Abstract
Carbon quantum dots-titanium dioxide composite (CQDs-TiO2) has been synthesized by a simple hydrothermal process. It was used as a heterogeneous catalyst for oxidation of alcohols using water as a solvent. The morphologies and composition of the resulting catalysts were characterized to show that the CQDs were uniformly dispersed on the surface of the TiO2 with well combination with each other. The obtained catalyst showed enhanced catalytic activities compared to the pure CQDs catalyst. The enhanced catalytic activities were mainly attributed to the highly dispersive CQDs with more active centers which caused by the TiO2 support. The CQDs-TiO2 catalyst can also be reused without any significant loss in their catalytic activities after five times.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The selective oxidation of alcohols to its aldehydes or ketones is playing a vital role in organic synthesis [1,2,3,4,5], because the obtained products are highly valuable intermediates for pharmaceutical, dyestuff, perfumery, and agrochemical industries. However, stoichiometric oxidants, such as permanganate and perchlorate, were broadly used in homogenous reaction, leading to lots of environmental pollutions in traditional industrial production. Therefore, noble metals (Pd [4, 6], Au [5, 7], Ag [8], Ru [9], etc.) and metal oxides (RuO2 [10] and MnO2 [11]) were developed. These catalysts showed high catalytic activities. But these noble metal based catalysts are so relatively expensive and generated a lot of environmental problems.
Carbon quantum dots (CQDs) is a new class of nanocarbons with size below 10 nm, which have been received increasing attention due to their unique chemical and physical properties. Compared to the traditional semiconductor quantum dots, CQDs possess low toxicity, good biocompatibility, highly aqueous solubility and also have high natural abundance [12]. Therefore, CQDs have gradually become promising carbon nanomaterials for lots of important applications, such as photocatalysis [13, 14], solar cells [15], fuel cell catalyst [16] and have been successfully utilized in bioimaging [17]. Considering the superior activities of the CQDs, our group has developed a transition metal-free CQDs catalyst for oxidation of alcohols with cheap NaClO oxidant using water as the only solvent [18]. The CQDs showed high catalytic activities for oxidation of alcohols. However, owing to the nanosize of the CQDs, the aggregation of the CQDs could not be avoided during the reaction process. Thus, in order to improve their catalytic performance, one of the solutions is that the CQDs should be dispersed on the surface of the support.
TiO2, one of the established photocatalysts under UV light, which has also been extensively probed in the research areas of energy conversion [19] and degradation of contaminants [20] because of its cost-effective nature and fine chemical stability. TiO2 by heteroatom doping possess highly value in solar energy utilization. For this reason, recently, much effort has been devoted to develop visible-light-active photocatalysts. On the side, modifying TiO2 with a carbonaceous substance on its surface can also induce visible-light-responsive activity. In recent years, CQDs-TiO2 composites had been largely reported as photocatalysts and showed enhanced photocatalytic performances [21, 22]. These researches pointed out that CQDs can be well dispersed on the surface of the TiO2. In contrast, CQDs-TiO2 nanocomposites have not been used as heterogeneous catalysts for organic transformations and synthesis.
To the best of our knowledge there are no reports in the literature concerning the use of CQDs-TiO2 composites as heterogeneous catalysts in the oxidation of alcohols. In this work, CQDs were prepared by a simple electrochemical method and then were hydrothermally combined with TiO2 to obtain a new composite, CQDs-TiO2. The composite was used as a catalyst of the oxidation of alcohols with cheap oxidant using only water as the solvent. The reasons for the enhanced catalytic activity of CQDs-TiO2 have been discussed based on the experimental results and on recent literatures.
2 Experimental
2.1 Catalyst Synthesis
2.1.1 Preparation of CQDs
All chemicals were commercially available and were used without further purification. Carbon nanotubes were got from Tsinghua University and Graphene were got from Ningbo institute of industrial technology. CQDs were prepared based on a facile one-pot electro-chemistry method [25, 28]. In a typical process, two graphite rods were inserted parallel into the deionized water as the electrode with a separation of 10 cm, static potentials of 15–60 V were applied to the two electrodes using a direct current (DC) power supply. After about 120 h continuous stirring, a dark-yellow solution appeared gradually in the reactor. The solution was then filtered with slow-speed quantitative filter paper, and the resultant solution was centrifuged at 10,000 rpm for 10 min to remove the precipitated graphite particles. Finally, the obtained solution was water soluble CQDs. Bulk quantities of CQDs can be recovered by drying at 363 K.
2.1.2 Preparation of CQDs Based Composites
The CQDs-TiO2 composite was prepared via a hydrothermal method. Different amount of the above of CQDs was added into 40 mL of distilled water and 20 mL ethanol. Then 100 mg of TiO2 were added and the resultant mixture was ultrasonicated for 10 min. The mixture was put into reaction at 393 K for 2 h in a hydrothermal reactor. The final mixture was dried at 353 K to obtain the desired CQDs-TiO2 composite. In addition, ZnO, Al2O3, Fe2O3 and CuO were used and the preparation of other composites is similar to this method.
2.2 Characterization of Catalyst
The morphologies and structure details of the as-synthesized samples were studied by using transmission electron microscopy (TEM, JEOL-JEM-2010F, Japan), with an accelerating voltage of 200 kV. The crystal structures of the samples were characterized by X-ray diffraction (XRD) (Bruker D8 Advance diffractometer, Germany) at a scanning rate of 4°/min (Cu Ka radiation, k = 0.15418 nm). X-ray photoelectron spectroscopy (XPS) analysis was performed with an ESCAL a-b220i-XL electron spectrometer (VG Scientific, England) using 300 W Al Ka radiation. Fourier transform infrared spectroscopy (FT-IR) experiments were conducted on a Nicolet 670 FT-IR (Thermoelectric, USA) spectrometer in the form of KBr pellets.
2.3 Catalytic Oxidation of Alcohols
The selective oxidation reactions were carried out in a 100 mL flask (for a condenser) including 1 mmol alcohol, 0.09 g CQDs-TiO2, and 25 mL deionized water as solvent. The alcohols used were benzyl alcohol, cinnamyl alcohol, cyclohexanol, n-army alcohol and n-heptanol. The mixture were stirred to form suspension. Then 7 mL of 10% NaClO was gradually added. The reactions were conducted at 50 °C under ambient pressure for 7 h. When the reaction was finished, the mixture was extracted by ethyl acetate (3 × 30 mL).Then ethyl acetate was removed in vacuum. Oxygenated products were analyzed by an Agilent 7890 A Gas Chromatographer (GC). Furthermore, for the test of reusability, the used catalyst was removed from the reaction by filtration and washed with deionized water and acetone, and dried in the air for reuse.
3 Results and Discussion
3.1 The Effect of the Supports
In order to prove that the addition of supports can improve the dispersion of CQDs, the oxidation of benzyl alcohol was tested to help choosing the suitable support from four different nano-metal oxide supports. The result is shown in Table 1. The results show that the nanocomposite catalysts possess higher catalytic activities compare with pure CQDs. Therefore, the existence of supports indeed can promote the dispersion of CQDs. Among the four supports, the CQDs-TiO2 showed the highest catalytic properties. Thus, the TiO2 was chosen as the support for CQDs.
3.2 Characterization of Catalysts
To verify the combination between TiO2 and CQDs, the morphologies of the CQDs and 67 wt% CQDs-TiO2 composites were first conducted and the results are clearly shown in Fig. 1. Figure 1a shows the TEM image of the TiO2, which was in shape of nanoparticle with average diameter about 40 nm. Figure 1b shows the obtained CQDs, revealing that the average diameters of CQDs are about 4 nm. As shown in Fig. 1c, after the hydrothermal reaction, the CQDs were uniformly deposited on the surface of the TiO2. The HR-TEM image from Fig. 1d exhibits the crystal lattice spacing around 0.31 nm agrees well with the (002) spacing of graphitic carbon (JCPDS card no. 46-0943) and the inter planar spacing of 0.20 nm is assigned to the (210) plane of rutile TiO2 (JCPDS card no. 89-4920). The above results indicated that the CQDs can be well dispersed on the surface of the CQDs, demonstrating the well combination between the CQDs and TiO2.
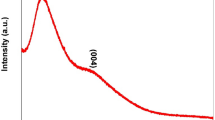
The XRD patterns of CQDs, TiO2 and 67 wt% CQDs-TiO2 composites were shown in Fig. 2. As shown in Fig. 2, the CQDs samples reveals that the electrochemical oxidation process leads to weak and broadened graphite peaks. The decrease in the peak intensity indicates the transformation of crystalline graphite into nano-size structure carbon, and the partial conversion of sp2-hybridized C=C in the aromatic ring into sp3-hybridized C=C in the CQDs [14, 18]. Figure 2b shows the XRD pattern of TiO2, showing the mixing phases of anatase and rutile titania, which can be assigned to the commercial P25. On the other hand, as shown in Fig. 2c, it is interesting to note that the intensity of the peaks at 21.9° and 26.5° are presented in 67 wt% CQDs-TiO2, thus suggesting that the CQDs were dispersed on the surface of TiO2 and can be well detected [23]. The powder XRD patterns for the CQDs and 67 wt% CQDs-TiO2 samples are in good agreement with the HR-TEM results shown above, thus showing the crystalline graphitic carbon structure and the good combination with each other.
FT-IR spectra were also carried out to further characterize the formation between the CQDs and TiO2 (Fig. 3). The characteristic absorption peaks of the 67 wt% CQDs-TiO2 at 3441, 2926, 2855, 1720 and 1387 cm−1 associated with the O–H, sp3 and sp2 C–H, C=O and C–OH in Fig. 3a. TiO2 spectrum displays three characteristic peaks at 3370, 1630 and 650 cm−1 associated with stretching vibrations of hydrogen-bonded water molecules and hydroxyl groups, bending vibrations of O–H group and Ti–O–Ti bridging stretching mode respectively. Comparison with the Fig. 3b, the characteristic absorption peaks of C=O has been reduced, indicating the formation of C=O–O–Ti group with TiO2, confirming the combination between CQDs and TiO2. The broad absorption band below 1000 cm−1 is attributed to the combination of the Ti–O–Ti and Ti–O–C vibrations [22].
The presence of CQDs was also detected by XPS in 67 wt% CQDs-TiO2 and not in TiO2 sample (Fig. 4a). This confirms that the CQDs are present in the 67 wt% CQDs-TiO2 composite. Figure 4a shows the XPS spectra of C1s, from which we can see that there were four clear peaks assigned to four types of carbon with different chemical valences: non-oxygenated ring C (284.6 eV), carbon in C–O bonds (285.1 eV), carbonyl C (288.0 eV), and carboxylate carbon (O–C=O) (288.5 eV) [24], which is consistent with the results from FI-IR. The Ti2p high resolution spectra for 67 wt% CQDs-TiO2 are shown in Fig. 4b. For the 67 wt% CQDs-TiO2, the binding energies of Ti2p3/2 and Ti2p1/2 are positioned at 458.8 and 464.7 eV, respectively. Compared to the TiO2, the Ti2p peaks of 67%CQDs-TiO2 are shifted towards a higher binding energy. The shift of 0.3–0.5 eV suggests that the chemical environment of Ti in the 67 wt% CQDs-TiO2 composites changed due to strong interaction between TiO2 and CQDs with formation of Ti–O–C bonds, which was in agreement with the FT-IR results [25].
3.3 Catalytic Performance
The catalytic activity of CQDs-TiO2 was first evaluated for the oxidation of benzyl alcohol with NaClO oxidant using water as an only solvent, as shown in Table 2. A control experiment in the absence of catalyst resulted in a conversion of about 3%. While in the presence of catalyst, product formation is observed. The reaction using TiO2 and CQDs as catalysts resulted in conversions of 5 and 59% after 7 h, respectively. Furthermore, the conversion of benzyl alcohol on CQDs-TiO2 gradually increased with the amount of CQDs used and reaches an optimum level. For instance, 33 wt% CQDs-TiO2 afforded a 25% conversion of benzyl alcohol after 7 h. In contrast, the 67 wt% CQDs-TiO2 catalyst drastically enhanced the conversion of benzyl alcohol to 88% after 7 h. Further increasing the loading of CQDs on TiO2 to 83 wt% CQDs-TiO2 did enhance the reaction further (90% conversion) after 7 h. However, the selectivity decreased and the byproduct was detected to be benzoic acid. Considering that carbon nanotubes (CNTs) and graphene were commonly used active catalyst for alcohol oxidation [26, 27], under identical reaction conditions, the catalytic activity of 67 wt% CQDs-TiO2 was compared with that of other carbon materials such as graphene and CNTs containing the same weight percentage of TiO2 and the observed results are summarized in Table 1. Conversions of 38 and 13% of benzyl alcohol were achieved with 67 wt% GO-TiO2 and 67 wt% CNTs-TiO2, respectively. In contrast, the physical mixture of CQDs (60 mg) and TiO2 (30 mg) showed 67% conversion under identical reaction conditions; this conversion is lower than that of 67 wt% CQDs-TiO2 but higher than that of 67 wt% GO-TiO2 and 67 wt% CNTs-TiO2 catalysts. These experiments clearly indicate the superior activity of 67 wt% CQDs-TiO2 due to the highly dispersive CQDs on TiO2 as well as optimal loading of CQDs to achieve maximum yield.
The 67 wt% CQDs-TiO2 catalyst was also found to be active for the oxidation of different alcohols, such as cinnamyl alcohol, n-amyl alcohol, and n-octanol and cyclohexanol. The catalyst performance data are given in Table 3. Cinnamic alcohol was oxidized into cinnamic aldehyde with a conversion of 67% in 7 h without any byproduct. In addition, n-amyl alcohol and n-octanol were oxidized into corresponding aldehydes with 100% conversion in 6 h. Cyclohexanol was significantly oxidized into cyclohexanone with a 80% conversion in 7 h.
Considering the active center of the reaction is the functional groups of the CQDs, the mechanism for oxidation of alcohols might also follow the SN1 mechanism [18, 28]. Firstly, a CQDs inclusion complex between CQDs and substrate was formed in situ, followed by the formation of carbonium ion. This carbonium ion was then attacked by ClO− anion on carbon atom. Further elimination of HCl gave the corresponding aldehydes. In addition, due to the presence of TiO2, which has a large number of surface defect adsorbed the CQDs on the surface and increased the amount of active site of CQDs. Hence, CQDs-TiO2 had a higher reactivity and conversion compared with the pure CQDs.
In order to evaluate the recyclability of the catalysts, the catalyst was separated from the reaction mixture after each experiment by filtration and washed with deionized water and acetone, then dried in air. It is apparent from Fig. 5 that the 67 wt% CQDs-TiO2 catalyst could be reused up to five cycles without obvious loss of catalytic activity, demonstrating the stability of the catalyst.
4 Conclusions
In summary, CQDs were successfully dispersed on the surface of the TiO2 to prepare the CQDs-TiO2 nanocomposites for oxidation of alcohols with NaClO oxidant using water as the only solvent. It is found that the catalytic activities of the CQDs-TiO2 nanocomposite were enhanced compared with that of the CQDs. The enhanced catalytic activities were mainly attributed to the highly dispersive CQDs with more active centers which caused by the TiO2 support. It can be recovered readily without any significant loss in their catalytic activities. Thus, the CQDs-TiO2 can be used as a recyclable catalyst for the oxidation of alcohols. In addition, the information obtained for this study may be useful for designing CQDs catalysts for application towards various other oxides.
References
Mallat T, Baiker A (2004) Oxidation of alcohols with molecular oxygen on solid catalysts. Chem Rev 104:3037–3058
Paraskevopoulou P, Psaroudakis N, Koinis S, Stavropoulos P, Mertis K (2005) Catalytic selective oxidation of benzyl alcohols to aldehydes with rhenium complexes. J Mol Catal A 240:27–32
Li G, Enache DI, Edwards J, Carley AF, Knight DW, Hutchings GJ (2006) Solvent-free oxidation of benzyl alcohol with oxygen using zeolite-supported Au and Au–Pd catalysts. Catal Lett 110:7–13
Chen YT, Lim HM, Tang QH, Gao YT, Sun T, Yan QY, Yang YH (2010) Solvent-free aerobic oxidation of benzyl alcohol over Pd monometallic and Au–Pd bimetallic catalysts supported on SBA-16 mesoporous molecular sieves. Appl Catal A 380:55–65
Zhan GW, Huang JL, Du MM, Sun DH, Abdul-Rauf I, Lin WS, Hong YL, Li QB (2012) Liquid phase oxidation of benzyl alcohol to benzaldehyde with novel uncalcined bioreduction Au catalysts: high activity and durability. Chem Eng J 187:232–238
Villa A, Wang D, Dimitratos N, Su DS, Trevisan V, Prati L (2010) Pd on carbon nanotubes for liquid phase alcohol oxidation. Catal Today 150:8–15
Choudhary VR, Dhar A, Jana P, Jha R, Uphade BS (2005) A green process for chlorine-free benzaldehyde from the solvent-free oxidation of benzyl alcohol with molecular oxygen over a supported nano-size gold catalyst. Green Chem 7:768–770
Yamamoto R, Sawayama Y, Shibahara H, Ichihashi Y, Nishiyama S, Tsuruya S (2005) Promoted partial oxidation activity of supported Ag catalysts in the gas-phase catalytic oxidation of benzyl alcohol. J Catal 234:308–317
Dijksman A, Marino-Gonzalez A, Payeras AMI, Arends I, Sheldon RA (2001) Efficient and selective aerobic oxidation of alcohols into aldehydes and ketones using ruthenium/TEMPO as the catalytic system. J Am Chem Soc 123:6826–6833
Fu XB, Yu H, Peng F, Wang HJ, Qian Y (2007) Facile preparation of RuO2/CNT catalyst by a homogenous oxidation precipitation method and its catalytic performance. Appl Catal A 321:190–197
Fu XB, Feng JY, Wang HA, Ng KM (2010) Fast synthesis and formation mechanism of gamma-MnO2 hollow nanospheres for aerobic oxidation of alcohols. Mater Res Bull 45:1218–1223
Xu H, Miao R, Fang Z, Zhong XH (2011) Quantum dot-based “turn-on” fluorescent probe for detection of zinc and cadmium ions in aqueous media. Anal Chim Acta 687:82–88
Ma Z, Ming H, Huang H, Liu Y, Kang ZH (2012) One-step ultrasonic synthesis of fluorescent N-doped carbon dots from glucose and their visible-light sensitive photocatalytic ability. New J Chem 36:861–864
Li HT, Sun CH, Ali MT, Zhou FL, Zhang XY, MacFarlane DR (2015) Sulfated carbon quantum dots as efficient visible-light switchable acid catalysts for room-temperature ring-opening reactions. Angew Chem 54:8420–8424
Mirtchev P, Henderson EJ, Soheilnia N, Yip CM, Ozin GA (2012) Solution phase synthesis of carbon quantum dots as sensitizers for nanocrystalline TiO2 solar cells. J Mater Chem 22:1265–1269
Shih ZY, Periasamy AP, Hsu PC, Chang HT (2013) Synthesis and catalysis of copper sulfide/carbon nanodots for oxygen reduction in direct methanol fuel cells. Appl Catal B 132:363–369
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem 49:6726–6744
Zhang X, Fu XB, Zhang YM, Zhu Y, Yang J (2016) Transition metal-free carbon quantum dots for selective liquid phase oxidation of alcohols using water as an only solvent. Catal Lett 146:945–950
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Liu ZY, Zhang XT, Nishimoto S, Murakami T, Fujishima A (2008) Efficient photocatalytic degradation of gaseous acetaldehyde by highly ordered TiO2 nanotube arrays. Environ Sci Technol 42:8547–8551
Zhang LW, Fu HB, Zhu YF (2008) Efficient TiO2 photocatalysts from surface hybridization of TiO2 particles with graphite-like carbon. Adv Funct Mater 18:2180–2189
Zhang H, Lv XJ, Li YM, Wang Y, Li JH (2010) P25-graphene composite as a high performance photocatalyst. ACS Nano 4:380–386
Josephine DSR, Sakthivel B, Sethuraman K, Dhakshinamoorthy A (2015) Titanium dioxide/graphene oxide nanocomposites as heterogeneous catalysts for the esterification of benzoic acid with dimethyl carbonate. ChemPlusChem 80:1472–1477
Li Y, Zhao Y, Cheng HH, Hu Y, Shi GQ, Dai LM, Qu LT (2012) Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J Am Chem Soc 134:15–18
Martins NCT, Angelo J, Girao AV, Trindade T, Andrade L, Mendes A (2016) N-doped carbon quantum dots/TiO2 composite with improved photocatalytic activity. Appl Catal B 193:67–74
Luo J, Yu H, Wang HJ, Wang HH, Peng F (2014) Aerobic oxidation of benzyl alcohol to benzaldehyde catalyzed by carbon nanotubes without any promoter. Chem Eng J 240:434–442
Luo J, Peng F, Yu H, Wang HJ (2012) Selective liquid phase oxidation of benzyl alcohol catalyzed by carbon nanotubes. Chem Eng J 204:98–106
Ji HB, Shi DP, Shao M, Li Z, Wang LF (2005) Transition metal-free and substrate-selective oxidation of alcohols using water as an only solvent in the presence of beta-cyclodextrin. Tetrahedron Lett 46:2517–2520
Acknowledgements
The authors are grateful to the supports from Natural Science Foundation of Guangdong (No. 2014A030313628) and National Natural Science Foundation of China (No. 21306061).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, P., Fu, X. & Zhang, Y. Carbon Quantum Dots-TiO2 Nanocomposites with Enhanced Catalytic Activities for Selective Liquid Phase Oxidation of Alcohols. Catal Lett 147, 1679–1685 (2017). https://doi.org/10.1007/s10562-017-2065-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2065-x