Abstract
Pd nanoparticles supported on humic acid-coated nanoferrites as magnetically-recoverable catalyst was expediently prepared from inexpensive precursors and characterized by techniques such as SEM, TEM, XPS, FT-IR, XRD, ICP-AES, EDX. The as made catalyst displayed an admirable catalytic activity towards the Suzuki–Miyaura cross-coupling reaction of aryl halides (or) aryl diazonium salts with aryl boronic acid in non-toxic solvent EtOH/water under mild conditions. The same catalyst showed high efficiency for the Heck reaction of aryl halides with styrene was carried out with high efficiency offering good yields under ligand-free conditions. Additionally, the reduction of olefins using molecular hydrogen was also achieved using the same catalyst under ambient and base-free conditions affording the products in good to excellent yields. The magnetic activity of nano-Fe3O4 allowed the effortless recovery of the catalyst and demonstrated subsequent recyclability up to 5 cycles without a significant loss in the activity.
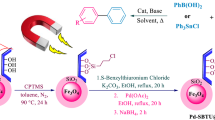
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transition-metal catalysts have played a major role in C–C [1] and C–hetero [2] bond forming reactions which have not only benefitted the chemical [3], pharmaceutical [4], polymer [5], and other allied industries for manufacturing a wide range of products, but also laboratory-scale organic synthesis [6,7,8]. Amongst all the Transition-metal catalysis, palladium based reactions i.e. Heck, Suzuki–Miyaura and other ancillary cross-coupling reactions are graded the most versatile transformations in organic synthesis owing to the efficiency and diversity [9,10,11,12]. These reactions have allowed construction of biphenyls and stilbenoids which are present in many natural products, drug molecules and biologically-active molecules (Scheme 1). Although these homogeneous catalysts demonstrate high activity and selectivity, the proclivity towards heterogeneous catalysts is rising primarily due to their ease of separation from products and reusability in successive reactions. Immobilization of active molecules or metal nanoparticles on a solid support to fabricate a heterogeneous catalytic system has gained significant importance in the past decades [10, 11]. These heterogeneous nanocatalysts are a subject of substantial research and are exemplar of “green chemistry [12,13,14,15,16,17,18].”
Owing to their nano-size, separation of nanocatalysts through filtration or centrifugation becomes laborious. To tackle these challenges, the development of magnetically-recoverable nanoparticles (MNPs) has proved invaluable. Among the myriad of available magnetically-recoverable nanomaterials, iron oxide has flourished tremendously chiefly because it is biocompatible, nontoxic, inexpensive and exhibits superparamagnetism [19]. However, the nanoparticles have a strong tendency to aggregate. This issue is overcome by using suitable stabilizing agents such as dopamine derivatives [20,21,22,23,24], silica [25,26,27,28], dendrimers [29,30,31,32], ionic liquids [33,34,35,36,37], graphene [38,39,40,41,42,43], natural polymers [44,45,46,47,48] which also have reactive sites that covalently or non-covalently bind to the catalytic units and thus can display numerous synthetic applications. Commonly, synthesis of these capped iron oxide nanoparticles requires harsh conditions, toxic precursors, inert conditions. Most essentially, they suffer from the need of sophisticated synthetic procedures for capping and stabilizing the nanoparticles [49,50,51]. Therefore, the development of robust catalysts which are environmentally-benign, versatile, inexpensive and expediently prepared from nontoxic precursors is vital.
In light of these facts, we focused our attention towards naturally available polyphenolic substances from soil. Humic acid (HA) is one such substance found abundantly in soil along with Humic substances (HS), fulvic acid (FA) and humin [52]. Humic acid possesses multiple hydroxyl and carboxylic groups which exhibit exceptional binding capability to metal oxide nanoparticles especially iron oxides and can significantly alter their physicochemical and sorption behavior [53,54,55]. Besides this, due to its planar structure, humic acid shows good interaction with organic compounds especially aromatic molecules by providing large surface area. Moreover, substantial research on humic acid for sequestering heavy-metal ions and polyaromatic hydrocarbons from aquatic sources is ongoing [56,57,58,59,60,61].
Considering the sequestering capability of humic acid towards late transition metal ions, we envisaged that humic acid can act as an excellent capping agent for iron oxide nanoparticles which can capture Pd(II) ions and further be reduced to Pd(0) NPs. Therefore, extending the methodology for magnetically-recoverable nanocatalyst, we herein present an environmentally-benign humic acid coated nanoferrites [62] supported by Palladium nanoparticles for C–C cross-coupling reactions (Suzuki and Heck reaction) and reduction of olefins using molecular hydrogen to confront the challenges of recoverability, reusability, and benign support.
2 Experimental Section
2.1 Synthesis of HA@Fe3O4
8.5 g of FeSO4.7H2O and 12.3 g of FeCl3.6H2O were dissolved in 200 mL of de-ionized water in a 1000 mL beaker and heated to 90 °C. The pH of the solution was regulated by adding 30% ammonia solution (25 mL) followed by addition of sodium salt of humic acid (1 g). The solution was stirred using an overhead stirrer for 40 min and then cooled to room temperature. The particles were recovered using an external magnet followed by washing with ethanol and de-ionized water. Later, the particles were dried in a vacuum oven.
2.2 Preparation of HA@Pd/Fe3O4
The Fe3O4 coated with humic acid (HA@Fe3O4) MNPs were obtained from the previously reported method. In a 1000 mL beaker, 1 g of HA@Fe3O4 MNPs were added to 400 mL of de-ionized water and sonicated for 30 min. Meanwhile, in a 15 mL round-bottom flask, 80 mg of PdCl2 and 90 mg of NaCl were dissolved in 6 mL of de-ionized water and heated to 60 °C with constant stirring till homogeneity and then, cooled to room temperature. This solution was added to the sonicated mixture of HA@Fe3O4 MNPs and stirred on an overhead stirrer at room temperature. Further, NaBH4 solution (76 mg of NaBH4 in 25 mL) was added to it portion-wise over a period of 30 min and stirred for 15 h. The resultant wet paste was recovered using an external magnet from the solution and washed with de-ionized water (50 mL × 3) and acetone (25 mL × 3). The catalyst was dried in a vacuum oven at room temperature.
2.3 General Procedure for Suzuki Cross-Coupling Between Aryl Boronic Acid and Aryl Halide
A 15 mL round bottom flask (back-filled with N2) was loaded with HA@Pd/Fe3O4 (50 mg). Aryl halide (1 equiv.), aryl boronic acid (1.1 equiv.) and potassium carbonate (2.0 equiv.) were added to the flask followed by 1:1 mixture of ethanol and water (4 mL). The resultant mixture was stirred at 50 °C for 4 h under nitrogen atmosphere. With the aid of an external magnet, the catalyst was recovered and the product was extracted using ethyl acetate (5 mL × 3). The organic layer was separated and dried over Na2SO4 followed by evaporation under reduced pressure and the mixture was purified using column chromatography on silica gel (Petroleum ether/Ethyl acetate).
2.4 General Procedure for Suzuki Cross-Coupling Between Aryl Diazonium Tetrafluoroborate and Aryl Boronic Acid
A 15 mL round bottom flask was loaded with HA@Pd/Fe3O4 (50 mg). Aryl diazonium tetrafluoroborate (1 equiv.), aryl boronic acid (1.1 equiv.) were added to the flask followed by methanol (4 mL). The resultant mixture was stirred at room temperature for 12 h in air. With the aid of an external magnet, the catalyst was recovered and the product was extracted using ethyl acetate (10 mL × 3). The organic layer was separated and dried over Na2SO4 followed by evaporation under reduced pressure and the mixture was purified using column chromatography on silica gel (Petroleum ether/Ethyl acetate).
2.5 General Procedure for the Heck Reaction
To a solution of aryl halide (1 equiv.), styrene (2 equiv.) and HA@Pd/Fe3O4 (50 mg) in DMF (4 mL), potassium carbonate (2.5 equiv.) was added. The resultant mixture was stirred under O2 atmosphere at 140 °C for 24 h. With the aid of an external magnet, the catalyst was recovered and water (5 mL) was added to the reaction mixture. Further, the product mixture was extracted using ethyl acetate (5 mL × 3). To remove traces of DMF, the organic layer was washed with water (4 mL × 2) and then the organic layer was dried over Na2SO4. The solvent was evaporated under reduced pressure and the mixture was purified using column chromatography on silica gel (Petroleum ether/Ethyl acetate).
2.6 General Procedure for Olefin Reduction Using Molecular Hydrogen
In a 25 mL round bottom flask equipped with Teflon coated magnetic needle, olefin (1 equiv.) was dissolved in 4 mL of methanol. Then, H2 was introduced in the flask followed by flushing it twice and the resulting mixture was stirred at room temperature under H2 atmosphere for 15 h. The solvent was then evaporated under reduced pressure and water (5 mL) was added to the residue to dissolve inorganic impurities. The product was then extracted using ethyl acetate (3 × 5 mL). The organic layer was dried over sodium sulphate and the solvent was evaporated under reduced pressure. The mixture was purified using column chromatography on silica gel (Petroleum ether/Ethyl acetate).
3 Results and Discussion
Initially, humic acid capped Fe3O4 NPs were prepared and were used to immobilize palladium nanoparticles on them. The as synthesized material was fully characterized by Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), X-Ray Diffraction (XRD), X-Ray Photoelectron Spectroscopy (XPS), Energy Dispersive Spectroscopy (EDS), Fourier Transformed Infrared Spectroscopy (FT-IR), and Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) analysis. The humic acid coating is indistinguishable from SEM images (Fig. 1). For a detailed knowledge on the surface morphology, Transmission Electron Microscopy (TEM) and High-Resolution TEM (HR-TEM) of HA@Pd/Fe3O4 was done (Fig. 2). It was observed that humic acid is uniformly coated along with evenly distributed Pd NPs of size less than 10 nm on the catalyst. Energy Dispersive X-Ray (EDX) mapping provided the elemental composition of HA@Pd/Fe3O4 (Fig. 6a). The presence of palladium and carbon peaks in the spectrum confirmed the humic acid coating as well as supported palladium on the catalyst. Upon ICP-AES analysis, it was discovered that 7.98 wt% of Pd was loaded. X-ray Photoelectron Spectroscopy (XPS) was carried out for native as well as recycled HA@Pd/Fe3O4 to identify the chemical state of Pd supported on the catalyst. XPS analysis of the native catalyst in Pd 3d region (Fig. 7) indicates that BE of Pd 3d5/2 at 335.4 eV and 3d3/2 at 340.6 eV, which is a characteristic emissions from metallic Pd (0). The XPS data for recycled catalyst showed that the peaks did not shift, thus suggesting the unaffected chemical state of Pd after the reaction. X-ray Diffraction is a valuable technique to understand the lattice structure of a material. The XRD pattern of the obtained HA, HA@Fe3O4, HA@Pd/Fe3O4 ative and recycled) are shown (Figs. 3, 8). The peaks in the XRD pattern of HA were not as prominent as the native catalyst peaks. Native HA@Pd/Fe3O4 shows characteristic broad and weak (1 1 1) reflection peak centered at 2θ = 39.5° indicate the presence of metallic Pd (0). This peak was visible in recycled HA@Pd/Fe3O4 hinting towards the presence of Pd NPs even after the 5th cycle. FT-IR of HA@Pd/Fe3O4 was recorded (Fig. S1, supporting information) to ascertain the functional groups in the support. The spectrum shows a strong and broad peak at 3400 cm−1 which is indicative of H-bonded hydroxyl groups whereas the 1720 cm−1 shoulder peak corresponds to the presence of ketonic or aldehydic C=O group. This was confirmed by the characteristic COO− peaks at 1600 cm−1 and 1390 cm−1. The band near 1600 cm−1 could be due to C=C aromatic vibrations and H-bonded C=O quinones. The strong peak at 530 cm−1 is characteristic of Fe–O stretching which confirms the interaction between iron oxide and humic acid [63]. All these characterization techniques conclusively authenticated the immobilization of Pd on the support. To investigate the catalytic activity of HA@Pd/Fe3O4, we attempted Suzuki–Miyaura cross-coupling reaction. In order to determine optimized reaction conditions, we chose the reaction between 4-iodoanisole and phenylboronic acid as a model reaction.
Several parameters such as solvent, bases and temperature of the reaction were varied to obtain the best yields. The combination of 1:1 of ethanol: water mixture with K2CO3 as base at 50 °C afforded the cross-coupled product in 94% yield (Table 1, entry 7). The identity of the product was confirmed by 1H and 13C NMR spectroscopy. The turnover number and turnover frequency of the synthesized PdNPs are found to be 14.329 and 1.0023 × 10− 3 s−1 respectively.
With the optimized conditions in hand, several substrates were screened for their conversion. The reaction of phenylboronic acid with aryl iodides possessing electron-withdrawing and electron-donating groups such as nitro, methoxy and cyano, afforded the products in good yields (95–89%). The aromatic heterocyclic iodides viz. 2-pyridyl iodide and 2-iodothiophene also gave excellent yields of 89% and 85% respectively (Tables 2, 1l and 1i).
We further extended the substrate scope by reacting substituted bromoarenes with phenylboronic acid. Different aryl bromides bearing electron-donating and electron-withdrawing groups also successfully afforded the products in 90–85% yield. The polyaromatic aryl products viz.,1-pyrenyl (Table 2, 1k) and 2-naphthyl (Table 2, 1f) obtained from the corresponding arylboronic acids and 4-iodoanisole were obtained in 94% and 92% yield respectively whereas the reaction of the same boronic acids with 4-bromoanisole formed products in 88% and 89% yield respectively. As anticipated the aryl halides possessing electron-donating substituents were less reactive in contrast to electron-withdrawing counterparts. For the same reason, the reaction of 4-chloroanisole with phenylboronic acid, was found to be sluggish even at higher temperature providing unsatisfactory yield of 32%. (Table 2, 1c).
It is noteworthy to mention that, the challenging reactions of aryl bromide, that too the ones with electron-donating substituents despite their low reactivity underwent conversion to afford products in good to excellent yields vouches the efficiency of the catalyst. Additionally, reactant/products bearing oxidation or hydrolysis-prone substituents such as cyano or ester groups were unaffected under these conditions and hence, no such side products formation was observed. Proficiency and mildness of the developed protocol can be advocated due to the conservation of sensitive groups and improved activation of substrates. To further examine the usefulness of the catalyst, we decided to employ it another cross-coupling reaction which is relatively unexploited with heterogeneous Pd catalysts. We carried out Suzuki–Miyaura reaction of aryldiazonium tetrafluoroborate and arylboronic acid using this catalytic system. Initially, 4-methoxyphenyldiazonium tetrafluoroborate and phenylboronic acid were chosen as model substrates for the optimization of the reaction. Parameters affecting the reaction such as solvent and temperature were varied to obtain the optimum yield of the products. The reaction when carried out in methanol as reaction solvent at 30 °C afforded the maximum yield of 88% of the biaryl product (Table 3).
To broaden the substrate scope, diverse aryldiazonium tetrafluoroborates and aylboronic acids were assessed for their conversion. Electron-withdrawing and electron-donating groups on both aryldiazonium tetrafluoroborates and arylboronic acids were well-tolerated offering yields ranging from 93 to 83% (Table 4). Only in case of 4-methoxyphenyldiazonium tetrafluoroborate and 4-nitrophenylboronic acid, the corresponding product was obtained in poor yield of 26% which shows sluggishness of the substrates to react via oxidative coupling mechanism (Table 4, 2e).
The Heck reaction is typically found to be difficult to accomplish using a supported palladium catalyst. Therefore, we decided to investigate the robustness of this catalytic system towards the Heck reaction of aryl halides namely, aryl iodides and aryl bromides. The optimized conditions were determined by taking 4-iodoanisole and styrene as the model substrates and varying the factors affecting the reaction such as solvent, base and temperature. The optimum yield of product was obtained when DMF was taken as the reaction solvent using K2CO3 as base at 140 °C under O2 atmosphere (Table 5).
The products were obtained in the range of 85–51% depending on the nature of aryl halide. As expected, a trend was observed with electron rich aryl halides which are less reactive than the electron deficient counterparts. The aryl bromides being less reactive, required longer reaction times and afforded only moderate yields of products (Table 6). However, the reaction did not proceed in the case of aryl chlorides.
Nowadays, versatile multi-modal catalysts are highly desirable due to increasing demand in sustainable chemistry leveraging the principle “one catalyst-many jobs [47, 48].” In this context, we evaluated the versatility of HA@Pd/Fe3O4 catalyst for the reduction of olefins using molecular hydrogen and trans-stilbene as the model substrate. In order to optimize the reaction conditions, parameters such as solvent and temperature were varied. The best outcome was observed when the reaction was carried out in methanol at room temperature in 12 h (Table 7).
Applying these optimized conditions, effectiveness of this catalyst towards diverse substrates was assessed. Substituted (E)-stilbene derivatives were employed in order to carry out this the task and it was found that good to excellent yields of products ranging from 96 to 85% were obtained. Remarkably, the reaction proceeded smoothly without the use of a base and under mild conditions. Electron-donating as well as electron-withdrawing substituents on the stilbene were well-tolerated. When reduction of 4-nitrostilbene was carried out, an inseparable mixture of products was observed, possibly due to lack of selectivity towards C = C and nitro group leading to the formation of saturated and unsaturated amines (Table 8).
For heterogeneous catalytic systems, the level of reusability and the lifetime of a catalyst are very crucial. Therefore, we probed the recyclability of the catalyst (Fig. 4) for Suzuki cross-coupling between 4-iodoanisole and phenylboronic acid by recovering the catalyst particles and reuse them in the next cycle. The recovery of the catalyst was effortless owing to the magnetic property exhibited by iron oxide nanoparticles. Unlike the conventional laborious methods such as filtration or centrifugation, the catalyst was recovered by means of an external magnet. The nanoferrite-supported palladium catalyst showed excellent efficiency and recoverability till the fifth cycle without any loss of activity. With the completion of recyclability study, we examined the change in surface morphology the catalyst after the 5th cycle as it poses a significant difficulty in heterogeneous catalytic systems. To gain more insights of heterogeneity of the catalyst, how palladium is operating in the reaction under the optimised reaction conditions, we performed a hot filtration test. For this the cross-coupling reaction between 4-iodoanisole and phenylboronic acid was carried out for 2 h executed 62% yield of desired coupled product, later the catalyst was removed/separated magnetically. The obtained filtrate was taken in a separate reaction flask and reaction continued at 50 °C for next 24 h, shows no increase in the coupled product yield. Another hot filtration test was carried out in the same optimized condition (reaction conversion after 2 h ~ 61%) but now catalyst filtered by centrifugation (3000 rpm) to prevent the back deposition of leached if any Pd species. This showed a no further increase in yield the and hence endorse catalyst’s heterogeneous property. In order to find out the amount of Palladium reamined intact on the support after the five cycles Pd was deliberately leached from the recycled HA@Pd/Fe3O4 catalyst and was found to be 7.93%, which shows no significant leaching occur during catalytic processes.
SEM analysis was done for the recycled catalyst which revealed that morphology of the catalyst was unchanged (Fig. 5). Moreover, EDX analysis of the recycled catalyst showed that the ratios of the peaks were very close compared to the native catalyst indicating that the elemental composition is intact (Fig. 6b). When XPS data of the catalyst after 5 cycles was examined, it revealed that Pd 3d region showed two peaks at BE 340.9 eV and at BE 335.7 eV corresponding to 3d3/2 and 3d5/2 respectively (Fig. 7). It can be inferred that since no other peaks were detected, the oxidation state of Pd is constant. The integrity of the lattice structure of the recycled catalyst was evaluated by XRD pattern. It was found that the lattice structure of the core nanoferrite and also Pd NPs is undamaged after 5 cycles (Fig. 8). The catalytic efficiency of various catalysts with respect to their product yield and other application in organic transformation, including this work is shown in Table 9 for comparison.
4 Conclusions
In conclusion, a novel and versatile Pd nanoparticles supported on humic acid stabilized nano-Fe3O4 (HA@Pd/Fe3O4) was prepared in a straightforward manner using nontoxic precursors. The catalyst displayed excellent catalytic activity towards Suzuki–Miyaura and Heck cross-coupling reactions by well-tolerating electron-donating and electron-withdrawing substituents on aryl iodides and bromides. Reduction of olefins was rendered possible at room temperature in nontoxic solvent and under base-free condition making it a highly sustainable methodology. Other advantages of this catalyst were facile recovery and extensive reusability. Moreover, humic acid played a major role in stabilizing the Fe3O4 as well as Pd nanoparticles.
References
Molnár (2011) Á Chem Rev 111:2251
Hartwig JF Nature (2008) 455: 314
Blaser HU, Catal Today (2000) 60:161
Busacca CA, Fandrick DR, Song JJ, Senanayake CH (2011) Adv Synt Catal 353:1825
Vandenberg EJ (1992) Catalysis in polymer synthesis. American Chemical Society, Washington, DC, pp 2–23
Feng J, Holmes M, Krische MJ (2017) Chem Rev 117:12564
Trost BM (1995) Angew Chem Int Ed 34:259
Fumagalli G, Stanton S, Bower J (2017) F, Chem Rev 117:9404
Chen X, Engle KM, Wang DH, Yu JQ (2009) Ang Chem Int Ed 48:5094
Polshettiwar V, Luque R, Fihri A, Zhu H, Bouhrara M, Basset J-M (2011) Chem Rev 111:3036
Wang D, Astruc D (2017) Chem Soc Rev 46:816
Zhang S, Nguyen L, Zhu Y, Zhan S, Tsung CK, Tao F (2013) Acc Chem Res 46:1731
Fihri A, Bouhrara M, Nekoueishahraki B, Basset JM, Polshettiwar V (2011) Chem Soc Rev 40:5181
Veisi H, Nasrabadi NH, Mohammadi P (2016) Appl Organomet Chem 30:890
Lebaschi S, Hekmati M, Veisi HJ (2017) Colloid Interface Sci 485:223
Veisi H, Faraji AR, Hemmati S, Gil A (2015) Appl Organomet Chem 29:517
Das SK, Parandhaman T, Pentela N et al (2014) J Phys Chem C 118:24623
Veisi H, Pirhayati M, Kakanejadifard A et al ChemistrySelect (2018) 3:1820
Karimi B, Mansouri F, Mirzaei HM, ChemCatChem (2015) 7:1736
Dubey AV, Kumar AV, Adv RSC (2016) 6:46864
Farzad E, Veisi H (2018) J Ind Eng Chem 60:114
Baig RBN, Varma RS (2012) Chem Commun 48:2582
Nasir Baig RB, Leazer J, Varma RS, Clean Technol Environ Policy (2015), 17:2073
Dam B, Patil RA, Ma YR, Pal AK (2017) New J Chem 41:6553
Jha A, Patil CR, Garade AC, Rode CV (2013) Ind Eng Chem Res 52:9803
Yi DK, Lee SS, Ying JY (2006) Chem Mater 18:2459
Sharma RK, Yadav M, Gawande MB (2016) In: Eds.: Sharma VK, Doong R, Kim H, Varma RS, Dionysiou DD ACS Symp Ser. American Chemical Society, Washington, DC, pp 1–38
Heidari F, Hekmati M, Veisi H (2017) J Colloid Interface Sci 501:175
Rehm TH, Bogdan A, Hofmann C, Löb P, Shifrina ZB, Morgan DG, Bronstein LM (2015) ACS Appl Mater Interfaces 7:27254
Deraedt C, Wang D, Salmon L, Etienne L, Labrugère C, Ruiz J, Astruc D, ChemCatChem (2015) 7:303
Asadi B, Mohammadpoor-Baltork I, Tangestaninejad S, Moghadam M, Mirkhani V, Landarani-Isfahani A (2016) New J Chem 40:6171
Paez JI, Froimowicz P, Landfester K, Brunetti V, Strumia M (2014) J Polym Sci Part A 52:3185
Safaei S, Mohammadpoor-Baltork I, Khosropour AR, Moghadam M, Tangestaninejad S, Mirkhani V, Kia R (2012) RSC Adv 2:5610
Zheng X, Luo S, Zhang L, Cheng JP (2009) Green Chem 11:455
Otokesh S, Kolvari E, Amoozadeh A, Koukabi N, RSC Adv (2015) 5:53749
Zhang Q, Su H, Luo J, Wei Y (2012) Green Chem 14:201
Mandal P, Chattopadhyay AP, Dalton Trans (2015), 44:11444
Jamatia R, Gupta A, Pal AK (2017) ACS Sustain Chem Eng 5:7604
Veisi H, Pirhayati M, Kakanejadifard A (2017) Tetrahedron Lett 58:4269
Zhang M, Liu YH, Shang ZR, Hu HC, Zhang ZH, Catal Commun (2017) 88:39
Patil MR, Kapdi AR, Vijay Kumar A (2018) ACS Sustain Chem Eng 6:3264
Ko S, Jang J (2006) Angew Chem Int Ed 45:7564
Jawale DV, Gravel E, Boudet C et al (2015) Catal Sci Technol 5:2388
Reddy LH, Arias JL, Nicolas J, Couvreur P (2012) Chem Rev 112:5818
Korneva G, Ye H, Gogotsi Y, Halverson D, Friedman G, Bradley JC, Kornev KG (2005) Nano Lett 5:879
Veisi H, Mirzaee N (2018) Appl Organomet Chem 32:e4067
Yang S, Zong P, Ren X, Wang Q, Wang X (2012) ACS Appl Mater Interfaces 4:6891
Veisi H, Mohammadi Biabri P, Falahi H (2017) Tetrahedron Lett 58:3482
Wang D, Liu W, Bian F, Yu W (2015) New J Chem 39:2052
Parandhaman T, Pentela N, Ramalingam B et al (2017) ACS Sustain Chem Eng 5:489
Veisi H, Najafi S, Hemmati S (2018) Int J Biol Macromol 113:186
Veisi H, Mirshokraie SA, Ahmadian H (2018) Int J Biol Macromol 108:419
Kharisov BI, Kharissova OV, Dias HVR (2014) Nanomaterials for environmental protection. Wiley, Hoboken, pp 483–501
Hu JD, Zevi Y, Kou XM, Xiao J, Wang XJ, Jin Y (2010) Sci Total Environ 408:3477
Ni L, Su L, Li S, Wang P, Li D, Ye X, Li Y, Li Y, Li Y, Wang C (2017) Environ Toxicol Chem 36:1856
Lippold H, Gottschalch U, Kupsch H (2008) Chemosphere 70:1979
Liu S, Zhu Y, Liu L, He Z, Giesy JP, Bai Y, Sun F, Wu F (2018) Environ Pollut 234:726
Tan L, Wang X, Tan X, Mei H, Chen C, Hayat T, Alsaedi A, Wen T, Lu S, Wang X (2017) Chem Geol 464:91
Tang Z, Zhao X, Zhao T, Wang H, Wang P, Wu F, Giesy JP (2016) Environ Sci Technol 50:8640
Liu J, Zhao Z, Jiang G (2008) Environ Sci Technol 42:6949
Stevenson FJ, Goh KM (1971) Geochim Cosmochim Acta 35:471
García-Suárez EJ, Balu AM, Tristany M, García AB, Philippot K, Luque R (2012) Green Chem 14:1434
García CS, Uberman PM, Martín SE, Beilstein (2017) J Org Chem 13:1717
Sobhani S, Zarifi F, Chinese J, Catal (2015), 36:555
Acknowledgements
AVD is grateful to University Grants Commision (UGC) for the research fellowship. AVK is grateful to DST, Govt. of India, for research funding, DST-SERB (YSS/2015/002064) and the Department of Pharmaceutical Science and Technology, ICT, for NMR analysis. We are also thankful to Sophisticated Analytical Instruments Facility (SAIF) at the Indian Institute of Technology, Mumbai (IITB) for providing the characterization data for the analysis of the catalyst.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chinchole, A.N., Dubey, A.V. & Kumar, A.V. Bioinspired Palladium Nanoparticles Supported on Soil-Derived Humic Acid Coated Iron-Oxide Nanoparticles as Catalyst for C–C Cross-Coupling and Reduction Reactions. Catal Lett 149, 1224–1236 (2019). https://doi.org/10.1007/s10562-019-02703-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02703-z