Abstract
In order to improve the photocatalytic degradation performance of Ag3PO4 and reduce the cost of photocatalyst made of Ag3PO4, a kind of Ag3PO4/TiO2 composite was successfully fabricated via a simply precipitation method for the Ag3PO4 nanoparticles being loaded on the surface of commercial titanium dioxide (P25) to form a heterostructure. The Ag3PO4/TiO2 composite were characterized by FT-IR, XRD, XPS, FE-SEM, HR-TEM, DRS and PL and applied for the degradation of formaldehyde solution under solar radiation. The results showed that the degradation rat of Ag3PO4/TiO2 composite (0.01796 min−1) was much higher than that of pure Ag3PO4 (0.00775 min−1), indicating that the Ag3PO4/TiO2 composite possessed better photocatalytic degradation activity for the formaldehyde solution than pure Ag3PO4. The composite significantly decreased the dosage of silver for photocatalyst under solar radiation, thereby reduced the cost of the photocatalyst made from silver.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the development of economy and the improvement of people’s living standard, the interior decoration was paid more attention, and lots of textiles were used. This inevitably results in indoor air pollution, among which formaldehyde is one of the most typical pollutants, and it is harmful to human body, prolonged exposure to formaldehyde may cause the immune system of body being injured, leading to central nervous system being damaged, growth disorders, blindness and respiratory diseases [1, 2], and may even cause cancer and probably leukemia [3]. It is urgent to remove formaldehyde efficiently, and to do it without secondary pollution.
Photocatalytic technology widely used as a kind of green technology in energy conversion, pollution treatment and air purification has attracted great attention in recent years. Among the numerous semiconductor photocatalysts, silver phosphate (Ag3PO4), a kind of materials with quantum efficiency up to 90% [4, 5], which can absorb sunlight with wavelength short than 520 nm [6], has been widely researched for its extremely high photooxidative capabilities for O2 evolution from water as well as the degradation of organic pollutants under visible light irradiation [7]. As a kind of narrow band gap photocatalysts [8], Ag3PO4, with higher photocatalytic activity than that of many other visible light photocatalysts such as TiO2–xNx, BiVO4 and WO3 [9], has a wide range of applications in the future. However, the silver belongs to noble metal, its price is relatively high. How to reduce the cost of its application without sacrificing its photocatalytic performance is still a challenge.
The titanium dioxide (TiO2) has been proved to be a prospect photocatalysts because of its low cost, nontoxicity and long-term photostability [10,11,12,13]. But the band gap of TiO2 is large, which makes it only absorb the lights in ultraviolet (UV) region. While the UV light occupied only 3–5% in the solar spectrum, this may limited the application of TiO2 in visible region [14]. To improve the utilization ratio of solar light, much efforts has been invested to expand the band gap of TiO2, including metal and nonmetal ion doping [14], organic dye sensitization, surface precious metal deposition, semiconductor composite and other means [15]. Obviously, the semiconductor composite has proved to be an efficient way to extend the absorption spectra of TiO2 into the visible region [16,17,18,19].
However, the conduction band of TiO2 is more negative than that of Ag3PO4, and the valence band of them are similar [20, 21], thus it is appropriate to construct heterojunction to make the composite response under visible light and reduce the cost of the photocatalyst made from silver [22]. Therefore, in this study, we use a simple precipitation method by depositing Ag3PO4 nanoparticles onto the surface of commercial TiO2 (P25) to prepare the Ag3PO4/TiO2 composite for reducing the cost of the photocatalysts made from silver while not affecting its photocatalytic property. Taking into account the convenience of the experiments, the composites were applied to degrade formaldehyde in aqueous solution, the photocatalytic property of the composite has been measured, and compared with that of pure Ag3PO4 and P25.
2 Experimental
2.1 Materials
Silver nitrate (AgNO3), formaldehyde solution (HCHO), isopropanol (IPA) and ammonia (NH3·H2O) were obtained from Hangzhou Gaojing Fine Chemical Co. Ltd. Sodium hydrogen phosphate (Na2HPO4·12H2O), ethylene diamine tetraacetic acid (EDTA) and benzoquinone (BQ) were purchased from Aladdin Reagent Co. Ltd. These above reagents were all analytical grade. Commercial titanium dioxide (P25, mixture of anatase and rutile) was achieved from Guangzhou Heqian Trading Co. Ltd. All of the experiments the deionized water were used. The reagents were used as received without further purification.
2.2 The Fabrication of Ag3PO4/TiO2 Composite
The Ag3PO4/TiO2 composite was fabricated by a simple precipitation method as following: 0.005 mol commercial TiO2 was dispersed in 30 mL deionized water to form a mixture, and the pH of the mixture was adjust to 10 with ammonia, then the mixture was sonicated for 20 min to obtain a white suspension. Subsequently a certain amount of AgNO3 (0.1 M) was added into the suspension, which was continuously stirred for 30 min. Then the Na2HPO4 (0.1 M) solution was added dropwise into the above solution, which was stirred for 5 h at room temperature. After that, the precipitate was obtained by centrifugated and rinsed thoroughly with deionized water until the solution near neutral, and it was dried in the vacuum at the temperature of 60 °C for 12 h. Finally the Ag3PO4/TiO2 composite was obtained. For comparison, the pure Ag3PO4 was obtained at the same conditions in the absence of commercial TiO2.
2.3 Characterizations
Fourier transform infrared (FT-IR) analysis was performed to evaluate the functional identification of the composite photocatalysts using a spectrophotometer (Nlcolet iS50, USA) in a wavenumber ranging from 400 to 4000 cm−1. X-ray diffraction (XRD) patterns were identified by a ARL XTRA diffractometer (Thermo ARL, Switzerland) to record the crystal structures over 2θ in the range of 20°–80° at a scanning rate of 4 °/min. X-ray photoelectron spectroscopy (XPS) was conducted on a K-Alpha spectrometer (Thermo Fisher Scientific, USA) to detect the chemical states and the elemental composition of the composite, and the binding energy was calibrated by the C1s peak of the contamination carbon. The surface morphology structure of the composite photocatalysts was observed by the Field emission scanning electron microscope (JSM-5610LV, JEOL, Japan). The lattice structure of the sample was examined by the high-resolution Transmission electron microscopy (GATAN, 832, USA). The diffuse reflectance spectra (DRS) was recorded on a UV–Vis spectrophotometer in the wavelength range of 325–700 nm. The photoluminescence spectroscopy (PL) was obtained by using the fluorescence spectrophotometer (F-46001, Japan). The electron spin resonance (ESR) signals of superoxide radical (·O2−) and hydroxyl radical (·OH) trapped by DMPO were conducted by a Bruker model spectrophotometer.
2.4 Photocatalytic Degradation Test
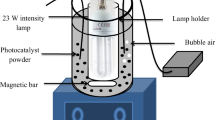
The photocatalytic activity of all samples was measured by the decomposition of formaldehyde solution in a reactor at room temperature during the process by circulating with cool water, with a 350 W xenon lamp as light source, 100 mg Ag3PO4/TiO2 composite was dispersed in 50 mL the as-prepared formaldehyde solution (3.2 mg/L), and before the photocatalytic reaction, first the suspension was stirred in the dark condition for 30 min for adsorption–desorption equilibrium, then, under the light source, the photocatalytic reaction experiment was carried out. Take 5 mL of the reaction liquid at any given time, then the reaction liquid was centrifugated to get the supernatant, the as-prepared acetylacetone solution which used as the colour reaction agent of the formaldehyde solution was added into the supernatant to get a pale yellow transparent solution. According to the change of the absorbance at the maximum absorption wavelength (413 nm), the change of the concentration of formaldehyde solution was determined, so as to judge the photocatalytic degradation performance of the photocatalyst. The residual rate of the solution was calculated by R = {1 − (C0 − Ct)/C0} × 100%, where C0 and Ct were the concentrations of the solution, when the reaction time was 0 and t respectively. According to the Langmuir–Hinshelwood kinetics model, the process of the photocatalytic reaction in accordance with first-order kinetics can be expressed as the following equation [23]:
3 Results and Discussion
3.1 Structure and Morphology Analysis of Ag3PO4/TiO2 Composite
The infrared spectra of Ag3PO4 and Ag3PO4/TiO2 composite photocatalysts were showed in Fig. 1. These infrared spectra were ranged from 400 to 4000 wavenumbers. Figure 1a shows two board peaks, the board peak around 3000 cm−1 was assigned to the O–H stretching vibration of the adsorbed water, the peak around 975 cm− 1 can be assigned to the PO43− stretching vibration [24], and compared with Fig. 1a, there were another two peaks in Fig. 1b, they are around 700 and 1100 cm−1, the board peak corresponds to the O–Ti–O bond stretching. Therefore the infrared spectra indirectly proved that the Ag3PO4/TiO2 composite photocatalysts had been achieved.
Figure 2a shows the XRD patterns of commercial TiO2 (P25), as-prepared Ag3PO4 and Ag3PO4/TiO2 composite. It is observed that the XRD pattern of Ag3PO4 was in good agreement with the XRD pattern of the standard Ag3PO4 (JCPDS no. 06-0505) [22], it was attributed to the body-centered cubic structure, the diffraction peak was sharp, and the half peak width was narrow, there was no impurity peak, it showed that Ag3PO4 has higher crystallinity and fewer crystal defects. The titanium dioxide was commercial TiO2 (P25), a mixture of anatase and rutile phase. The Ag3PO4/TiO2 composite showed the characteristic diffraction peaks of Ag3PO4 and TiO2, there were no other impurity peaks, the result revealed that there was no chemical reaction between Ag3PO4 and P25.
In order to further analysis the chemical states and the elemental composition of the composite, XPS measurements were carried out, and the result can be seen in Fig. 2b–f. From the survey spectrum the primarily peaks are correspond to Ti, O, Ag, C and P elements. The element of carbon was attributed to the calibration carbon. The peak centered at 133.48 eV was related to the p5+ of P 2p in Ag3PO4 [25], and from the picture the peaks at 368.08 eV and 373.98 eV were seen which were corresponded to the 3d3/2 and 3d5/2 of Ag+ respectively [26], there was no peak found at 369.2 eV and 375.8 eV which proved that there is no Ag0 exist. The peak located at 458.88 eV and 464.58 eV could be assigned to Ti 2p1/2 and Ti 2p3/2 respectively [20], and the splitting between them is 5.7 eV, indicating that the element of Ti was in the normal state of Ti4+. The element of oxygen have three different forms of binding energy on the surface of the composite, they were 529.78 eV, 530.58 eV and 532.18 eV respectively, and the peaks at 529.78 eV and 530.58 eV were attributed to the oxygen lattices in Ag3PO4 and TiO2 [27], the other one was ascribed to the present of hydroxyl groups on the surface of the composite. The results of XPS further proved that we had obtained the Ag3PO4/TiO2 composite.
Figure 3a showed the morphological characteristics of the Ag3PO4/TiO2 composite when the molar ratio of Ag3PO4 and TiO2 was 5:5. From the image, it could be seen that there were two kinds of particles (big particles and small particles), and the smaller nanoparticles were loading on the surface of the bigger one. Figure 3b, c show the TEM images of TiO2 and Ag3PO4/TiO2 composite. For further observation, Fig. 3d showed the high magnification TEM image of Ag3PO4/TiO2 composite. The image of Fig. 3b revealed that the average particle size of TiO2 was about 25 nm, and from the image of Fig. 3d it could be seen clearly that there were two types of lattice fringes. One of the observed lattice spacing of 0.350 nm was in good agreement with the (101) plane of anatase TiO2 (JPCDS no. 21-1272) [21], and the other lattice spacing of 0.268 nm was attributed to the (210) plane of Ag3PO4 (JPCDS no. 06-0505) [22]. Therefore it can be conclude that the Ag3PO4 nanoparticles were successfully loading on the surface of TiO2, and the TEM image was shown in Fig. 3c, the size of these Ag3PO4 nanoparticles was about 5–9 nm, it was much smaller than that of pure Ag3PO4 with the average particle size being about 1261 nm. This may be due to the electrostatic repulsion between TiO2 and HPO4−, which slowed the reaction between Ag+ and PO43−, therefore, the size of Ag3PO4 on the surface of TiO2 was relatively small.
The UV–Vis diffuse reflectance spectroscopy of Ag3PO4, commercial TiO2 and Ag3PO4/TiO2 composite were shown in Fig. 4. It could be seen clearly that TiO2 could only absorb the light in UV region, barely absorb the light in visible light region. The UV–Vis diffuse reflectance spectroscopy of Ag3PO4 indicates that it could absorb the solar energy with the absorption edge about 506 nm. From the picture it could be observed that the Ag3PO4/TiO2 composite showed a red shift in its absorption bands being compared with TiO2, and it had a strong absorption in solar, which was because of the Ag3PO4 nanoparticles loading on the surface of TiO2 to form a heterostructure.
3.2 Photocatalytic Degradation Performance Test Under Solar Radiation
The photocatalytic degradation performance of Ag3PO4/TiO2 composite were shown in Fig. 5. To silver phosphate, after solar radiation for 70 min, almost 50% of the formaldehyde had been degraded, this proved that silver phosphate had a certain extent capability of degrading formaldehyde solution, but it is not ideal, and the silver belonged to noble metal, its price is relatively high. In this article, the cheaper TiO2 was chosen to composite with Ag3PO4 for reducing the dosage of Ag3PO4 for preparing photocatalyst, and it was found that the Ag3PO4/TiO2 composite had a better photocatalytic activity compared with Ag3PO4 as photocatalyst, after solar radiation for 70 min almost 80% of the formaldehyde could be degraded. With the increase of Ag3PO4 in the composite, the photocatalytic activity increase, especially when the molar ratio of Ag3PO4 and TiO2 was 5:5, the degradation rate (0.01796 min−1) of formaldehyde over Ag3PO4/TiO2 are 2.32 times higher than that (0.00775 min−1) of Ag3PO4, when the Ag3PO4 being increased continuously the photocatalytic activity of Ag3PO4/TiO2 composite decrease. For further analysis the photocatalytic activity of the photocatalysts, the PL spectrum analysis was carried out to investigate the separation efficient of photogenerated electrons and holes. Generally speaking, the lower PL emission signal indicates the higher separation efficient of photogenerated electrons and holes, and resulting in higher photocatalytic activity. The PL emission of TiO2, Ag3PO4 and Ag3PO4/TiO2 composite was shown in Fig. 6. Compared with pure TiO2 and Ag3PO4, the PL intensity of Ag3PO4/TiO2 composite decreased significantly, this clearly reveal that the Ag3PO4 composite with TiO2 can obtain a higher charge separation efficiency, and the Ag3PO4 deposited on the surface of TiO2 can greatly inhibited the recombination of photogenerated electrons and holes under solar radiation. The PL intensity decreased with the increase of Ag3PO4 being loaded on the surface of TiO2 and reach a minimum when the molar ratio of Ag3PO4 and TiO2 was 5:5, which indicating the better photocatalytic activity of the Ag3PO4/TiO2 composite. However, the intensity of PL increased when the Ag3PO4 being increased continuously. This may be ascribed to the factor that the spare Ag3PO4 become the recombination centre of photogenerated electrons and holes. The PL results were in consistent with the photocatalytic activity of the Ag3PO4/TiO2 composite.
3.3 Stability of Ag3PO4/TiO2 Composite in Recycling Reaction for Formaldehyde Degradation
Apart from the photocatalytic degradation performance, the recycling stability of photocatalyst is another important performance for the practical application. The recycling experiments were carried out to compare the stability between Ag3PO4/TiO2 composite and pure Ag3PO4 for the photodegradation of formaldehyde solution under solar radiation, as shown in Fig. 7. After three recycling runs, from the corresponding results we can know that the photocatalytic degradation performance of Ag3PO4/TiO2 composite was better than pure Ag3PO4 in all these recycling experiments. The photocatalytic activity of Ag3PO4 and Ag3PO4/TiO2 composite were all only slightly reduced after three consecutive recycling experiments, indicating that the combination of Ag3PO4 and TiO2 didn’t affect the cyclic stability of the composite. At the same time, the results indicate that the Ag3PO4/TiO2 composite photocatalyst has a better photocatalytic activity and recycle stability compared with Ag3PO4.
3.4 The Mechanism of Photocatalytic Activity of Ag3PO4/TiO2 Composite
In the process of photocatalytic reaction, the main factor that affects the activity of the photocatalysts is the separation and transfer of the photogenerated electrons (e−) and holes (h+). The photogenerated electrons tend to combine with O2 in the solution to form superoxideradical (·O2−), and the holes tend to combine with water molecules and hydroxyl ion to produce hydroxyl radical (·OH). In order to study the mechanism of the degradation process, the scavenging experiments were conducted on Ag3PO4/TiO2 composite which the mole ratio of Ag3PO4 and TiO2 was 5:5 for the degradation of formaldehyde solution under solar radiation. In this study, three different chemicals ethylene diamine tetraacetic acid (EDTA), benzoquinone (BQ) and isopropanol (IPA) was used as scavengers for holes (h+), superoxideradical (·O2−) and hydroxyl radical (·OH) respectively. As shown in Fig. 8a, b, it can be found that the photocatalytic degradation rate decreased in the order for decrease degree: IPA (·OH) < BQ (·O2−) < EDTA (h+), when IPA was added in, the photocatalytic activity was slightly decreased, which indicate that ·OH was not the main reactive species during the photocatalytic reaction. On the contrary, when EDTA and BQ were added in, the photocatalytic activity was significantly decreased, which indicated that h+ and ·O2− were the main reactive species during the photocatalytic reaction in the Ag3PO4/TiO2 system. Furthermore, the main photocatalytic activity species ·OH and ·O2− of the Ag3PO4/TiO2 composite were also detected by ERS spin-trap technique (with DMPO), as shown in Fig. 8c, d, there are six characteristic peaks of the DMPO-·O2− radicals under solar radiation that can not be detected under dark, and there is hardly any peak of the DMPO-·OH radicals whether under solar radiation or dark. Thus, h+ and ·O2− can be considered as the main reactive specie rather than ·OH in Ag3PO4/TiO2 composite photocatalytic reactions.
a Photocatalytic activities of Ag3PO4/TiO2 composite for the degradation of formaldehyde solution in the presence of different scavengers under solar radiation, b the apparent pseudo-first-order rate constant kapp with Ag3PO4/TiO2 composite in the presence of different scavengers for the degradation of formaldehyde solution, DMPO spin-trapping ESR spectra of Ag3PO4/TiO2 composite photocatalytic c·O2− and d ·OH
The probably mechanism of photocatalytic activity of Ag3PO4/TiO2 composite was showed in Fig. 9. As shown in Fig. 9, under solar radiation, the Ag3PO4 and TiO2 absorbed the photons, produced the photo-generated electrons in the conduction band (CB) and photo-generated holes in the valence band (VB), because of the potential valence band and conduction band of TiO2 were more negative than that of Ag3PO4 [7, 28], then photo-generated holes quickly transferred to the surface of TiO2, photo-generated electrons transferred to the surface of Ag3PO4, at the same time the photo-generated electrons absorbed O2 in the solution to form ·O2−, in such a way, prevented the recombination of the photo-generated electrons and photo-generated holes to promote the separation of them, thus improved the photocatalytic activity of the Ag3PO4/TiO2 composite photocatalyst.
4 Conclusion
The Ag3PO4/TiO2 composite was successfully fabricated via a simply precipitation method. The Ag3PO4 nanoparticles were loaded on the surface of commercial titanium dioxide (TiO2), and the size of Ag3PO4 nanoparticles was about 5–9 nm. From the images of the HR-TEM it could be seen that Ag3PO4 were dispersed on the surface of TiO2 to form the formation of heterojunctions. The Ag3PO4 nanoparticles on the surface of TiO2 made the absorption bands of TiO2 have a red shift, thus improved the photocatalytic performance of the composite. The composite showed much higher photocatalytic activity under solar radiation than pure Ag3PO4 and TiO2 for Ag3PO4/TiO2 composite was a formation of heterojunctions. Therefore it was showed that the Ag3PO4 composited with TiO2 can not only enhance the photocatalytic activity, but also reduce the cost of Ag3PO4 as photocatalyst under solar radiation.
References
Ensafi AA, Honarmand E (2005) Anal Sci 21(5):545
Jones SB, Terry CM, And TEL (1999) Anal Chem 71(18):4030
Tang X, Bai Y, Duong A (2009) Environ Int 35(8):1210
Yan T, Guan W, Li W (2014) RSC Adv 4(70):37095
Guo X, Chen C, Yin S (2015) J Alloys Compd 619:293
Hua X, Jin Y, Wang K (2014) Catal Commun 52(8):49
Yi Z, Ye J, Kikugawa N (2010) Nat Mater 9(7):559
Zhao FM, Pan L, Wang S (2014) Appl Surf Sci 317:833
Vu TA, Dao CD, Hoang TT T (2013) Mater Lett 92(1):57
Lim YWL, Tang Y, Cheng YH (2010) Nanoscale 2(12):2751
Fujishima A, Honda K (1972) Nature 37(1):238
Ohno T, Sarukawa K, Tokieda K (2001) J Catal 203(1):82
Pan L, Zhang J, Jia Xu, Ma Y-H, Zhang X, Wang L, Zou J-J (2017) Chin J Catal 38(2):253
Cong Y, Zhang J, Chen F (2007) J Phys Chem C 111(19):6976
Yu J, Dai G, Huang B (2009) J Phys Chem C 113(37):16394
Tong ZW, Yang D, Sun YY (2015) Phys Chem Chem Phys 17(18):12199
Wang M, Han J, Xiong H (2015) Langmuir 31(22):6220
Wang X, Utsumi M, Yang Y (2015) Appl Surf Sci 325:1
Pan L, Wang S, Xie J (2016) Nano Energy 28:296
Xie J, Yang Y, He H (2015) Appl Surf Sci 355:921
Liu R, Hu P, Chen S (2012) Appl Surf Sci 258(24):9805
Teng W, Li X, Zhao Q (2013) J Mater Chem A 1(32):9060
Yang ZM, Huang GF, Huang WQ (2014) J Mater Chem A 2(6):1750
Thomas M, Ghosh SK, George KC (2002) Mater Lett 56(4):386
Pelavin M, Hendrickson DN, Hollander JM (1970) J Phys Chem 74(5):1116
Zhang H, Wang G, Chen D (2008) Chem Mater 20(20):6543
Li Y, Yu L, Li N (2015) J Colloid Interface Sci 450:246
Serpone N, Maruthamuthu P, Pichat P (1995) J Photochem Photobiol A 85(3):247
Acknowledgements
This study was kindly supported by Key Research and Development Program of Science and Technology Department of Zhejiang Province (NO. 2018C03004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Yu, D., Wu, M. et al. Fabrication of Ag3PO4/TiO2 Composite and Its Photodegradation of Formaldehyde Under Solar Radiation. Catal Lett 149, 882–890 (2019). https://doi.org/10.1007/s10562-019-02654-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02654-5