Abstract
Bismuth oxychloride (BiOCl) single crystal nanosheets with controllable and highly exposed {001} facets have been synthesized via hydrolytic methods without any surfactants. The BiOCl nanosheets had a thickness from 30 to 120 nm and were synthesized by tuning evaporation temperature, while the ratio of {001} facets varies from 98 to 49%. A growth rate in {001} facets agrees with the Boltzmann function and a possible growth mechanism has been proposed. Moreover, the existence of highly exposed {001} facets could gradually narrow the band gap of BiOCl nanosheets. This work provides a new insight for controllable synthesis of layered materials with different facets, and provides a flexible strategy for exploring and enhancing photoactivity based on the facets engineering.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Crystalline materials with different exposed facets possess various geometric and electronic structures, which endow them with distinctive properties [1,2,3]. Increasing research interests have been devoted to the synthesis of single crystalline nano-materials with tunable crystallographic orientation to improve the facet dependent photocatalytic, photoelectric, and other related physical and chemical properties [4,5,6,7]. The crystal orientation is especially important since the active facet is vital to the photocatalytic activity due to the facets dependent effective adsorption of reactant molecules and the sensitive active reaction sites [8,9,10]. Zhao et al. [9] reported that BiOCl single-crystalline nanosheets possess surface structure-dependent molecular oxygen activation properties under UV light. Pan et al. [10] reported that the facet with a higher percentage of undercoordinated atoms is more reactive in heterogeneous reactions in TiO2. Undoubtedly, engineering crystal facets provides us a systematic way for exploring highly effective photocatalysts and also allows for the investigation between surface properties and the photoactivity.
Bismuth oxychloride (BiOCl), as one of the most important two dimensional layered materials, has attracted much attention for its unique layered structure and high chemical stability [11, 12]. Recent investigations revealed that BiOCl nanoplates with exposed {001} facets exhibit higher photoactivity than exposed other facets. Jiang et al. [5] reported the internal electric field in {001} facets is beneficial for the separation of the photo-excited electron–hole pairs and present high photocatalytic activities. Wang et al. [13] also reported the exposed {001} facets are not only favorable for the adsorption of dye but also promote the electron transfer between the dye and BiOCl, resulting in an enhanced dye self-sensitized degradation efficiency. Although great efforts have been devoted to controllable synthesis of BiOCl with highly exposed {001} facets with the assistance of the surfactants, such as thiourea [14], mannitol molecules [11], sodium citrate [15], N-hexadecyltrimethylammonium chloride (CTAC) [16, 17], polyvinylpyrrolidone (PVP) [18], there are several shortcomings due to the introduction of heterogeneous impurities and strong effect on the surface sensitive active sites, which are vital to the photoactivity.
In this paper, BiOCl nanosheets with highly exposed {001} facets were synthesized via dominating the nucleation by tuning the evaporation temperature during hydrothermal process. The rate of {001} exposed facets can reach to 98% and maintain the natural state. Moreover, our results suggest that the surface area decreases as the percentage of the {001} facets increase, and the morphology of the BiOCl sheets has much strong effect on the photocatalytic activity.
2 Experimental
2.1 Synthesis
All chemical agents used in this experiment were analytical grade purity and without further purification. In a typical procedure, 0.01 mol Bi2O3 was dissolved in 20 mL 37% HCl solution with fully stirring until a transparent BiCl3–HCl mixed solution was received. Then the above solutions were completely evaporated at different temperature (100, 200, 300, 400 °C respectively) and cooled to room temperature naturally. The resulting powder was washed with deionized for several times until the pH value is about 7.0 and finally dried at 70 °C for 12 h. The samples obtained were named as BOC-1, BOC-2, BOC-3 and BOC-4, corresponding to the evaporation temperature at 100, 200, 300, 400 °C, respectively.
2.2 Characterization
The X-ray diffraction (XRD) measurements were conducted on an Empyrean diffractometer with monochromatic Cu Kα radiation (λ = 0.15418 nm). The morphology of as-prepared samples was obtained with the ZEISS AURIGA field-emission scanning electron microscopy (SEM) and ZEISS LIBRA 200 FEG transmission electron microscopy (TEM). UV–visible diffused reflectance spectra were obtained by a UV2100 UV–visible spectrophotometer using Bi2SO4 as a reference.
2.3 Photocatalytic Measurements
The photocatalytic activity was evaluated by photocatalytic degradation of 10 mg/L rhodamine-B (RhB) and methylene orange (MO) solution under 300 W mercury lamp. In a typical procedure, 100 mg of catalyst was added into 200 mL RhB or MO solution. Prior to illumination, the solution was continuously stirred for 1 h at 800 r/min in dark to establish the adsorption–desorption equilibrium. 5 mL of suspension was taken out every 20 min to monitor the concentration by UV–vis Spectrophotometer (Shimadzu UV-2100).
3 Results and Discussion
3.1 Characterization
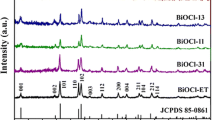
The crystallinity and phase purity of the as-prepared samples are examined by XRD analysis. Figure 1 shows typical XRD patterns of the samples obtained at different evaporation temperature. All the diffraction peaks are well indexed to standard tetragonal phase of BiOCl (JCPDS No. 06-0249, Space group P4/nmm). No impurity peaks can be observed, indicating a high purity of the samples. It is worth noting that a significant increase in the intensity of the {001} facets diffraction peaks with the increasing of evaporation temperature, which signifies the preferential orientation of the {001} facets. As the evaporation temperature increases from 100 to 400 °C, the percentage of {001} facets increases from 49 to 98% (Table S1). Considering the dramatically difference between BOC-2 and BOC-3 samples, the samples has also been synthesized at 230 and 260 °C and the results are also confirmed the increasing trends (Table S1; Fig. S1), which will be discussed later.
The morphology of the different BiOCl samples was characterized by SEM and TEM imaging, as shown in Fig. 2. The BiOCl are square-like nanosheets with a size of 200–400 nm and an average size of about 120 nm in thickness when the evaporation temperature is 100 °C (Fig. 2a). As the evaporation temperature increases, the width of the nanosheets becomes larger and the thickness of the nanosheets becomes thinner (Fig. 2b–d). The largest width of the ultrathin nanosheets can reach up to 10 μm while the thickness is <30 nm when the temperature increases to 400 °C. The side view of the nanosheets displays the step by step overlap and several isolated small sheets on the surface, suggesting an expansion parallel to the surface (Fig. 2e, f).
A typical HRTEM image (Fig. 3a) shows that nanosheets exhibit two sets of clear lattice fringes with inter planar lattice spacing of 0.275 nm, corresponding to the (110) facets of the tetragonal system of BiOCl. The corresponding selected area electron diffraction (SAED) patterns display a regular spot pattern (Fig. 3b), indicating the single-crystalline characteristic. The diffraction patterns can be indexed to (110) and (200) facets of the BiOCl of tetragonal phase, implying that the set of diffraction spots is indexed as the [001] zone axis. Based on the above results, it can be concluded that the BiOCl nanosheets are {001} facets exposed with the top and bottom, as shown in the insert in Fig. 3b. When the evaporation temperature increases, the BiOCl nanosheets were found to grow along the [110] direction, resulting the smaller thickness and larger size. Both of the SEM and TEM results are in good agreement with the XRD patterns and thus the {001} facets are the preferred orientation of the BiOCl nanosheets.
A possible growth mechanism of BiOCl nanosheets is illustrated in Fig. 4 based on the above experimental results. According to the simulation of the percentage and temperature, our results suggest that the growth rate of BiOCl with {001} exposed facet can be well fitted by Boltzmann function [19] (Rsq = 0.995), suggesting that the appearance of {001} facets are thermal energy dependent, as shown in Fig. 4a. As the evaporation temperature increases, the growth of {001} facets may exceed the other facets due to the highest thermal dynamic stability energy [20]. Figure 4b illustrates the process of generation of crystalline nuclei and then gradually grow up at different temperature. The nanosheets with larger size are formed with the high-speed growth of {001} facets, and consequently, the thickness of the nanosheets is reduced.
3.2 Photocatalytic Activity
The photocatalytic performances of the obtained samples were evaluated for the degradation of RhB. The concentration variation of RhB solution (i.e. C/C0, where C is the concentration of RhB at time t and C0 is the concentration of RhB at the beginning of irradiation) versus irradiation time of as-prepared BiOCl samples are shown in Fig. 5. A blank experiment without photocatalyst was also performed under the same conditions for comparison. It should be noted that no degradation can be observed without photocatalyst, while the degradation can reach to ~80% after irradiation for 30 min, indicating the high photoactivity of the BiOCl. In contrast to the previous report that the {001} exposed facets has a higher photoactivity than other facets [5, 13, 21], the degradation rate decreases as the percentage of {001} facets increases. It is found that only 55%, 22% and 8% of RhB is degraded by BOC-2, BOC-3 and BOC-4, respectively. The corresponding degradation rate decreases to 30.0 × 10−4 min−1 from 972.1 × 10−4 min−1 (Fig. 5b). It is reported that BiOCl has a much higher oxygen atoms density in (001) facets, in which the oxygen atom is a negative electric center, should prefer to absorb the positively charged RhB [22]. Thus the absorption and the activity should be lower in the negative charged dyes. Herein, the photocatalytic degradation of the negative charged MO is evaluated, as shown in Fig. 5c, d. However, the BiOCl also present high photocatalytic activity and similar decreasing tendency with higher {001} facets exposed.
a The degradation curves of RhB under UV-light with BOC-1–BOC-4. b Comparison of the reaction rate constants for photocatalytic degradation of RhB via BiOCl. c The degradation curves of MO under UV-light with BOC-1–BOC-4. d Comparison of the reaction rate constants for photocatalytic degradation of MO via BiOCl
There are several reasons which could affect the activity, including surface area, bandgap, charge transport, and surface charge [23,24,25,26,27]. Therefore, the specific surface area and UV–vis diffuse reflectance spectra of the BiOCl samples are characterized. As shown in Fig. 6 and Table S2, the optical band gap of obtained samples should be calculated via the formula (αhν)n = A(hν−E g), where α, h, ν, E g and A are the absorption coefficient, plank constant, light frequency, band gap and constant, respectively, and n equals 2 for direct inter-band transition and 1/2 for indirect inter-band transition. Since BiOCl is an indirect-gap semiconductor, n equals 1/2. The band gap values of the samples are 3.28, 3.21, 3.16 and 3.08 eV for samples BOC-1, BOC-2, BOC-3 and BOC-4, respectively. Our results agree with the report of Gao et al. [28] and Peng et al. [29] that the bandgap of BiOCl has a slightly decrease upon the increasing of {001} facets ratios. Generally, the optical band gap of the crystals is anisotropic and depends on the direction of the crystal [28]. The amplitude of atomic vibrations would increase as the thickness decrease, result in larger interatomic spacing [29, 30], and consequently narrow the band gaps of BiOCl. It is worth noting that the BOC-1 has the largest surface area among the four samples, as shown in Table 1, suggesting that BOC-1 has a higher percentage of exposed surface during the photocatalysis. It is thereby concluded that the excellent photocatalytic activity of BiOCl is mainly attributed to its surface area for dye adsorption and the surface area plays a key role in improving the photocatalytic activity.
4 Conclusions
In summary, BiOCl nanosheets with highly exposed {001} facets have been synthesized via tuning evaporation temperature during hydrolytic process. It is demonstrated that the BiOCl with more {001} facets exposed (up to 98% at 400 °C) can be obtained at higher temperature due to the highest thermal dynamic stability energy of (001) facets. During the high-speed extension of the {001} facets in BiOCl nanosheets, the growth mode was found to be confined along the [001] direction, leading to the formation of the nanosheets with larger lateral length and smaller thickness. While the ratio of {001} facets varies from 49 to 98%, the thickness decreases from 120 to 30 nm. BiOCl with highly exposed {001} facets was also found to have a decrease in the photocatalytic activity, suggesting that the morphology of the photocatalysts has a dominant effect on the photocatalytic activity than exposed highly active facets. The specific surface area plays a key role in enhancing the photocatalytic activity. This work provides new insights for controllable synthesis of layered materials with different facets and broadens the understanding of the major effects of surface structure in BiOCl on its photocatalytic activity.
References
Jiang Z-Y, Kuang Q, Xie Z-X, Zheng L-S (2010) Adv Funct Mater 20:3634
Seker F, Meeker K, Kuech TF, Ellis AB (2000) Chem Rev 100:2505
Wu S, Wang C, Cui Y (2014) Appl Surf Sci 289:266
Xiong J, Cheng G, Qin F, Wang R, Sun H, Chen R (2013) Chem Eng J 220:228
Jiang J, Zhao K, Xiao X, Zhang L (2012) J Am Chem Soc 134:4473
Ye L, Tian L, Peng T, Zan L 2011 J Mater Chem 21:12479
Tian F, Zhang Y, Li G, Liu Y, Chen R (2015) New J Chem 39:1274
Xu Y, Xu S, Wang S, Zhang Y, Li G (2014) Dalton Trans 43:479
Zhao K, Zhang L, J Wang, Q Li, W He, JJ Yin (2013) J Am Chem Soc 135:15750
Pan J, Liu G, Lu GQ, Cheng HM (2011) Angew Chem Int Ed 50:2133–2137
Xiong J, Cheng G, Li G, Qin F, Chen R (2011) RSC Adv 1:1542
Wu S, Xiong J, Sun J, Hood ZD, Zeng W, Yang Z, Yang SZ (2017) ACS Appl Mater Inter 9:16620
Wang DH, Gao GQ, Zhang YW, Zhou LS, Xu AW, Chen W (2012) Nanoscale 4:7780
Ye L, Zan L, Tian L, Peng T, Zhang J (2011) Chem Commun 47:6951
Hu X, Xu Y, Zhu H, Hua F, Zhu S (2016) Mater Sci Semicond Process 41:12
Ao Y, Tang H, Wang P, Wang C (2014) Mater Lett 131:74
Bai Y, Wang P-Q, Liu J-Y, Liu X-J (2014) RSC Adv 4:19456
Lei Y, Wang G, Song S, Fan W, Zhang H (2009) CrystEngComm 11:1857
Cercignani C (1988) The boltzmann equation and its applications. Springer, New York, pp 40–103
Zhang H, Liu L, Zhou Z 2012 RSC Adv 2:9224
Haider Z, JY Zheng, Kang YS (2016) Phys Chem Chem Phys 18:19595
Guan M, Xiao C, Zhang J, Fan S, An R, Cheng Q, Xie J, Zhou M, Ye B, Xie Y (2013) J Am Chem Soc 135:10411
Lei A, Qu B, Zhou W, Wang Y, Zhang Q, Zou B (2012) Mater Lett 66:72
Zhang J, Sun J, Maeda K, Domen K, Liu P, Antonietti M, Fu X, Wang X (2011) Energy Environ Sci 4:675
Zhang Z, Luo Z, Yang Z, Zhang S, Y Zhang, Zhou Y, Wang X, Fu X (2013) RSC Adv 3:7215
Kim DS, Kwak S-Y (2007) Appl Catal A 323:110
Kawahara K, Suzuki K, Ohko Y, Tatsuma T (2005) Phys Chem Chem Phys 7:3851
Gao M, Zhang D, Pu X, Li M, Yu YM, Shim JJ, Seo HJ (2015) J Am Ceram Soc 98:1515
Peng S, Li L, Zhu P, Wu Y, Srinivasan M, Mhaisalkar SG, Yan Q (2013) Chem-Asian J 8:258
Zhao T, Zai J, Xu M, Zou Q, Su Y, Wang K, Qian X (2011) CrystEngComm 13:4010
Acknowledgements
We gratefully acknowledge the helpful and informative discussions with Dr. Yuan Yuan and Mr. Zachary H. Hood. We also thank the Fundamental Research Funds for the Central Universities (No.106112015CDJXY130010) and the National Natural Science Foundation of China (No. 51302329) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yi Jiang, Jianguo Sun have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, Y., Sun, J. & Wu, S. BiOCl Nanosheets with Controlled Exposed Facets and Improved Photocatalytic Activity. Catal Lett 147, 2006–2012 (2017). https://doi.org/10.1007/s10562-017-2118-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2118-1