HJ (2011) Bull Korean Chem Soc 32:1327–1330
Nicolás MB, Andrés FT, Carlos RA, Alberto JM (2013) Appl Catal A Gen 458:28–38
Lange JP, Mesters CMAM (2001) Appl Catal A Gen 210:247–255
Jean-Paul L, Vincent O (2006) J Catal 238:6–12
Mimuraa N, Takaharaa I, Saitoa M, Hattorib T, Ohkumac K, Andod M (1998) Catal Today 45:61–64
Venkata Rao M, Venkateshwarlu V, Thirupathaiah K, Ashok Raju M, Nagaiah P, Murali K, David Raju B, Rama Rao KS (2017) Arab J Chem. https://doi.org/10.1016/j.arabjc.2017.07.014
Kumarsrinivasan S, Akrati V, Chinnakonda SG (2012) Green Chem 14:461–471
Kustrowski P, Segura Y, Chmielarz L, Surman J, Dziembaj R, Cool P (2006) Catal Today 114:307–313
Gaspar NI, Cohen Vadekar AD, Pasternak IS (1975) Can J Chem Eng 53:79–82
Jean-Paul L, Vincent O (2007) Ind Eng Chem Res 46:6899–6903
Addiego WP, Liu W, Boger T (2001) Catal Today 69:25–31
Mitchell JE Jr (1946) Trans Am Inst Chem Eng 42:293–308
Venkata Rao M, Peddinti N, Challa P, Vasikarappa K, Ajmeera N, Burri David R, Rama Rao KS (2018) Arab J Chem. https://doi.org/10.1016/j.arabjc.2018.07.018
Christian N, Valeriya Z, Ignacio MC, Hero JH, Freek K, Michiel M (2013) Catal Sci Technol 3:519–526
Chiou JYZ, Yang SY, Lai CL, Kung HY, Tang CW, Wang CB (2013) Mod Res Catal 2:13–21
Brabant C, Khodakov A, Constant AG (2017) C R Chim 20:40–46
Jongsomjit B, Panpranot J, Goodwin JG (2001) J Catal 204:98–109
Zhang NW, Huang CJ, Zhu XQ, Xu JD, Weng WZ, Wan HL (2012) Chemistry 7:1895–1901
Caia Z, Lia J, Liewb K, Hua J (2010) J Mol Catal A: Chem 330:10–17
Song H, Zhang L, Ozkan US (2007) Green Chem 9:686–694
Batista MS, Santos RKS, Assaf EM, Assaf JM, Ticianelli EA (2004) J Power Sources 134:27–32
Taghavimoghaddam J, Knowles GP, Chaffee AL (2012) J Mol Catal A: Chem 358:79–88
Guo J, Lou H, Zhao H, Wang X, Zheng X (2004) Mater Lett 58:1920–1923
Chandradass J, Ki Hyeon K (2010) J Ceram Process Res 11:96–99
Mostafa YN, Ibrahim SA, Ihab S (2014) Spectrochim Acta Part A Mol Biomol Spectrosc 131:329–334
Chang JS, Vislovskiy VP, Park MS, Hong DY, Yoo JS, Park SE (2003) Green Chem 5:587–590
David Raju B, Choi KM, Han SC, Abhishek B, Park SE (2007) J Mol Catal A: Chem 269:58–63
Hong DY, Vislovsky VP, Park SE, Park MS, Yoo JS, Chang JS (2005) Bull Korean Chem Soc 26:1743–1748
Reddy BM, Lee SC, Han DS, Park SE (2009) Appl Catal B Environ 87:230–238
David Raju B, Choi KM, Lee JH, Han DS, Park SE (2007) Catal Commun 8:43–48
Mohan V, Pramod CV, Suresh M (2012) Hari Prasad Reddy K, David Raju B, Rama Rao KS. Catal Commun 18:89–92
Navaei Alvar E, Rezaei M, Navaei Alvar H, Feyzallahzadeh H (2009) Yan ZF 196. Chem Eng Commun 196:1417–1424
Xingnan Y, Ning M, Weiming H, Yinghong Y, Changxi M, Zaiku X, Zi G (2004) J Mol Catal A: Chem 217:103–108
Balkrishna BT, Rabindran JB, Alam K, Luqman AA, Hidenori Y, Tetsuya S, Katsuomi T, Sulaiman SA (2011) Appl Catal A Gen 407:118–126
Ye X, Hua W, Yue Y, Dai W, Miao C, Xie Z, Gao Z (2004) N J Chem 28:373–378
Sun A, Qin Z, Chen S, Wang J (2004) J Mol Catal A: Chem 210:189–195
Mathew T, Malwadkar S, Pai S, Sharanappa N, Peter Sebastian C, Satyanarayana CVV, Bokade VV (2003) Catal Lett 91:217–224
Geisler S, Vauthey I, Farusseng D, Zanthoff H, Muhler M (2003) Catal Today 81:413–424
Carja G, Nakamura R, Aida T, Niiyama H (2003) J Catal 218:104–110
Sun A, Qin Z, Wang J (2002) Appl Catal A Gen 234:179–189
Arishtirova K, Kovacheva P, Predoeva A (2003) Appl Catal A Gen 243:191–196
Saito M, Kimura H, Mimura N, Wu J, Murata K (2003) Appl Catal A Gen 239:71–77
Huerta L, Meyer A, Choren E (2003) Microporous Mesoporous Mater 57:219–227
Bispo JRC, Oliveira AC, Correa MLS, Fierro JLG, Marchetti SG, Rangel MC (2002) Studies in surface science and catalysis, vol 142. Elsevier, Amsterdam, pp 517–524
Keller N, Maksimova NI, Roddatis VV, Schur M, Mestl G, Butenko YV, Kuznetsov VL, Schlogl R (2002) Angew Chem Int Ed 41:1885–1888
Assabumrungrat S, Suksomboon K, Praserthdam P, Tagawa T, Goto S (2002) J Chem Eng Jpn 35:263–273
Miyakoshi A, Ueno A, Ichikawa M (2001) Appl Catal A Gen 219:249–258
Surman J, Majda D, Rafalska LA, Kustrowski P, Chmielarz L, Dziembaj R (2001) J Therm Anal Calorim 65:445–450
Kong C, Lu J, Yang J, Wang J (2007) J Membr Sci 306:29–35
Delgado JJ, Chen XW, Su DS, Hamid Sharifah BA, Schloegl R (2007) J Nanosci Nanotechnol 7:3495–3501
Su D, Maksimova NI, Mestl G, Kuznetsov VL, Keller V, Schloegl R, Keller N (2007) Carbon 45:2145–2151
Wang L, Zhang J, Su Dang S, Ji Y, Cao X, Xiao FS (2007) Chem Mater 19:2894–2897
Acknowledgements
The author, MVR is grateful to the University Grants Commission and Council of Scientific and Industrial Research, New Delhi, India respectively for the award of fellowship and the services provided by the Analytical Division; CSIR-IICT is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Madduluri, V.R., Rama Rao, K.S. Role of Cobalt Oxide-Based Catalysts for Styrene Production: A Review on Significance of Various Promoters, Calcination Temperature, Chemical Behavior of Support Materials and Synthesis Procedure. Catal Surv Asia 23, 290–310 (2019). https://doi.org/10.1007/s10563-019-09286-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10563-019-09286-0