Abstract
Uniform rutile SnO2 nanoparticles with small size (ca. 3 nm) were highly dispersed on both sides of GO sheets through electrostatic interactions, giving pseudo-homogeneous catalysts of SnO2/GO nanocomposites. The SnO2-decorated GO nanocomposites, especially, SnO2 (15 wt%)/GO was highly efficient and reusable in Baeyer–Villiger oxidation of ketones with H2O2.
Graphical Abstract
SnO2/GO nanocomposites, where uniform SnO2 nanoparticles were tightly gripped on both sides of GO sheets by surface oxygenated functional groups through electrostatic interaction, have proved to be a versatile, efficient and reusable catalyst for the BV oxidation of ketones with H2O2.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
B–V oxidation of ketones with H2O2 is of great importance in organic chemistry, not only due to the environment friendly oxygen source, but also because obtained lactones or esters are valuable synthetic intermediates in agrochemical, chemical and pharmaceutical industries [1–9]. In 2001, Corma et al. reported the tetravalent tin (SnIV)-containing beta zeolite as a highly active and chemoselective catalyst for B–V oxidation of ketones with H2O2 [10]. SnIV centers, tetrahedrally coordinated in beta zeolite framework, acted as Lewis acids to activate the carbonyl group of ketones through coordination, facilitating the nucleophilic attack on this group by H2O2. Despite high yields of lactones, in some cases, microporosity of zeolite hinders the diffusion of bulky molecules, resulting in lower activity and faster deactivation of catalyst [11]. To eliminate the diffusion limitation, mesoporous silica such as MCM-41, have been employed to incorporate the SnIV species [2, 12, 13]. The mesoporous matrix indeed allowed for the free diffusion of regents, but the amorphous structure of silica wall often incorporates SnIV species disorderly. As a result, not all SnIV centers were equally active and/or accessible to the reactants for efficient B–V oxidation [14–16]. Recently, layered double hydroxides (LDHs), a class of two-dimensional anionic clays, have emerged as the alternative support for SnIV species through ion-exchange [17, 18]. The key structural characteristic that MII and MIII cations were distributed in a uniform manner in the hydroxide layers resulted in the formation of SnIV/LDH with specific morphology/surface structure and high dispersion [19]. Furthermore, ‘flexible’ interlayer spaces of the layered SnIV/LDH catalyst not only fitted substrates either bulky or less, but also allowed the free access of SnIV center during reaction. It thus represented a fascinating strategy for developing highly efficient and stable Tin-based catalysts for B–V oxidation with H2O2.
Graphene, a novel layered carbon material with a tight packing of honeycomb lattice, has become one of the rising stars in material science [20]. Its intriguing properties, such as unique layered structure, high surface area, high flexibility and mechanical strength, made it very attractive in heterogeneous catalysis [21–24]. Although many SnO2/graphene nanocomposites have been recently proposed as an anode material for Li-ion batteries [25–28], the employment of SnO2/graphene nanocomposites in catalysis was scarcely reported. For higher catalytic performance, it was desirable to have smaller particle size and higher dispersion of SnO2 NPs in the composite. However, it was difficult to control the dispersion state of loaded NPs on graphene surfaces, mainly due to the lack of strong interactions between them [29]. Furthermore, layer graphene sheets tended to stack with each other in solution, thus losing their high surface area and intrinsic chemical and physical properties. An ideal solution to above problems was supporting SnO2 NPs on a graphene oxide (GO) sheet instead of a graphene sheet. Different from graphene, GO sheet consisted of intact graphitic regions interspersed with sp3-hybridized carbons containing carboxyl, hydroxyl, and epoxide functional groups on the edge, top, and bottom surface of each sheet. The abundant surface oxygenated functional groups ensured GO to grip SnO2 NPs tight on the surface through electrostatic interactions, which prevented not only the aggregation of SnO2 NPs, but also the aggregation of graphene sheets. In addition, all the SnO2 NPs located on the GO surface were exposed to the reagents in catalysis, which may further enhance the catalytic efficiency of SnO2/GO nanocomposites.
Herein, SnO2 NPs were grown on the GO surfaces via a hydrothermal method using SnCl4·5H2O as a metal precursor. Then SnO2 NPs were formed, in situ, and anchored by the oxygenate groups on the GO layer, forming uniform loading of SnO2 NPs on GO sheets. It has proved that SnO2 NPs were kept small in size and dispersed uniformly on the GO sheets. The resultant SnO2/GO nanocomposites were demonstrated the efficient and selective solid Lewis acid catalysts for B–V oxidation with H2O2. Quantitative yield of lactones was obtained within 2 h even in the case of bulky 2-adamantanone. Furthermore, the catalyst could be easily separated for reuse by centrifugation.
2 Experimental
2.1 Materials and Methods
Cyclopentanone and high-purity graphite (99.9999 %, 200 mesh) were purchased from Alfa Aesar. Cyclohexanone and adamantanone were obtained by J&K. Other commercially available chemicals were laboratory grade reagents from local suppliers. All of the solvents were purified by standard procedures.
Surface structure of the samples was measured using a TEM (JEOL JEM-3010). X-ray diffraction (XRD) patterns were recorded on a Philips X'PERT-Pro-MPD diffractometer using Cu Kα radiation (λ = 1.542 Å). A continuous scan mode was used to collect the 2θ from 5o to 80o. X-ray Photoelectron Spectroscopy (XPS) data were obtained with an ESCALab220i-XL electron spectrometer from VG Scientific using 300 W Al Kα radiation. The base pressure was about 3 × 10−7 Pa. SnO2 NPs contents were analyzed by TGA using a NETZSCH STA 449C thermal analyzer. Samples (ca. 0.01 g) were heated from room temperature up to 800 oC with 10 K/min under air flow using alumina sample holders. Thin layer chromatography (TLC) was conducted on glass plates coated with silica gel GF254. The conversion and ee values were measured by a 6890 N gas chromatograph (Agilent Co.) equipped with a capillary column (HP19091G-B213, 30 m × 0.32 mm × 0.25 μm).
2.2 Preparation of SnO2(x)/GO (Where x is the Mass Ratio of SnO2 NPs)
GO was prepared by the oxidation of high-purity graphite powder (99.9999 %, 200 mesh) with H2SO4/KMnO4 according to the Hummers method [30], and then was subjected to dialysis for 7 days to completely remove metal ions and acids. The resulting product was dried at room temperature under vacuum overnight, giving GO as yellowish-brown powder. FT-IR (KBr): 3187, 3132, 1735, 1621, 1224, 1050 and 581 cm−1.
The dried GO (1.0 g) was sonicated in deionized water (100 mL) for 0.5 h to ensure most GO being fully exfoliated. Various amount of SnCl4·5H2O (0.125, 0.25, 0.375, 0.50 and 0.75 g) in deionized water (50 mL) was then added into the homogeneous dispersion. After further ultrasonic treatment for 0.5 h, the mixtures were refluxed at 100 °C for 24 h, resulting in a black suspension. The precipitate was collected by centrifugation and washed with deionized water. The products were dried at room temperature overnight under vacuum, giving SnO2/GO nanocomposites with various SnO2 loading (c a. 7.5, 15, 20, 30 or 40 wt%).
2.3 B–V Oxidation of Ketones with H2O2
SnO2/GO nanocomposites (0.05 g) and ketones (1.0 mmol) were mixed in 1,2-dichloroethane (15 mL) under vigorously stirring. H2O2 (30 wt%, 2.0 mmol) was then added dropwise at 90 °C. The reaction progress was monitored by GC. After the reaction, catalyst was separated by centrifugation. The liquid reaction mixture was extracted by ethyl ether and then quantitatively analyzed by a 6890 N gas chromatograph (Agilent Co.) equipped with the capillary column (HP19091G-B213, 30 m × 0.32 mm × 0.25 μm). The recovered catalyst was washed with ethanol and distilled water repeatedly, and dried at 40 oC overnight for reused.
3 Results and Discussions
3.1 Preparation of SnO2/GO Nanocomposites
GO, an oxygenated derivative of graphene, contains abundant oxygen-containing groups such as carboxyl, carbonyl, hydroxyl and ether on the surface. The oxygen-containing groups were capable of gripping metal oxide NPs through electrostatic interactions, which thus facilitated high dispersion of metal oxide NPs on both sides of GO sheet, and also prevented them from aggregation [31–33]. With this point in mind, we decided to locate SnO2 NPs on the surface of GO sheets through electrostatic interactions between the oxygenated functional groups and SnO2 NPs. A convenient hydrothermal method was employed for the hybridization, as shown in Scheme 1. GO sheets were readily dispersed in water due to surface oxygenate species, forming a uniform GO nanosheets suspension. When GO solution was mixed with SnCl4·5H2O solution, the SnIV cation was selectively bonded with the negative oxygenated groups by electrostatic force. After incubated at 100 °C for 24 h, anchored SnIV cation was in situ converted to SnO2 NPs, and gripped by the oxygenated functional groups through electrostatic interaction. The driving force for the gripping of SnO2 NPs in the nanocomposite structures may be subdivided into the following categories: (I) dative bonds between oxygen atom in oxygenated functional groups and Sn atoms of SnO2, (II) hydrogen-bond between oxygen atom of SnO-2 NPs and hydrogen atom in hydrogen-containing oxygenated functional groups. As a result, uniform SnO2 NPs with small size should be highly dispersed on both sides of GO sheets, as proposed in Scheme 1. This unique hybrid architecture gave rise to an efficient and stable Lewis acid catalyst for B–V oxidation.
3.2 Characterization of Samples
3.2.1 XPS
XPS was employed as an optimal characterization technique to provide the direct evidence for the conjugation of SnO2 NPs and GO nanosheets (Fig. 1). Figure 1a shows the XPS survey spectra of GO and typical SnO2(15 wt%)/GO nanocomposite, which were normalized with respect to the respective C 1s peaks. Obviously, besides C (C 1s, 286.1 ev) and O (O 1s, 532.0 ev) peaks [34, 35], the nanocomposite exhibited typical Sn (Sn 3p, 3d, 4s, 4p, 4d) signals in the survey spectrum [36], suggesting the existence of Tin species on GO sheets. The Sn 3d XPS spectrum of composite was presented in Fig. 1b, where the peaks of Sn 3d5/2 (487.20 eV) and Sn 3d3/2 (495.53 eV) were distinct. They had a spin energy separation of 8.33 eV and an area ratio of 1:1.5, which were in good accordance with the reported XPS data of SnO2 [37, 38]. It suggested that SnO2 NPs were in situ grown on GO surfaces during hydrothermal synthesis. While, we noticed that the obtained nanocomposite showed slight increase in binding energy of Sn 3d, as compared with commercial SnO2 NPs (Fig. 1b). Higher binding energy should be related with the coordination between oxygenated functional groups of GO and Sn atoms of SnO2. The deduction could also be drawn from the evidence that the O1s binding energy of oxygenated functional groups in SnO2/GO composite, shifted from 532.74 to 532.62 eV, compared with that in GO (Fig. 1c). Furthermore, an additional O1 s signal (531.28 eV) associated with O-SnIV species of SnO2 [39] was also observed in the O1s XPS spectrum of SnO2(15 wt%)/GO composite. This was unambiguous evidence for a SnO2/GO composite structure. Notably, the O 1s binding energy (531.28 eV, Fig. 1c) of O-SnIV species in nanocomposite slightly decreased as compared with that in commercial SnO2 NPs (532.20 eV, Fig. 1c inset). It was probably due to a hydrogen-bonding between oxygen atom of the SnO-2 NPs and the hydrogen atom of hydrogen-containing oxygenated functional groups on GO sheets. We could thus deduce that SnO2 NPs were tightly bonded to the GO layer by oxygenate groups through dative bond and/or hydrogen bond. Actually, similar interactions have been reported in other metal oxide/GO hybrids, such as CuO/GO [40], MnO2/GO [41], and Fe3O4/GO [31] etc. These facts were consistent with the synthesis design, and confirmed the successful dispersion of SnO2 NPs on the basal planes of GO nanosheets, as shown in Scheme 1.
Figure 1d showed the C1s deconvolution spectra of SnO2(15 wt%)/GO composite and pristine GO. As compared with GO, the C1s XPS spectrum of SnO2/GO nanocomposite exhibited an additional C1s signal at 291.46 eV, which was probably associated with the Sn–O–C carbonaceous bonds due to the interaction between Sn atom and oxygen-containing functional groups of GO as mentioned above. Moreover, decreased intensity of carbon binding to oxygenated functional groups was observed in the C1s XPS spectrum of SnO2/GO. Such results indicate that partial oxygen-containing functional groups were eliminated during the hydrothermal process. It thus tuned a hydrophobic surrounding for SnO2 NPs, suppressing the undesired competitive coordination of water to the Lewis acid center. The remaining oxygenated groups helped to grip metal oxide NPs tight on the GO surface through electrostatic interactions, which were crucial to the high dispersion of SnO2 NPs on GO sheets.
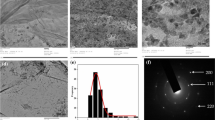
3.2.2 XRD
The crystal structure of SnO2 NPs placed on GO sheets was determined by XRD. Figure 2 shows powder XRD patterns of SnO2/GO nanocomposites and pristine GO material. OB-Viously, pristine GO displayed a strong (002) peak (*) centered at 10.5o, suggesting the layered structure with an average interlayer distances of ca. 0.85 nm (Fig. 2a). While, the sharp diffraction peak disappeared, accompanied by the appearance of several new peaks (#) at 2θ of 26.56, 34.22, 51.50, and 65.42o in XRD patterns of SnO 2 (x)/GO nanocomposites (Fig. 2b–e), which were attributed to (100), (101), (211), and (112) planes of tetragonal rutile SnO2 (JCPDS Card No. 41-1049), respectively [28, 42]. Therefore, rutile SnO2 crystals were deposited on GO sheets during the hydrothermal process, and layer-stacking regularity of GO sheets was disturbed by the decorated rutile SnO2 NPs. The exfoliated GO sheets should be beneficial for free access of SnO2 NPs to reagents during the oxidation. An average crystal size of SnO2 in typical SnO2(15 wt%)/GO was estimated using Scherrer equation of d = Kλ/βcosθ and was found to be 2.6 nm at 2θ = 26.56°, where d is the average crystal size, K = 0.89 is the Scherrer constant, λ = 0.154 nm is the wavelength of X-ray, β is the width (in radian)of the XRD peak at half maximum intensity and θ = 26.56°/2 is the Bragg diffraction angle found from XRD data.
3.2.3 Morphological Analyses
The high dispersion of uniform SnO2 NPs on exfoliated GO sheets was further proved by morphological analyses. Figure 3 shows the TEM images of SnO2/GO nanocomposites with various SnO2 loading. Obviously, TEM image of SnO2(15 wt%)/GO showed thin layered GO sheets, on which uniform SnO2 NPs were distributed homogeneously with the size of ca. 3 nm (Fig. 3b). The size obtained from TEM was approach to that determined by XRD (2.6 nm). It was direct visual evidence that SnO2 NPs were evenly anchored by the oxygenated groups on GO surface rather than disordered physical adsorption on the basal planes. The strong interaction between them prevents the NPs from aggregating and increases the stability of SnO2 NPs. As the oxygenate groups are uniformly distributed on two sides of the GO sheets, SnO2 NPs should be gripped on both sides of GO sheets, resulting in a sandwich-like structure where layer of GO sheet alternated by SnO2 NPs sheet. Aggregation problem of GO could be also prevented or at least minimized, which should be beneficial for mass transfer during the heterogeneous catalysis. Increasing the mass ratio of SnO2 from 15 to 40 wt% led to the aggregation or even accumulation of NPs on the basal planes (Fig. 3b–e). It was probably due to that SnO2 Nps were too much to be gripped by the surface oxygenated groups. Sparse SnO2 Nps was observed on the GO sheets when mass ratio of SnO2 decreased to 7.5 wt% (Fig. 3a). Low local concentration of active sites was detrimental to efficient catalysis. Therefore, SnO2(15 wt%)/GO composite has the most reasonable structure that sufficient uniform SnO2 NPs were homogeneously distributed onto GO sheets, which led to highly efficient solid Lewis acid catalyst for B–V oxidation with H2O2.
3.2.4 TGA
Thermal analysis was performed to assess the thermal decomposition behaviour of SnO2/GO nanocomposites, as well as to quantify the mass percentage of SnO2 in the nanocomposites. The results obtained are depicted in Fig. 4. Pristine GO sheets exhibited three distinct steps of weight loss in the combined TG–DTG curves (Fig. 4a). The first weight loss before 100 oC corresponded to removal of the surface-adsorbed and/or interlayer water molecules. A second large weight loss centered at 205 oC, which was assigned to the pyrolysis of the labile oxygen-containing functional groups [43]. The third step assigned to the successive cleavage of carbon sketch appeared at 550 oC. The weight loss extended up to ca. 730 oC until the GO was completely decomposed under air flow. A similar thermal behaviour was observed for the SnO2/GO samples (Fig. 4b–e). Three mass loss steps associated with the removal of water molecules, oxygen-containing functional groups and carbon sketch are distinguished in the combined TG–DTG curves of SnO2/GO samples. The non-removable residue belonged to incorporated SnO2 NPs which was quite stable with negative mass loss during the temperature range (Fig. 4f). Thus, the mass percentages of SnO2 in corresponding composites could be accordingly estimated to be 15 (Fig. 4b), 20 (Fig. 4c), 30 (Fig. 4d) and 40 (Fig. 4e) wt%, respectively. Furthermore, we noticed that the decomposition temperature of oxygenated groups gradually increases with the increase of SnO2 content in composites (Fig. 4b–e). The observation suggested the interaction between SnO2 NPs and oxygenated groups, which enhanced thermal stability of the oxygen-containing functional groups.
3.3 Catalytic Performances
The featured structure that small sized SnO2 NPs are uniformly dispersed on the surface of GO sheets turns the SnO2/GO nanocomposite into the efficient solid Lewis acid catalysts for the B–V oxidation of ketones with H2O2. The catalytic efficiency of the resultant SnO2/GO nanocomposite catalyst was investigated in B–V of bulky 2-adamantanone with H2O2 (30 wt%) in 1,2-dichloroethane at 90 oC. The results are presented in Table 1. GO material and commercial SnO2 NPs were also examined for comparison purposes.

Obviously, commercial SnO2 NPs were inactive in B–V oxidation with H2O2, giving practically no lactone of 2-oxatricyclo [4. 3. 1. 14,8]-undecan-9-one even if double H2O2 was used (Table 1, entry 1). While, the catalytic efficiency benefited from dispersing SnO2 NPs on GO sheets, although GO itself was also inactive (Table 1, entry 2). 48-97 % Conversion of 2-adamantanone was obtained over the obtained SnO2/GO nanocomposites (Table 1, entries 3-7). The results demonstrated the positive effect of GO sheets on catalytic performance of SnO2(x)/GO nanocomposites. Abundant oxygenate groups on GO sheets ensured high dispersion of uniform SnO2 NPs on both sides of the GO layer with small size. Delamination of the nanocomposites allowed catalytic reactions to be carried out under pseudo-homogeneous reaction conditions, thereby significantly increasing the reaction rate. More importantly, the synergistic effect between SnO2 NPs and GO support effectively promoted further enhancement of catalytic activity. The deduction can be drawn from the evidence that SnO2 directly grafted onto mesoporous MCM-41 yielded negligible 2-adamantanone conversion under analogous reaction conditions (Table 1, entry 8), as reported by Román-Leshkov et al. [5]. Notably, SnO2/GO nanocomposite containing 15 wt% SnO2 NPs showed the most remarkable activity in the B–V oxidation. Almost quantitative conversion of 2-adamantanone with excellent selectivity to lactone (>99 %) was achieved over 0.025 g of SnO2 (15 wt%)/GO nanocomposite (2.0 mol%, regarding catalytic Tin sites) within 2 h (Table 1, entry 4). While, the conversion gradually decreased as SnO2 loading increased (Table 1, entries 4–7). Only 48 % conversion of 2-adamantanone was observed when SnO2 loading was increased to 40 wt% (Table 1, entry 7). Higher loading of SnO2 resulted in the lower dispersion state of SnO2 NPs, as shown in TEM images, which was detrimental to the catalysis. These results thus prompted us to further decrease SnO2 loading from 15 to 7.5 wt%. Unexpectedly, although uniform NPs, SnO 2 (7.5 wt%)/GO nanocomposite was unsatisfied for the B–V oxidation of 2-adamantanone, likely due to the insufficient active sites (Table 1, entry 3). Interestingly, the selectivity to lactone was almost 100 % irrespective of the Tin concentration with no formation of any side-products.
Apart from enhanced catalytic efficiency, the reusability of SnO2/GO composites in B–V oxidation was also enjoyable. Typical SnO2(15 wt%)/GO nanocomposite was employed to investigate the reusability in B–V oxidation of 2-adamantanone with H2O2, and the results were listed in Fig. 5. Simply by centrifugation, the SnO2(15 wt%)/GO catalyst could be facilely recovered for reuse. To our delight, it could be reused for at least ten times with no appreciable decrease in catalytic activity and selectivity. Therefore, the SnO2(15 wt%)/GO catalyst was quite stable in the oxidation. The stability was further identified by TEM image of recovered SnO2(15 wt%)/GO nanocomposite (Fig. 3b′ vs b). No significant change in layered structure of catalyst, as well as dispersed state and particle size of SnO2 NPs was observed even after reused for ten times. The results indicated that tightly pinning SnO2 NPs on GO sheets could effectively enhance the dimensional stability of NPs during cycling. Leaching of SnO2 NPs was also avoided by the electrostatic interaction between SnO2 NPs and oxygenated groups on GO sheets, as indicated by the identical SnO2 content in recovered catalyst (15 wt% loading). More importantly, the organic deposits, a main reason for poisoning the Tin-containing catalyst [11, 12], can also be avoided because of the free traffic of reactants and products in the layered SnO2/GO nanocomposite. Therefore, the novel SnO2(15 wt%)/GO nanocomposite was efficient and reusable in the typical B–V oxidation of 2-adamantanone with H2O2.
It should be more attractive if the nanocomposite can be applied to various ketones. Table 2 summarized the B–V oxidation of various ketones in the presence of SnO2(15 wt%)/GO. Besides 2-adamantanone, cyclobutanone was also successfully oxidized to lactone with high efficiency and excellent selectivity under identical condition (Table 2, entry 2). The oxidation of other cyclic ketones (5- or 6- membered rings) also occurred smoothly to afford the corresponding lactones with excellent selectivity, although longer reaction time was required (Table 2, entries 4, 6, and 8). Interestingly, the substituent in six-membered cyclic ketone influenced the conversion of ketone in B–V oxidation (Table 2, entries 7 and 8). Cyclohexanone with methyl substituent at para-position was less reactive than cyclohexanone, due to the steric effect, as well as electron-donating inductive effect of methyl group (Table 2, entry 8 vs 6). Notably, although the reaction rate depends on the chemical structure of ketones, it can be concluded that the SnO2(15 wt%)/GO nanocomposite could oxidize the various ketones to corresponding lactones with excellent selectivity (>99 %) (Table 2, entries 1, 2, 4, 6, and 8). The high selectivity obtained is attributed to the carbonyl group activation mechanism proposed by Corma et al., which involves initial selective activation of the carbonyl group, followed by reaction with non-activated H2O2 [10].
Superiority of the SnO2(15 wt%)/GO nanocomposite over other reported catalysts, such as Sn-Montmorillonite [44], Sn-palygorskite [18], Sn/PS [45], and Silica-AlCl3, is seen in Table 3. Obviously, SnO2(15 wt%)/GO was far more efficient than these reported catalysts either layered or porous in B–V oxidation of cyclopentanone with H2O2, due to the pseudo-homogeneous reaction conditions, as well as synergistic effect between SnO2 NPs and GO support (Table 3, entry 1 vs entries 2-5). Especially, silica-AlCl3 gave only 37 % conversion of cyclopentanone with low selectivity (70 %) to lactone (Table 3, entry 5). Furthermore, SnO2(15 wt%)/GO nanocomposite also exhibited much higher activity than tin containing mesoporous silicas even in the case of bulky 2-adamantanon (Table 3, entry 6 vs entries 7, 8) [10]. The results demonstrated the advantages of SnO2/GO nanocomposite used for baeyer-villager oxidation reaction. Aapart from higher efficiency, the reusability of SnO2(15 wt%)/GO was also superior to that of the compared catalysts. Blocking of the active sites by either products or intermediates, a main reason for deactivation of the Tin-containing catalyst, was avoided by the flexible layered structure of SnO2/GO nanocomposite. Furthermore, leaching of SnO2 NPs was also minimized due to the electrostatic interaction between SnO2 NPs and oxygenated groups on GO sheets.
4 Conclusions
With the aid of oxygenated functional groups, small rutile SnO2 NPs (ca. 3 nm) have been controllably anchored on GO sheets evenly and tightly for the formation of SnO2/GO nanocomposites. Characterization results confirmed the loading of SnO2 NPs on both sides of the exfoliated GO sheets through electrostatic interactions. Benefiting from the flexible GO support, high dispersion of SnO2 NPs, as well as the intimate interaction between SnO2 NPs and GO, the resultant SnO2/GO composites, especially SnO2 (15 wt%)/GO, exhibited excellent performance as the solid Lewis acid catalysts in B–V oxidation of ketones with H2O2. Furthermore, catalyst poisoning by organic deposits could also be effectively avoid by free traffic of reactants and products in the layered SnO2/GO nanocomposite. The heterogeneous catalyst was stable in the reaction system and could be easily recovered for efficient reuse. The design and controlled synthesis of SnO2/GO nanocomposites provided a facile, economic, and versatile approach to promote the catalytic performance of other metal oxides and can be scaled up easily for industrial production.
References
Conte V, Floris B, Galloni P, Mirruzzo V, Scarso A, Sordi D, Strukul G (2005) Green Chem 7:262
Jiménez-Sanchidrián C, Ruiz JR (2008) Tetrahedron 64:2011
Sasakura N, Nakano K, Ichikawa Y, Kotsuki H (2012) RSC Adv 2:6135
Hammond C, Conrad S, Hermans I (2012) Angew Chem Int Ed 51:11736
Luo Y, Bui L, Gunther WR, Min E, Román-Leshkov Y (2012) ACS Catal 2:2695
Hara T, Hatakeyama M, Kim A, Ichikuni N, Shimazu S (2012) Green Chem 14:771
Ma Q, Xing W, Xu J, Peng X (2014) Catal Commun 53:5
Uyanik M, Ishihara K (2013) ACS Catal 3:513
Chen S, Zhou X, Li Y, Luo R, Ji H (2014) Chem Eng J 41:138
Corma A, Nemeth LT, Renz M, Valencia S (2001) Nature 412:423
Wang Y, Yokoi T, Otomo R, Kondo JN, Tatsumi T (2015) Appl Catal A 490:93
Corma A, Navarro MT, Nemeth L, Renz M (2001) Chem Commun 21:2190
Corma A, Navarro MT, Renz M (2003) J Catal 219:242
Li L, Stroobants C, Lin KF, Jacobs PA, Sels BF, Pescarmona PP (2011) Green Chem 13:1175
Osmundsen CM, Holm MS, Dahl S, Taarning E (2012) Proc R Soc A 468:2000
Cho HJ, Dornath P, Fan W (2014) ACS Catal 4:2029
Pillai UR, Sahle-Demessie E (2003) J Mol Catal A 191:93
Lei Z, Zhang Q, Luo J, He X (2005) Tetrahedron Lett 46:3505
He S, An Z, Wei M, Evans DG, Duan X (2013) Chem Commun 49:5912
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Nature 422:282
Kakaei K, Dorraji M (2014) Electrochim Acta 143:207
Kakaei K (2015) Electrochim Acta 165:330
Lan D, Yang F, Luo S, Au C, Yin S (2014) Carbon 73:351
Zheng W, Tan R, Yin S, Zhang Y, Zhao G, Chen Y, Yin D (2015) Catal Sci Technol 5:2092
Tan C, Zhao S, Yang G, Hu S, Qin X (2015) Ionics 21:987
Thomas R, Rao KY, Rao GM (2014) Mater Express 4:65
Patil S, Patil V, Sathaye S, Patil K (2014) RSC Adv 4:4094
Zhou X, Wan L, Guo Y (2013) Adv Mater 25:2152
Deosarkar MP, Pawar SM, Sonawane SH, Bhanvase BA (2013) Chem Eng Process 70:48
Hummers WS, Offeman RE (1958) J Am Chem Soc 80:1339
Yang X, Zhang X, Ma Y, Huang Y, Wang Y, Chen Y (2009) J Mater Chem 19:2710
Wang H, Cui L, Yang Y, Casalongue HS, Robinson JT, Liang Y, Cui Y, Dai H (2010) J Am Chem Soc 132:13978
Bai H, Li C, Shi G (2011) Adv Mater 23:1089
Li Z, Wu S, Ding H, Zheng D, Hu J, Wang X, Huo Q, Guan J, Kan Q (2013) New J Chem 37:1561
Luo S, Xu X, Zhou G, Liu C, Tang Y, Liu Y (2014) J Hazard Mater 274:145
Li Y, Lv X, Lu J, Li J (2010) J Phys Chem C 114:21770
Seema H, Christian KK, Chandra V, Kim KS (2012) Nanotechnol 23:355705
Tang L, Nguyen VH, Lee YR, Kim J, Shim JJ (2015) Synth Met 201:54
Zhu Y, Wang Y, Xie J, Cao G, Zhu T, Zhao X, Yang H (2015) Electrochim Acta 154:338
Zhu J, Zeng G, Nie F, Xu X, Chen S, Han Q, Wang X (2010) Nanoscale 2:988
Qu J, Shi L, He C, Gao F, Li B, Zhou Q, Hu H, Shao G, Wang X, Qiu J (2014) Carbon 66:485
Liu H, Liu T, Dong X, Lv Y, Zhu Z (2014) Mater Lett 126:36
Tang XZ, Li W, Yu ZZ, Rafiee MA, Rafiee J, Yavari F, Koratkar N (2011) Carbon 49:1258
Lei Z, Ma G, Jia C (2007) Catal Commun 8:305
Zhang Q, Wen S, Lei Z (2006) React Funct Polym 66:1278
Lei Z, Wei L, Wang R, Ma G (2008) Catal Commun 9:2467
Acknowledgments
The project was financially supported by the National Natural Science Foundation of China (Grant No. 21476069), the Scientific Research Fund of Hunan Provincial Education Department (13B072), the Program for Excellent Talents in Hunan Normal University (ET14103), the Hunan Provincial Innovation Foundation for Postgraduate (CX2013B209) and the Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zheng, W., Tan, R., Luo, X. et al. SnO2 Nanoparticle-Decorated Graphene Oxide Sheets Efficiently Catalyze Baeyer–Villiger Oxidation with H2O2 . Catal Lett 146, 281–290 (2016). https://doi.org/10.1007/s10562-015-1639-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1639-8