Abstract
Here, a novel nanostructured catalyst based on CuO included hallow mesoporous silica spheres (CuO–HMSS) was prepared for synthesis of 1,2,3-triazole compounds. The hallow silica spheres were synthesized via the hydrothermal procedure. The characterization of prepared catalyst was also performed applying several analysis techniques such as TEM and SEM, EDX, XRD, and ICP.
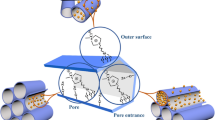
Graphical Abstract
CuO active sites in a hollow mesoporous silica shell are stably and efficiently attainable for conversion of precursors to triazole compounds.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The various metal/metal oxide loaded mesoporous silica nanostructures have attracted several attentions during the past decades especially in the field of heterogeneous catalysis [1, 2]. In addition, the application of mesoporous silica structure is familiar in various scientific fields such as drug delivery, drug release [3, 4], gene delivery [5], biosensing [6], and as adsorption media for removing of environmental pollutants [7, 8]. The wide range of mesoporous silica nanostructures with various morphologies, sizes, pore structures and pore sizes could be synthesized [9]. In addition, thanks to the mechanical, thermal and chemical stability, controllable synthesis, high adsorption capacity, facilitated diffusion of substances as well as the high surface area, mesoporous silica nanomaterials are known as one of the best catalyst supports. In addition to the various favorable features due to the presence of mesoporous channels, further improvement of mass transfer could be expected for hollow structures. Moreover, the enrichment of reactants in the cavities as well as the arrangement of active sites interior the hollow structure, the enhancement of the catalytic activity could be resulted [10,11,12]. In some cases, due to the specific role of the hollow catalyst, the whole structure could be regarded as a nanoreactor [13]. On the other hand, by restriction of the catalyst active sites into an outer shell of mesoporous silica, the chemical stability, dispersibility and also the more tolerance toward the unfavorable oxidation and aggregation phenomena could be resulted [14,15,16,17]. The synthesis of silica mesoporous structure could be done by applying the modified Stöber method in the presence of cetyltrimethylammonium bromide (CTAB) as a cationic surfactant [18, 19].
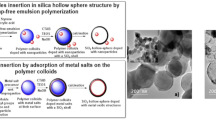
The hollow silica nanostructures could be synthesized using both hard and soft templates [9]. Carbon microspheres (CMS) could be introducing as suitable hard (solid) templates for synthesis of hollow silica structures [20]. The surface of carbon spheres is rich of hydroxyl and carboxyl functional groups and thus it is favorably hydrophilic [21]. In addition, the high interactions between CMS surface with metal cations could be induced by the presence of these functional groups [21]. Moreover, the removal of CMSs is facile and efficient by calcination under the air atmosphere. By applying CMSs as template, metal oxide hollow shells also could be synthesized by the one pot synthesis procedure using CMS as sacrificial template. To this reason, a solution containing certain metal salt and the carbohydrate precursor is treated under the optimized hydrothermal conditions. The product of the hydrothermal process is CMSs with outer hydrophilic shell occupied with the cations of the metal. The hollow metal oxide shells are achieved during the removal of carbon cores under the air atmosphere calcination process [22]. Several metal oxide hollow shells such as Fe2O3, MgO, CeO2, NiO, Co3O4 and CuO were reported to be prepared by this method [22].
1,2,3-Triazole compounds are known as valuable chemicals in various fields such as biologically active pharmaceutical, artificial receptors, photographic material, dyes, organic semiconductor, electroluminescent material, and corrosion inhibitors [23]. As a common way for synthesis of triazole compounds, the Click reaction could be accelerated by copper species as catalyst [24,25,26,27,28]. Although Cu(I) species are known as common catalysts for Click reaction, some reports were focused on the Cu(0) nanoparticles [29, 30], Cu(II) compounds [31, 32] and even Cu based bimetallic catalysts [33]. The Cu catalysis of Click reaction was also reported using various nanostructures such as CuO hollow nanostructures [34], CuO nanowires [35] and nanoporous Cu structures [36]. However, novel Cu catalysts by using various substrates such as MOFs [37, 38], activated carbon [39, 40], zeolite [41], agarose [42], and etc. have been reported. Specially, using silica as support, several CuO/SiO2 catalysts were reported for applications in several gas phase and liquid phase important reactions such as the production of fatty alcohols from natural oil [43], methanol conversion and hydrogen production [44], hydrogenation of reactants to alcohols [45, 46], gas phase synthesis of ethanol via syngas [47], and etc.
Following our previous researches in the field of heterogeneous catalysis of the main organic reactions [27, 48,49,50,51,52,53,54,55,56,57,58,59], the catalyst based on CuO mesoporous silica was prepared for catalyzing of 1,2,3-triazole production via the cycloaddition reaction. The unique structural feature of catalyst were characterized via several techniques and some catalytic aspects such as reactivity enhancement, recyclability and also the heterogeneous action of CuO based catalyst have been studied.
2 Experimental
2.1 Materials and Apparatus
The phase analysis of catalyst was studied using XRD with a Bruker D8 ADVANCE diffractometer. NMR spectra were obtained applying Bruker AMX 300 MHz instruments. FE-SEM images were recorded using a TESCAN, Model: MIRA3 scanning electron microscope operating at an acceleration voltage of 30.0 kV. Elemental compositions were determined with an SC7620 energy-dispersive X-ray analysis (EDX) presenting a 133 eV resolution at 20 kV. Transmission electron microscopy (TEM) was performed with a Leo 912 AB (120 kV) microscope (Zeiss, Germany). All compounds were obtained from Merck in analytical grade and were used without any further purification.
2.2 Synthesis of Cu Included Carbon Microspheres
Here, for synthesis of CuO–HMSS, the Cu included carbon microspheres (Cu–CMS) was prepared at first via an optimized one step hydrothermal procedure. Practically, a 6 ml aliquot of solution of 1 M of Cu(NO3)2 was added to 30 ml solution of 0.5 M of glucose. The resultant mixture was reached to the volume of 90 ml by adding the doubled distilled water and was transferred into a Teflon lined stainless steel autoclave vessel. By adjusting a heating program, the precursor mixture was heated up from room temperature to 190 °C along 5 h and was kept at 190 °C for 24 h. Finally, the mixture was cooled to room temperature (25 °C). The resulting particles was filtered and washed three times with double distilled water and finally dried at 60 °C overnight (sample is denoted as Cu–CMS).
2.3 Synthesis of SiO2 Coated Cu–CMS
For synthesis of silica shell over the Cu–CMS, a modified Stöber method was used. Briefly, 0.5 g of as-synthesized Cu–CMS was well dispersed into the 25 ml of double distilled water. 15 ml of ethanol, 0.07 g of CTAB, 0.25 ml of 0.25% solution of NH4OH and 120 µl of TEOS were added and the resultant mixture was sonicated for 30 min and then stirred for 6 h at room temperature. Finally, the product was filtered and washed three times with double distilled water and ethanol and finally dried at 60 °C overnight.
2.4 Synthesis of CuO–HMSS Catalyst
The SiO2 coated Cu–CMS sample is converted to CuO–HMSS as a final product via two steps of calcination under Ar and air atmospheres, respectively. Along calcination step, by elimination of CMS template and CTAB as organic constituents, the mesoporous hollow structure of Cu containing silica is obtained. However, the oxidation of Cu species to CuO as well as the annealing of SiO2 shell are performed during the calcination procedure.
2.5 Synthesis of 1,2,3-Triazole Compounds
Cautions: because of the heat and shock sensitivity of sodium azide, the required caution should be exercised for prevention of explosion.
1 mmol of phenylacetylene, 1 mmol of alkyl halide/epoxide, 1.2 mmol of sodium azide, 0.02 g of CuO–HMSS and 3 ml of water were delivered into the round bottle flask and the mixture of reactants was heated at 80 °C until the reaction completeness. The CuO–HMSS catalyst was separated via centrifugation and was dried after washing with ethylacetate to be applied in the next runs. Here, some water insoluble or sticky products could be dissolve into the ethylacetate. Thus, this ethylacetate portion was added to the final extract of the reaction mixture to attain the complete recovery of product. After separation of CuO–HMSS catalyst, the product was extracted from the reaction mixture by using ethylacetate. Finally, the product was purified via the recrystallization procedure. All the products were confirmed by the spectroscopic method using 1H and 13C NMR. (See Supporting Information, Spectral data).
2.5.1 1-(4-Methylbenzyl)-4-phenyl-1H-1,2,3-triazole: (Table 2, Entry 2)
1H NMR (DMSO-d6, 300 MHz, δ, ppm): 2.29 (s, 3H), 5.60 (s, 2H), 7.18–7.30 (m, 4H), 7.30–7.47 (m, 3H), 7.84–7.87 (m, 2H), 8.62 (s, 1H). 13C NMR (75 MHz, DMSO-d6, δ, ppm): 21.1, 53.3, 121.7, 125.6, 128.3, 129.3, 129.8, 131.4, 133.5, 137.9.
2.5.2 1-(4-Bromobenzyl)-4-phenyl-1H-1,2,3-triazole: (Table 2, Entry 3)
1H NMR (DMSO-d6, 300 MHz, δ, ppm): 5.66 (s, 2H), 7.20–7.35 (m, 3H), 7.41 (t, 2H, J = 9.0 Hz), 7.56 (d, 2H, J = 9.0 Hz), 7.81 (d, 2H, J = 6.0 Hz), 8.82 (s, 1H). 13C NMR (75 MHz, DMSO-d6, δ, ppm): 52.6, 121.8, 122.3, 125.6, 128.4, 129.4, 129.8, 130.8, 131.1, 132.1, 135.6, 147.0.
2.5.3 1-(4-Nitrobenzyl)-4-phenyl-1H-1,2,3-triazole: (Table 2, Entry 4)
1H NMR (DMSO-d6, 300 MHz, δ, ppm): 5.86 (s, 1H), 7.31–7.36 (m, 1H), 7.45 (t, 2H, J = 7.2 Hz), 7.58 (d, 2H, J = 8.7 Hz), 7.81 (d, 2H, J = 9.0 Hz), 8.25 (d, 2H, J = 9.0 Hz), 8.71 (s, 1H). 13C NMR (75 MHz, DMSO-d6, δ, ppm): 52.6, 122.4, 124.4, 125.5, 128.4, 129.3, 130.0, 143.8, 147.7.
3 Results and Discussion
The Cu included carbon microsphere (Cu–CMS) was synthesized at first via the one step hydrothermal procedure. Then, a protective shell of SiO2 in the presence of CTAB (via the modified Stöber method) is formed over the Cu–CMSs. Finally, by elimination of CMSs and CTAB residuals (as organic constituents) via the air calcination, the novel hallow mesoporous silica particles—containing cavities including CuO species (CuO–HMSS)—was resulted. The above mentioned steps were presented in Scheme 1.
Figure 1 represents the SEM micrographs of Cu–CMS (micrograph a) and CuO–HMSS (micrograph b, c). The regular solid spheres of Cu–CMS with the smooth surfaces are observable in Fig. 1a. In the case of CuO–HMSS, due to the application of CMSs as a hard template for synthesis of HMSS structures, the approximately uniform size distribution of particles with average diameter around 1.3 µm is achievable. In contrast to Cu–CMS, the surface of CuO–HMSS particles is appeared to become roughened which could be referred to the SiO2 shell morphology. Additionally, large cavities on some particles in the image (specified by arrows) are remarkable. However, due to improper position of other particles in the image, some cavities could not be observed.
For better clarification of the structure of CuO–HMSS, the TEM micrograph was also studied. Figure 2a, b shows the corresponding TEM images with two magnifications. Similar to SEM micrographs, the approximately narrow size distribution of spherical particles with diameter around 1.3 µm is observable. However, some cavities are observable which are specified by circles in the Fig. 2a. Additionally, the mesoporous structure of SiO2 could be clearly distinguished from the magnified image represented in Fig. 2b.
Using the BET analysis (the adsorption–desorption isotherm in Fig. S1) of CuO–HMSS catalyst, the BET surface area of 141.8 m2 g−1 was obtained. The investigable type IV isotherm as well as the hysteresis shape could be referred to the presence of mesoporous structure. Additionally, the adsorption average pore width was obtained to be 3.0 nm. Thus according to nitrogen adsorption studies as well as TEM analysis, it can be concluded that, the silica structure is mesoporous in nature (See Supporting Information, Fig. S1).
Figure 3 represents the XRD spectra of CMS template (spectrum a), Cu–CMS before any calcination process (spectrum b) and the final product CuO–HMSS after the calcination and removal of CMS template. The characteristic peaks of Cu species are obvious in diffractograms b and c. According to JCPDS #00-004-0836, the diffraction peaks at 2θ values of 43.3, 50.4 and 74.1 in diffractogram b, could be referred to (111), (200) and (220) planes of Cu(0), respectively. The reduction of metal salt and the stabilization of Cu(0) species is done by reductive nature of glucose precursor which is occurred during the hydrothermal production of CMS. The diffraction peaks in diffractogram c could be attributed to CuO, according to JCPDS #00-045-0937. Herein, during the calcination under the air atmosphere, the conversion of zero-valent Cu species to CuO could be resulted. It means that after the calcination step, the CuO is the dominant form of copper in the catalyst.
The elemental composition of catalyst was also characterized via the EDX technique. According to ICP analysis, the weight percentage of 9.75% was obtained for Cu as the active component of the catalyst (See Supporting Information, Fig. S2).
Additionally, according to elemental mapping data in Fig. 4, the dispersion of Cu species is approximately homogeneous over the particles and no aggregated Cu species was distinguished. In addition, the presence of SiO2 shell could be resulted from the association of Si and O atoms over the selected particle.
The catalytic performance of the CuO–HMSS was examined for the synthesis of 1,4-disubstituted-1,2,3 triazole. In order to achieve the most suitable conditions for the designed protocol, reaction between benzyl bromide, phenylacetylene and sodium azide was chosen as a model reaction and was studied considering various parameters such as the solvent and the amount of catalyst (Table 1). As shown in Table 1, H2O was found to be the most effective solvent which provides the fast reaction rate and also the high product yield (Table 1, entry 6). In the presence of other solvents such as CH3CN, THF, DMF, EtOH and H2O/EtOH, the longer reaction completion times and the lower yields were obtained (Table 1, entry 1–5). During our optimization studies, various amounts of catalyst were tested and results showed that the reaction proceeds with excellent yield when the amount of 0.02 g of the catalyst was utilized (Table 1, entry 6). The further increase in the amount of CuO–HMSS catalyst just had negligible effects on the efficiency of the reaction (Table 1, entry 7). In the absence of any catalyst no progress in reaction was observed. Furthermore, for catalyst values below 0.02 g, the lower yields and the longer reaction times have been recorded.

As shown in Table 2, under the optimized conditions, we investigated the reaction of epoxides or alkyl or benzyl halides with sodium azide and phenylacetylene. The results of Table 2 indicate that cycloaddition reaction with various benzyl halides bearing either both electron-donating and with-drawing substituents were completed in a short time with excellent yields (Table 2, entry 1–5). However, in the case of electron-donating group, the rate of reaction was increased (Table 2, entry 2). Alkyl halides such as 1-chlorohexane, 2-bromoethyl benzene, 3-bromo-2-methylprop-1-ene and 1-bromo-2-methylpropane were also converted in high yields to the desired products. However, in comparison with benzyl halides, longer reaction times were required (Table 2, entry 6–9). Next, we explored the synthesis of symmetrical bis-triazoles starting from the 1,2-dibenzyl bromide, by the successive 1,3-dipolar cycloaddition reactions with phenylacetylene. The satisfactory yields were also obtained for synthesis of symmetrical bis-triazoles (Table 2, entry 10). Then the reactivity of substituted epoxides with sodium azide and phenylacetylene was studied. The aliphatic epoxides react relatively fast and provided the corresponding products with high yields. Other epoxides such as 2-((4-allyl-2-methoxyphenoxy)methyl)oxirane and 7-oxabicyclo[4.1.0]heptane were also react with sodium azide and phenylacetylene to produce corresponding products with the yields of 80 and 85%, respectively (Table 2, entry 11–16).
The recyclability of CuO–HMSS catalyst was also studied. For this reason, after the termination of each synthesis run, the CuO–HMSS catalyst was separated, washed and dried to be applied in the next synthesis run. This procedure was repeated successively for seven runs of synthesis. Figure 5 shows the SEM of CuO–HMSS catalyst after the seven runs of triazole synthesis reaction. It shows that the catalyst framework was preserved during the seven runs of reaction. In addition, according to ICP data, the Cu content of recycled catalyst (after seven runs) was determined to be 9.35%. Conclusively, just the loss of 4.5% of Cu as active ingredient after seven runs of reaction was recognized. This remarkable stability could be referred to the unique structure of the catalysts which is composed of a protective thick shell of mesoporous silica. As stated before, due to the mesoporous nature of SiO2 as well as the presence of cavities in catalyst structure, the mass transfer to active sites is facilitated regardless of the thickness of the silica shell.
The CuO–HMSS catalyst performance during the successive cycles was represented in Fig. 6. Clearly, the loss of reactivity in the presence of catalyst is favorably low even at 7th run of reaction.
To approve the heterogeneous nature of the Cu–HMSS for catalysis of cycloaddition reaction, the hot filtration test was performed. Briefly, after 50% progress of reaction, the catalyst was separated from the reactants vessel and then was allowed to proceed for excess 1 h. Here, in the absence of any CuO–HMSS catalyst, no progress in the reaction was observed. According to this observation, it could be resulted that the tested Click reaction over the CuO–HMSS catalyst, was proceeds via the heterogeneous route (Supporting Information, Fig. S3).
4 Conclusion
Here, the novel heterogeneous nanocatalyst based on CuO loaded mesoporous SiO2 was prepared for synthesis of 1,2,3 triazoles via the Click reaction in water. The favorable structural features such as microporosity, presence of cavities in catalyst spheres and the excellent dispersion of CuO species were characterized. In addition, the high chemical and mechanical stability was obtained for CuO–HMSS catalyst under the optimized reaction condition. Conclusively, the CuO–HMSS catalyst could be suggested for catalyzing of cycloaddition reaction for preparation of 1,2,3-triazole compounds under the satisfactory mild condition.
References
Luque R, Balu AM, Campelo JM et al (2012) Catalytic applications of mesoporous silica-based materials. Catalysis 24:253–280
Liang J, Liang Z, Zou R et al (2017) Heterogeneous catalysis in zeolites, mesoporous silica, and metal–organic frameworks. Adv Mater 29(30):1701139
Slowing II, Trewyn BG, Giri S et al (2007) Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv Funct Mater 17(8):1225–1236
Zhu Y, Shi J, Shen W et al (2005) Stimuli-responsive controlled drug release from a hollow mesoporous silica sphere/polyelectrolyte multilayer core–shell structure. Angew Chem 117(32):5213–5217
Kim MH, Na HK, Kim YK et al (2011) Facile synthesis of monodispersed mesoporous silica nanoparticles with ultralarge pores and their application in gene delivery. ACS Nano 5(5):3568–3576
Ispas C, Sokolov I, Andreescu S (2009) Enzyme-functionalized mesoporous silica for bioanalytical applications. Anal Bioanal Chem 393(2):543–554
Sayari A, Hamoudi S, Yang Y (2005) Applications of pore-expanded mesoporous silica. 1. Removal of heavy metal cations and organic pollutants from wastewater. Chem Mater 17(1):212–216
Franchi RS, Harlick PJ, Sayari A (2005) Applications of pore-expanded mesoporous silica. 2. Development of a high-capacity, water-tolerant adsorbent for CO2. Ind Eng Chem Res 44(21):8007–8013
Wu SH, Mou CY, Lin HP (2013) Synthesis of mesoporous silica nanoparticles. Chem Soc Rev 42(9):3862–3875
Wang X, Feng JI, Bai Y et al (2016) Synthesis, properties, and applications of hollow micro-/nanostructures. Chem Rev 116(18):10983–11060
Prieto G, Tüysüz H, Duyckaerts N et al (2016) Hollow nano-and microstructures as catalysts. Chem Rev 116(22):14056–14119
Li Y, Shi J (2014) Hollow-structured mesoporous materials: chemical synthesis, functionalization and applications. Adv Mater 26(20):3176–3205
Ding S, Chen JS, Qi G et al (2010) Formation of SnO2 hollow nanospheres inside mesoporous silica nanoreactors. J Am Chem Soc 133(1):21–23
Salgueiriño-Maceira V, Spasova M, Farle M (2005) Water-stable magnetic silica–cobalt/cobalt oxide–silica multishell submicrometer spheres. Adv Funct Mater 15(6):1036–1040
Liu J, Yang HQ, Kleitz F et al (2012) Yolk–shell hybrid materials with a periodic mesoporous organosilica shell: ideal nanoreactors for selective alcohol oxidation. Adv Funct Mater 22(3):591–599
Ge J, Zhang Q, Zhang T et al (2008) Core–satellite nanocomposite catalysts protected by a porous silica shell: controllable reactivity, high stability, and magnetic recyclability. Angew Chem 120(46):9056–9060
Joo SH, Park JY, Tsung CK et al (2009) Thermally stable Pt/mesoporous silica core–shell nanocatalysts for high-temperature reactions. Nat Mater 8(2):126
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26(1):62–69
Guo X, Liu X, Xu B et al (2009) Synthesis and characterization of carbon sphere-silica core–shell structure and hollow silica spheres. Colloids Surf A 345(1–3):141–146
Sun X, Liu J, Li Y (2006) Use of carbonaceous polysaccharide microspheres as templates for fabricating metal oxide hollow spheres. Chemistry—Eur J 12(7):2039–2047
Hu J, Chen M, Fang X et al (2011) Fabrication and application of inorganic hollow spheres. Chem Soc Rev 40(11):5472–5491
Titirici MM, Antonietti M, Thomas A (2006) A generalized synthesis of metal oxide hollow spheres using a hydrothermal approach. Chem Mater 18(16):3808–3812
Zhou HC, Wang Y (2012) Recent researches in triazole compounds as medicinal drugs. Curr Med Chem 19(2):239–280
Agalave SG, Maujan SR, Pore VS (2011) Click chemistry: 1, 2, 3-triazoles as pharmacophores. Chemistry—Asian J 6(10):2696–2718
Sharghi H, Khalifeh R, Doroodmand MM (2009) Copper nanoparticles on charcoal for multicomponent catalytic synthesis of 1,2,3-triazole derivatives from benzyl halides or alkyl halides, terminal alkynes and sodium azide in water as a “green” solvent. Adv Synth Catal 351(1-2):207–218
Sharghi H, Beyzavi MH, Safavi A et al (2009) Immobilization of porphyrinatocopper nanoparticles onto activated multi-walled carbon nanotubes and a study of its catalytic activity as an efficient heterogeneous catalyst for a click approach to the three-component synthesis of 1, 2, 3-triazoles in water. Adv Synth Catal 351(14–15):2391–2410
Sharghi H, Hosseini-Sarvari M, Moeini F et al (2010) One-Pot, Three-Component Synthesis of 1-(2-Hydroxyethyl)-1H-1, 2, 3-triazole Derivatives by Copper-Catalyzed 1, 3-Dipolar Cycloaddition of 2-Azido Alcohols and Terminal Alkynes under Mild Conditions in Water. Helvetica Chimica Acta. 93(3):435–449
Sharghi H, Khoshnood A, Doroodmand MM et al (2012) 1, 4-Dihydroxyanthraquinone-copper (II) nanoparticles immobilized on silica gel: a highly efficient, copper scavenger and recyclable heterogeneous nanocatalyst for a click approach to the three-component synthesis of 1, 2, 3-triazole derivatives in water. J Iran Chem Soc 9(2):231–250
Alonso F, Moglie Y, Radivoy G et al (2009) Copper nanoparticles in click chemistry: an alternative catalytic system for the cycloaddition of terminal alkynes and azides. Tetrahedron Lett 50(20):2358–2362
Alonso F, Moglie Y, Radivoy G (2015) Copper nanoparticles in click chemistry. Acc Chem Res 48(9):2516–2528
Brotherton WS, Michaels HA, Simmons JT et al (2009) Apparent copper (II)-accelerated azide–alkyne cycloaddition. Org Lett 11(21):4954–4957
Haldon E, Nicasio MC, Perez PJ (2015) Copper-catalysed azide–alkyne cycloadditions (CuAAC): an update. Org Biomol Chem 13(37):9528–9550
Pérez JM, Cano R, Ramón DJ (2014) Multicomponent azide–alkyne cycloaddition catalyzed by impregnated bimetallic nickel and copper on magnetite. RSC Adv 4(46):23943–23951
Kim J, Park J, Park K (2010) CuO hollow nanostructures catalyze [3 + 2] cycloaddition of azides with terminal alkynes. Chem Commun 46(3):439–441
Wang C, Yang F, Cao Y et al (2017) Cupric oxide nanowires on three-dimensional copper foam for application in click reaction. RSC Adv 7(16):9567–9572
Jin T, Yan M, Minato T et al (2011) Nanoporous copper metal catalyst in click chemistry: nanoporosity-dependent activity without supports and bases. Adv Synth Catal 353(17):3095–3100
Luz I, i Xamena FL, Corma A (2010) Bridging homogeneous and heterogeneous catalysis with MOFs: “Click” reactions with Cu-MOF catalysts. J Catal 276(1):134–140
Mukherjee N, Ahammed S, Bhadra S et al (2013) Solvent-free one-pot synthesis of 1, 2, 3-triazole derivatives by the ‘Click’reaction of alkyl halides or aryl boronic acids, sodium azide and terminal alkynes over a Cu/Al2O3 surface under ball-milling. Green Chem 15(2):389–397
Alonso F, Moglie Y, Radivoy G et al (2013) Alkenes as azido precursors for the one-pot synthesis of 1, 2, 3-triazoles catalyzed by copper nanoparticles on activated carbon. J Org Chem 78(10):5031–5037
Alonso F, Moglie Y, Radivoy G et al (2011) Multicomponent click synthesis of 1, 2, 3-triazoles from epoxides in water catalyzed by copper nanoparticles on activated carbon. J Org Chem 76(20):8394–8405
Chassaing S, Sani Souna Sido A, Alix A et al (2008) “Click chemistry” in zeolites: copper (I) zeolites as new heterogeneous and ligand-free catalysts for the huisgen [3 + 2] cycloaddition. Chemistry—Eur J 14(22):6713–6721
Gholinejad M, Jeddi N (2014) Copper nanoparticles supported on agarose as a bioorganic and degradable polymer for multicomponent click synthesis of 1, 2, 3-triazoles under low copper loading in water. ACS Sustain Chem Eng 2(12):2658–2665
Wu L, Li L, Li B et al (2017) Selective conversion of coconut oil to fatty alcohols in methanol over a hydrothermally prepared Cu/SiO2 catalyst without extraneous hydrogen. Chem Commun 53(45):6152–6155
Wu L, Li B, Zhao C (2018) Direct synthesis of hydrogen and dimethoxylmethane from methanol on copper/silica catalysts with optimal Cu+/Cu0 sites. ChemCatChem 10(5):1140–1147
Huang X, Ma M, Miao S et al (2017) Hydrogenation of methyl acetate to ethanol over a highly stable Cu/SiO2 catalyst: reaction mechanism and structural evolution. Appl Catal A 531:79–88
Zhao Y, Li S, Wang Y et al (2017) Efficient tuning of surface copper species of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol. Chem Eng J 313:759–768
Gong J, Yue H, Zhao Y et al (2012) Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0–Cu+ sites. J Am Chem Soc 134(34):13922–13925
Rajabzadeh M, Khalifeh R, Eshghi H et al (2018) A facile hydrothermal synthesis of novel hollow triple-shell CuNiFe2O4 nanospheres with robust catalytic performance in the Suzuki–Miyaura coupling reaction. J Catal 360:261–269
Rajabzadeh M, Eshghi H, Khalifeh R et al (2018) 2-Hydroxyethylammonium formate ionic liquid grafted magnetic nanoparticle as a novel heterogeneous catalyst for the synthesis of substituted imidazoles. Appl Organomet Chem 32(2):e4052
Rajabzadeh M, Eshghi H, Khalifeh R et al (2016) Generation of Cu nanoparticles on novel designed Fe3O4@SiO2/EP. EN. EG as reusable nanocatalyst for the reduction of nitro compounds. RSC Adv 6(23):19331–19340
Lamei K, Eshghi H, Bakavoli M et al (2017) Carbon coated copper nanostructures as a green and ligand free nanocatalyst for Suzuki cross-coupling reaction. Catal Commun 92:40–45
Lamei K, Eshghi H, Bakavoli M et al (2017) Magnetically recoverable gold nanorods as a novel catalyst for the facile reduction of nitroarenes under aqueous conditions. Catal Lett 147(2):491–501
Sharghi H, Khalifeh R (2007) Eco-friendly synthesis of novel lariat ethers via Mannich reaction under solventless. Heterocycles 71(7):1601–1614
Sharghi H, Khalifeh R (2008) Reaction on a solid surface—a simple, economical, and efficient Mannich reaction of azacrown ethers over graphite. Can J Chem 86(5):426–434
Khalifeh R, Sharghi H, Rashidi Z (2013) Synthesis of [Zn (ΙΙ) BHPPDAH] as new heterogeneous catalyst without being immobilized on any support and applied for Mannich reaction. Heteroat Chem 24(5):372–383
Sharghi H, Khalifeh R, Rashidi Z (2013) Synthesis of chromeno [3,4-bb] quinoline derivatives by heterogeneous [Cu (II) BHPPDAH] catalyst without being immobilized on any support under mild conditions using PEG 300 as green solvent. Mol Divers 17(4):721–730
Khalifeh R, Ghamari M (2016) A multicomponent synthesis of 2-amino-3-cyanopyridine derivatives catalyzed by heterogeneous and recyclable copper nanoparticles on charcoal. J Braz Chem Soc 27(4):759–768
Sharghi H, Khalifeh R, Mansouri SG et al (2011) Simple, efficient, and applicable route for synthesis of 2-aryl (heteroaryl)-benzimidazoles at room temperature using copper nanoparticles on activated carbon as a reusable heterogeneous catalyst. Catal Lett 141(12):1845–1850
Sharghi H, Khalifeh R, Moeini F et al (2011) Mannich reaction of secondary amines, aldehydes and alkynes in water using Cu/C nanoparticles as a heterogeneous catalyst. J Iran Chem Soc 8(1):S89–S103
Acknowledgements
We gratefully acknowledge the support of this work by the Shiraz University of Technology and Ferdowsi University of Mashhad Research Council (Grant No: 3/39578).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interests regarding the publication of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rajabzadeh, M., Khalifeh, R., Eshghi, H. et al. Design and Preparation of Hallow Mesoporous Silica Spheres Include CuO and Its Catalytic Performance for Synthesis of 1,2,3-Triazole Compounds via the Click Reaction in Water. Catal Lett 149, 1125–1134 (2019). https://doi.org/10.1007/s10562-019-02666-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02666-1