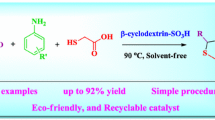
Abstract
An efficient and facile protocol has been documented for the synthesis of 4H-chromene and xanthen-1-one derivatives via a three component one pot reaction of salicyladehyde, malononitrile/dimedone and indole utilizing biomimetic catalyst. Affiliation between biomimetic and organocatalyst exhibits a versatile and high catalytic activity emerging to be a sustainable alternative to other catalysts. The significant features of this protocol are compatibility with various types of indole and active-methylene compound, accomplishment of high yields, cleaner reaction condition and avoidance of the use of costly catalysts. Recyclability of β-cyclodextrin a “biomimetic catalyst” is a significant feature of this protocol.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cyclodextrin (CD) is a cyclic oligosaccharides of d(+)-glucopyranosyl units connected by α-1,4-glycosidic bond and represented as narrow, shortened cone with Cn symmetry [1–9]. It obtained from the enzymatic degradation of starch. It has strongly hydrophobic central cavity and hydrophilic outer surface cavities which have attracted much attention of research community. They serve as solution-based hosts for inclusion complex formation with a broad range of guest molecules [10, 11]. They have cylindrical shape having primary hydroxyl groups at the more restricted rim of the cylinder [12, 13]. The broad opening of CD is encompassed by the secondary –OH groups on carbon-2 and carbon-3, while the narrower opening incorporates all the primary 6-OH group [1, 2]. Affiliation of organic molecules which are not soluble in water into their cavity results in the formation of inclusion complex. The formation of inclusion complex enhance the substrate local amount and immobilizes the alongside active catalytic centre, which further increase the reaction rate, outstanding substrate selectivity, enantioselectivity and regioselectivity [14]. CD binds to reactants discriminately and promotes a broad range of photochemical and chemical reactions by supramolecular catalysis; involving the formation of host–guest complexes as present in enzymes [15–18]. These attractive features of CDs encouraged us to further examined and explore the reactions under biomimetic conditions. Because of these properties CDs and their derivatives also have been widely applied as a phase transfer catalysts, biomimetic catalysts and molecular chelating agents. Thus formation of complex CD is familiar as an efficient protocol for amplifying finishing quality of less soluble organic compounds [14]. The probe of prosperous structures in drug discovery frequently becomes prominent theme in pharmaceutical chemistry [19]. Chromene and its derivatives belong to such an exceptionally elite class of heterocyclic scaffold [20]. They show their presence in many natural products [21] owing to their potent activity like antibiotic rhodomyrtone [22], HA14-1, a glycosidase inhibitor myrtucommulone-E, anti-viral [23] and apoptosis inducer [24]. Their importance is also due to their wide implementation in the treatment of tumor and microbial disease, and they are also present in anti-convolusant1 [25] and anti-anaphylactic drugs [26]. When chromene attached with other nulcleophiles which are bioactive in nature they also found their wide use as antioxidants, anti-tubercular and anti-HIV activity that accelerates and signifies their synthetic utility (Fig. 1). In addition 2-aminochromenes are also extensively utilized as pigments, agrochemicals and cosmetics [23, 24]. Lately the application of organocatalyst (DABCO) in synthesis process gained much attention because of their less toxic nature, stable nature in water and reusability [27]. According to a survey numerous methods have been present in the literature for the synthesis of chromene, xanthen-1-one derivatives and different catalysts have been examined such as l-proline [28, 29], indium trichloride (InCl3) [30], DBU, [31] ZnO nanoparticle, [24] FeCl3 [32], TBAB [33], [TBA][Gly] [34], RGO/ZnO [35], EDDF [36], l-cysteine functionalized magnetic nanoparticles [37] and polystyrene-supported p-toluenesulfonic acid [38].
2 Result and Discussion
Keeping all facts in our mind and in continuation of our previous work [39] we designed a unique synthetic protocol for the privileged heterocyclic scaffolds of medicinal value combined with the environmental benefit of using water as a reaction medium (scheme 1). We carried out the reaction of salicyladehyde (1a), malononitrile (2) and indole (3a) as a model reaction (scheme 2).
Indoles form an elite class of nucleophiles. They show their presence in many bioactive compounds and natural products. They use in medicinal chemistry as antitumour and antimicrobial agents. Many of them are applied as estrogens metabolism in human being. Hence we used indole as a nucleophile in our model reaction [40, 41]. Water is investigated a green reaction medium for number of biological and chemical reactions [42]. Breslow [43, 44] attributed that hydrophobic effect of water appreciably increase the rate of reactions. Because of the fact that cyclization reactions occur more efficiently in polar solvents and aqueous medium is the best solvent for solution-phase chemistry. We choose water as the reaction medium [45, 46] hence we started our experiment with use of water which resulted in a very poor yield of product and reaction was not finished even in 12 h. To overcome these difficulty different types of phase transfer of catalysts were scrutinized such as surfactants and non- ionic CD. Due to the fact that it is very difficult to remove surfactants from the reaction mixture. We carried out model the reaction in the presence of CD in aqueous medium. The product was obtained in a satisfactory amount as compared to that obtained with surfactant. (Table 1, entries 1–8) 

After realising the important role of β-CD in increasing the yield of the product we carried out the reaction in presence of different amounts of β-CD ranging from 10 mol% to stoichiometric amount in aqueous medium. Maximum amount of product was obtained when we use 20 mol% of β-CD. No change in amount of product was noticed when amount of β-CD was further enhanced (Table 1, entries 2–6). Encouraged by these results and in order to obtain better to excellent yields of the product and for decreasing the reaction time we performed the test reaction in presence of different type of acidic catalyst such as iodine, indium chloride but it did not catalyze the reaction well. Further we used some basic catalysts for example NaOH, KOH, Et3N, DMAP and DABCO. Results are tabulated in (Table 2) distinctly shows that DABCO is the finest promoter for this synthetic process. Therefore DABCO was found to work best in combination with CD.
Subsequently the model reaction between salicyladehyde (1a), malononitrile (2), indole (3a) and different amounts of DABCO as catalyst in presence of β-CD in water was carried out. The yield of product was increased as the amount of catalyst increased from 10 to 20 mol% (Table 2, entries 1–5). No further increase in yield of product was observed with further increase in the amount of catalyst (Table 2, entries 4 and 5). One of the most attractive feature of this protocol is that it saves 80 % of the catalyst because in this method consumes only 20 % of the catalyst for the synthesis of the desired product. In addition different CDs such as α-CD, γ-CD and β-CD were investigated for their efficiency as a phase transfer catalyst. It was observed that yield of the product highly depends on the magnitude of the angular aperture of the CD. We found that β-CD was most efficient for our model reaction due to optimal size of cavity [47]. Moreover it is easily available, cost-effective, less annoying, generally most applicable and easily form complex with aromatic compounds [48]. Further investigations revealed that water serve to be the best solvent as compared to different type of organic solvent for the test reaction. Results are summarized in (Table 3, entries 1–5). Temperature plays very important role [49, 50] because at low temperature yield of the product was not satisfactory. But as the temperature increases up to 60 °C amount of the product increases and reaction time also decreases.
With these optimized conditions in hand we further examined the scope and generality of our reaction. The replacement of malononitrile with dimedone/cyclohexane-1,3-dione was also examined. (Scheme 1). Based on the reports documented in literature and our observation in the present protocol, a plausible reaction mechanism has been proposed in presence of β-CD and DABCO in water (Scheme 3). Diversely substituted salicyladehyde (1) underwent Knoevenagel condensation with malononitrile (2) to give Knoevenagel product which followed by cyclisation to give 2-iminochrome intermediate (A) via Pinner reaction [36–38]. In second step indole (3) behave as nucleophile which adds on the (A) via Michael addition resulted in final product (4). β-CD and DABCO play a significant role in the synthesis of 4H-chromene-3-carbonitrile and tetrahydro-1H-xanthen-1-one derivatives. All the used functionalities were examined to be reconcilable under optimized reaction condition. Results were concluded in (Tables 4, 5). Substituents on salicyladehyde (1) show no significant effect on the yield of product. After completion of reaction, the solid product was filtered and the filtrate having β-CD was reused in the next run. β-CD was reused three times [49, 50] involving the use of fresh reaction media for the synthesis of the product. No significant loss was observed in the first three cycles (Table 6). The reusability was performed with the test reaction (Table 6, entries 1–4). Recyclability of catalyst showed minimal decrease from 80 to 70 % in the amount of the product. It should be observed that under the above optimized condition, model reaction was scaled up to 10 mmol gave uniform result [24, 32].


In summary we have developed a cost effective, eco-efficient, metal free and mild protocol for the synthesis of our target molecule via sequential, multicomponent and one pot reaction using salicyladehyde, active methylene compound and nucleophile. The combination of DABCO and β-CD in water was proved to be an effective medium to facilitate Knoevenagel condensation followed by addition of nucleophile. The simplicity and easy availability of reacting materials make this procedure better than other conventional methods. Variation of active methylene compounds was proven possible and therefore; this method not only documented the synthetic utility of this reaction, but also discovers a new era for effective way for synthesizing some biologically active compounds.
2.1 General Information
Reagents were obtained from commercial suppliers (Sigma Aldrich, Loba Chemie and Alfa Aesar) and used without further purification unless otherwise specified by a reference. All reactions were Performed using oven dried glassware. Organic solutions were concentrated using a Buchi rotary evaporator. TLC was performed using silica gel GF254 (Merck) plates. 1H NMR spectra were recorded on a Bruker Advance II 400 spectrometer in CDCl3/DMSO using TMS as internal reference with chemical shift value being reported in ppm. All coupling constants (J) are reported in Hertz (Hz). 13C NMR spectra were recorded on the same instrument at 100 MHz in CDCl3/DMSO and TMS was used as the internal reference. Mass (EI) spectra were recorded on a JEOL D-300 mass spectrometer.
3 Experimental
3.1 Materials and Methods
3.1.1 General Method
β-CD (20 mol%) was dissolved in water (25 ml) by warming to 60 °C until a clear aqueous solution was formed. Salicyladehyde (1.0 mmol), active methylene compound (1.0 mmol) and DABCO (20 mol%) was added, followed by indole (1.0 mmol) to the solution phase of β-CD. The mixture was stirred at 60 °C until the reaction was complete (as monitored by thin layer chromatography (TLC). The reaction mixture was cooled and β-CD was filtered. The aqueous layer was extracted with ethyl acetate. The combined organic layers were washed with water, saturated with brine solution, and dried over anhydrous Na2SO4. The combined organic layers were evaporated under reduced pressure and the resulting crude product was purified by column chromatography by using ethyl acetate and hexane. The identity and purity of the product were confirmed by 1H, 13C NMR, and mass spectra.
3.1.2 Spectral Data for Synthesized Compounds
-
1.
2-Amino-4-(1H-indol-3-yl)-4H-chromene-3-carbonitrile (4a): Light yellow solid;.1H NMR (400 MHz, CDCl3) δ = 10.94 (1H, s), 7.34 (1H, d J = 8.0 Hz), 7.31 (1H, d J = 1.6 Hz), 7.26–7.16 (2H, m), 7.08–6.98 (4H, m), 6.88 (1H, t), 6.83 (2H, s), 4.97 (1H, s) ppm; 13C NMR (100 MHz, DMSO) δ = 161.56, 149.10, 136.90, 130.01, 129.23, 126.09, 125.24, 124.90, 123.90, 121.30, 120.90, 119.90, 120. 96, 118.88, 117.24, 113.18, 55.88, 33.44 ppm. ESI–MS (m/z): 287 (M)+.
-
2.
2-Amino-4-(5-bromo-1H-indol-3-yl)-4H-chromene-3-carbonitrile (4b): Light yellow solid; 1H NMR (400 MHz, CDCl3) δ = 8.32 (1H, s), 7.41 (1H, s), 7.24–7.16 (3H, m), 7.15–7.12 (1H, m), 7.07–6.97 (3H, m), 5.03 (1H, s), 4.69 (2H, s) ppm; 13C NMR (100 MHz, DMSO) δ = 161.68, 147.96, 135.16, 128.88, 127.52, 125.80, 125.80, 126.10, 123.22, 123.01, 121.90, 121.48, 119.58, 116.36, 115.40, 112.78, 55.54, 33.78 ppm; ESI–MS (m/z): 365 (M)+.
-
3.
2-Amino-4-(5-methyl-1H-indol-3-yl)-4H-chromene-3-carbonitrile (4c): Light yellow solid;1H NMR (400 MHz, DMSO) δ = 10.77 (1H, s), 7.22 (3H, m), 7.07–7.00 (3H, m J = 7.6 Hz), 6.97 (1H, t), 6.86 (1H, d J = 8.4 Hz), 6.81 (2H, s), 4.96 (1H, s), 2.26 (3H, s) ppm; 13C NMR (100 MHz, DMSO) δ = 161.55, 149.88, 136.76, 128.71, 127.90, 127.38, 126.85, 124.68, 124.43, 123.24, 123.10, 122.36, 119.42, 118.64, 117.90, 112.80, 55.64, 33.64, 22.83 ppm; ESI–MS (m/z): 301 (M)+.
-
4.
2-Amino-4-(5-chloro-1H-indol-3-yl)-4H-chromene-3-carbonitrile (4d): Dark yellow solid; 1H NMR (400 MHz, DMSO) δ = 11.18 (1H, s), 7.41 (1H, d, J = 2.24 Hz), 7.36 (1H, d, J = 8.4 Hz), 7.27–7.18 (2H, m), 7.12–6.98 (4H, m), 6.90 (2H, s), 5.02 (1H, s) ppm; 13C NMR (100 MHz, DMSO) δ = 161.64, 149.83, 136.90, 128.84, 128.64, 126.88, 126.53, 125.74, 122.64, 122.54, 120.88, 120.36, 119.40, 118.98, 115.28, 112.82, 55.43, 33.22 ppm; ESI–MS (m/z): 321 (M)+.
-
5.
2-Amino-4-(6-chloro-1H-indol-3-yl) -6-methoxy-4H-chromene-3-carbonitrile (4e): Dark yellow solid; 1H NMR (400 MHz, CDCl3) δ = 8.14 (1H, s), 7.32 (1H, s), 7.22 (1H, d, J = 8.4 Hz), 7.15 (1H, s), 6.98-6.99 (2H, m,), 6.72 (1H, dd, J = 8.8, 2.8 Hz), 6.54 (1H, d, J = 2.4 Hz), 4.98 (1H, s), 4.56 (2H, s), 3.61(3H,s) ppm; 13C NMR (100 MHz, DMSO) δ = 161.60, 157.68, 142.15, 137.85, 126.98, 124.71, 124.43, 124.22, 121.73, 120.63, 118.93, 116.91, 115.19, 112.51, 111.48, 55.64, 55.64, 33.18 ppm; ESI–MS (m/z): 351 (M)+.
-
6.
2-Amino-4-(6-chloro-1H-indol-3-yl)-4H-chromene-3-carbonitrile (4f): Yellow solid; 1H NMR (400 MHz, DMSO): δ = 11.08 (1H, s), 7.38 (1H, d, J = 1.6 Hz), 7.36 (1H, d, J = 2.4 Hz), 7.21 (2H, dd, J = 10.4, 4.8), 7.05 (2H, d, J = 8 Hz), 7.01 (1H, t, J = 7.6 Hz), 6.92 (1H, dd, J = 8.4, 1.6 Hz), 6.87 (2H, s), 5.01 (1H, s) ppm. 13C NMR (100 MHz, DMSO) δ = 161.64, 149.76, 136.65, 128.66, 128.42, 127.36, 125.88, 125.48, 123.96, 123.31, 122.21, 121.16, 119.42, 118.33, 115.34, 112.64, 55.63, 33.55 ppm; ESI–MS (m/z): 321 (M)+.
-
7.
2-Amino-4-(6-methoxy-1H-indol-3-yl)-4H-chromene-3-carbonitrile (4g): Light yellow solid; 1H NMR (400 MHz, DMSO) δ = 10.71 (1H, s), 7.17 (1H, t, J = 7.6 Hz), 7.15 (1H, s,), 7.08 – 7.00 (3H, m), 6.98 (1H, d, J = 7.5 Hz), 6.82 (1H, s), 6.80 (2H, s), 6.52 (1H, d, J = 8.8 Hz), 4.92 (1H,s), 3.71 (3H,s) ppm; 13C NMR (100 MHz, DMSO) δ = 161.52, 154.12, 147.71, 139.75, 128.62, 127.72, 125.68, 124.43, 122.72, 121.43, 120.79, 119.31, 119.03, 115.98, 108.39, 96.61, 55.36, 55.21, 33.07 ppm; ESI–MS (m/z): 317 (M)+.
-
8.
2-Amino-4-(5-methoxy-1H-indol-3-yl)-4H-chromene-3-carbonitrile (4h): 1H NMR (400 MHz, DMSO) δ = 10.76 (1H, s), 7.20 (3H, dd, J = 18.4, 8.4 Hz), 7.11 (1H, d, J = 7.6 Hz), 7.06 (1H, d, J = 8.0 Hz), 7.01 (1H, t, J = 7.6 Hz), 6.83 (2H, s), 6.76 (1H, s), 6.71 (1H, dd, J = 8.8, 2.0 Hz), 4.97 (1H, s), 3.65 (3H, s) ppm; 13C NMR (100 MHz, DMSO) δ = 161.54, 152.90, 148.81, 132.72, 128.64, 129.27, 126.36, 123.73, 124.29, 123.14, 120.84, 119.19, 116.14, 113.62, 111.88, 109.36, 100.84, 54.90, 55.72, 33.75 ppm; ESI–MS (m/z): 317 (M)+.
-
9.
2-Amino-4-(-1H-indol-3-yl)-8-methyl-4H-chromene-3-carbonitrile (4i): Dark yellow solid; 1H NMR (400 MHz, CDCl3) δ = 8.09 (1H, s), 7.34 (2H, t, J = 8.4 Hz), 7.15-7.09 (2H, m), 7.01 (2H, t, J = 7.6 Hz), 6.93 (1H, d, J = 7.2 Hz), 6.85 (1H, t, J = 7.6 Hz), 5.06 (1H, s), 4.61 (2H, s), 2.34 (3H, s) ppm; 13C NMR (100 MHz, CDCl3): δ = 158.51, 148.95, 136.88, 128.80, 126.53, 126.27, 125.78, 125.23, 123.09, 121.28, 120.65, 120.42, 119.15, 119.01, 110.18, 59.31, 33.80, 14.75 ppm; ESI–MS (m/z): 301 (M)+.
-
10.
2-Amino-4-(1H-indol-3-yl)-6-methoxy-4H-chromene-3-carbonitrile (4j): 1HNMR (400 MHz, DMSO): δ = 10.92 (1 H, s,), 7.36 (1 H, d, J = 8 Hz), 7.31(1 H, d, J = 2.0 Hz), 7.28 (1H,d, J = 8.0 Hz), 7.02 (, 2 H, m), 6.88 (1 H, t, J = 7.6 Hz), 6.82–6.73 (3H, m,), 6.62 (1 H, d, J = 2.8 Hz), 4.97 (1 H, s), 3.60 (3H, s) ppm; 13C NMR (100 MHz, DMSO). δ = , 161.53, 155.06, 143.80, 136.13, 125.15, 124.25, 123.35, 120.52, 120.10, 119.03, 118.80, 118.35, 116.15, 115.52, 113.35, 111.52, 55.53, 55.20, 7, 33.29 ppm; ESI–MS (m/z): 317 (M)+.
-
11.
6-Hydroxy-9-(1H-indol-3-yl)-3,3-dimethyl-2,3,4,9-tetrahydro-1H-xanthen-1-one (4k): White solid 1H NMR (400 MHz, DMSO-d6): δ = 10.77 (1H, s); 9.56 (1H, s), 7.39 (1H, d, J = 8.0 Hz), 7.26 (1H, d, J = 8.4 Hz), 7.18 (1H, s), 6.96-7.02 (2 H, m,), 6.89 (t, 1H, J = 8.4 Hz), 6.53 (s, 1H), 6.47 (dd, J = 2.6 Hz, J = 5.9 Hz, 1H) 5.05 (1 H, s), 2.58 (2H, s), 2.26 (1H, d, J=16.0 Hz,), 2.07 (1H, d, J = 16.0 Hz), 1.05 (s, 3H,), 0.92 (s, 3H,) ppm; 13C NMR δ = : 197.04, 164.08, 157.16, 148.54, 136.9, 131.70, 126.3, 124.45, 121.58, 120.6, 119.40, 118.5, 117.5, 113.45, 112.40, 103.73, 51.21, 41.8, 30.32, 29.82, 28.8, 27.7 ppm; ESI–MS (m/z): 359 (M)+.
-
12.
9-(5-Bromo-1H-indol-3-yl)-7-chloro-2,3,4,9-tetrahydro-1H-xanthen-1-one (4l):
-
i.
White solid; 1H NMR (400 MHz, DMSO-d 6): δ 11.14 (s, 1H,), 7.74 (s, 1H,),7.14-7.30 (m, 5H,), 7.36(s, 1H), 5.25 (s, 1H), 2.65–2.77 (m, 2H), 2.24–2.37 (m, 2H), 1.88–2.01 (m, 2H) ppm; 13CNMR(100 MHz, DMSO-d 6): δ; 196.08, 165.97, 148.74, 134.99, 128.09, 127.26, 127.83, 127.55, 127.03, 124.84, 123.42, 120.63, 120.24, 118.18, 113.65, 112.88, 111.39, 36.52, 28.09, 27.03, 20.08 ppm; (ESI, m/z): 429 (M+).
-
i.
-
13.
7-Chloro-9-(1H-indol-3-yl)-2,3,4,9-tetrahydro-1H-xanthen-1-one (4m): White solid; 1H NMR (400 MHz, DMSO-d 6): δ 10.93 (s, 1H), 7.48 (d, J = 7.7 Hz, 1H), 7.36 (s, 1H), 7.33 (d, J = 8.4 Hz, 1H), 7.18–7.24 (m, 3H), 7.04 (t, J = 8.2 Hz, 1H), 6.95 (t, J = 8.2 Hz, 1H), 5.26 (s, 1H), 2.63–2.78 (m, 2H), 2.22–2.37 (m, 2H), 1.81–1.99 (m, 2H) ppm 13C NMR (100 MHz, DMSO-d 6): δ; 196.06, 165.88, 147.76, 136.46, 129.09, 128.17, 128.05, 127.36, 125.17, 123.02, 120.89, 119.08, 118.69, 118.14, 118.08, 112.74, 111.69, 36.57, 28.55, 27.03, 20.09 ppm (ESI, m/z): 349 (M+).
-
14.
9-(5-Bromo-1H-indol-3-yl)-6-methoxy-2,3,4,9-tetrahydro-1H-xanthen-1-one (4n): Brown solid; 1H NMR (400 MHz, DMSO-d 6): δ 11.06 (s, 1H), 7.66 (s, 1H), 7.27 (d, J = 8.5 Hz, 1H), 7.11–7.17 (m, 3H), 6.77 (s, 1H), 6.66 (dd, J = 2.7 Hz, J = 6.3 Hz, 1H), 5.15 (s, 1H), 3.72 (s, 3H), 2.65–2.77 (m, 2H), 2.23–2.37 (m, 2H), 1.87–2.02 (m, 2H) ppm; 13CNMR (100 MHz, DMSO-d6): δ; 196.24, 166.03, 158.58, 149.55, 134.98, 130.24, 127.18, 124.45, 123.21, 120.74, 120.02, 117.58, 113.52, 111.57, 111.17, 101.08, 55.38, 36.58, 27.62, 27.14, 20.1 ppm; (ESI, m/z): 423 (M+).
-
15.
5-Hydroxy-9-(1H-indol-3-yl)-2,3,4,9-tetrahydro-1H-xanthen-1-one (4o): White solid; 1H NMR (400 MHz, DMSO-d 6): δ 10.83 (s, 1H), 9.71 (s, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.31 (d, J = 8.2 Hz, 1H), 7.15 (s, 1H), 7.03 (d, J = 7.0 Hz, 1H), 6.95 (t, J = 7.2 Hz, 1H), 6.82 (t, J = 8.2 Hz, 1H), 6.73 (d, J = 7.8 Hz, 2H) 5.19 (s,1H), 2.67–2.83 (m, 2H), 2.24–2.36 (m, 2H), 1.88–2.01 (m, 2H,) ppm; 13C NMR (100 MHz, DMSO-d 6): δ; 196.29, 166.21, 145.23, 137.89, 136.48, 127.02, 125.44, 124.38, 122.64, 120.78, 119.78, 119.32, 118.49, 113.99, 113.14, 111.56, 36.68, 28.64, 27.28, 20.27; ppm; (ESI, m/z): 331 (M+).
References
Vilar M, Navarro M (2012) Electrochim Acta 59:270
Cao Y, Xiao X, Li R, Guo Q (2003) J Mol Struct 660:73
Bram G, Sansoulet J, Galons H, Bensaid Y, Combet-Farnoux C, Miocque M (1985) Tetrahedron Lett 26:4601
Durst HD (1974) Tetrahedron Lett 15:2421
Kimura Y, Kirszensztejn P, Regen SL (1983) J Org Chem 48:385
Huang J, Huang Y (1991) J Organomet Chem 414:49
Guo R, Wilson LD (2013) Curr Org Chem 17:14
Ma L, Zhou C, Yang Q, Yang X, Zhang C, Liao L (2014) Curr Org Chem 18:1937
Marchetti L, Levine M (2011) ACS Catal. 1:1090
Hu YL, Jiang H, Lu M (2011) Green Chem 13:3079
Villalonga R, Cao R, Fragoso A (2007) Chem Rev 107:3088
Rekharsky MV, Inoue Y (1998) Chem Rev 98:1875
Connors KA (1997) Chem Rev 97:1325
Shen H-M, Ji H-B (2012) Tetrahedron Lett 53:3541
Sridhar R, Srinivas B, Surendra K, Srilakshmi Krishnaveni N, Rama Rao K (2005) Tetrahedron Lett 46:8837
Shin J-Ah, Lim Y-G, Lee K-H (2012) J Org Chem 77:4117
Singh SB, Tiwari K, Verma PK, Srivastava M, Tiwari KP, Singh J (2013) Supramol Catal 25(5):255
Londhe BS, Pratap UR, Mali JR, Mane RA (2010) Bull Korean Chem Soc 31:2329
Wang C, Xu C, Yang G, Fan S, Xin L, Guo T (2014) Molbank 2014(3):M830. doi:10.3390/M830
Thomas N, Mary Zachariah S (2013) Asian J Pharm Clin Res 6:11
Chen W, Cai Y, Fu X, Liu X, Lin L, Feng X (2011) Org Lett 13:4910
Limsuwan S, Trip EN, Kouwen TRHM, Piersma S, Hiranrat A, Mahabusarakam W, Voravuthikunchai SP, van Dijl JM, Kayser O (2009) Phytomedicine 16:645
Kemnitzer W, Drewe J, Jiang S, Zhang H, Crogan-Grundy C, Labreque D, Bubenick M, Attardo G, Denis R, Lamothe S, Gourdeau H, Tseng B, Kasibhatla S, Cai SX (2008) J Med Chem 51:417
Ghosh PP, Das AR (2013) J Org Chem 78:6170
Bhatacharjee S, Das DK, Khan AT (2013) Synthesis 45:A–H
Hardcastle IR, Cockcroft X, Curtin NJ, El-Murr MD, Leahy JJ, Stockley M, Golding BT, Rigoreau L, Richardson C, Smith GCM, Griffin RJ (2005) J Med Chem 48:7829
Jain S, Rajguru D, Keshwal BS, Acharya DA (2013) ISRN Org Chem 185120:5
Li M, Zhang B, Gu Y (2012) Green Chem 14:2421
Ganguly NC, Roy S, Mondal P, Saha R (2012) Tetrahedron Lett 53:7067
Shanthi G, Perumal PT (2007) Tetrahedron Lett 48:6785
Khurana JM, Nand B, Saluja P (2010) Tetrahedron 66:5637
Lia M, Gua Y (2012) Adv Synth Catal 354:2484
Khurana JM, Kumar S (2009) Tetrahedron Lett 50:4125
Rajesh UC, Kholiya R, Thakur A, Rawat DS (2015) Tetrahedron Lett 56:1790
Rajesh UC, Wang J, Prescott S, Tsuzuki T, Rawat DS (2015) ACS Sustain Chem Eng 3:9
Thakur A, Linga RP, Tripathi M, Rawat SD (2015) New J Chem 39:6253
Khalafi-Nezhad A, Nourisefat M, Panahi F (2015) Org Biomol Chem 13(28):7772–7779
Shinde VV, Jeong YS, Jeong YT (2015) Mol Divers 19:367
Srivastava M, Rai P, Singh J, Singh J (2013) RSC Adv 3:16994
Kaushik NK, Kaushik N, Attri P, Kumar N, Kim CH, Verma AK, Choi EH (2013) Molecules 18:6620
Swetha A, Babu BM, Meshram HM (2015) Tetrahedron Lett 56:1775
Gawande MB, Bonifácio VDB, Luque R, Branco PS, Varma RS (2013) Chem Soc Rev 42:5522
Rideout DC, Breslow R (1980) J Am Chem Soc 102:7816
Breslow R (1991) Acc Chem Res 24:159
Srivastava M, Singh J, Singh SB, Tiwari K, Pathak VK, Singh J (2012) Green Chem 14(4):901–905
Gu Y (2012) Green Chem 14:2091
Monflier E, Tilloy S, Castanet Y, Mortreux A (1998) Tetrahedron Lett 39:2959
Takahashi K (1998) Chem Rev 98:2013
Tayade YA, Padvi SA, Wagh YB, Dalal DS (2015) Tetrahedron Lett 56:2441
Ramesh K, Karnakar K, Satish G, Reddy KHV, Nageswar YVD (2012) Tetrahedron Lett 53:6095
Acknowledgments
The author Pratibha Rai and Madhulika Srivastava grateful to UGC, New Delhi, for the award of Senior Research Fellowship and Snehlata Yadav thankful to UGC, New Delhi, for the award of Research Fellowship. We also sincerely thank SAIF, Punjab University, Chandigarh, for providing micro-analyses and spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rai, P., Srivastava, M., Yadav, S. et al. β-Cyclodextrin: A Biomimetic Catalyst used for the Synthesis of 4H-chromene-3-carbonitrile and Tetrahydro-1H-xanthen-1-one Derivatives. Catal Lett 145, 2020–2028 (2015). https://doi.org/10.1007/s10562-015-1588-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1588-2