Abstract
A variety of polyethylene glycol-400-functionalized dicationic acidic ionic liquids (ILs) (PEG400-DAILs) were synthesized and tested in the dehydration of fructose. Among these catalysts, hydrogen sulfate anion-based ILs showed the higher catalytic performance in the conversion of fructose. The highest HMF yield of 96.5 % with 100 % consumption of fructose were obtained after 60 min at 110 °C. The effects of catalyst concentration, reaction time, reaction temperature, and solvents were systematically investigated. In addition, the generality of the catalysts was examined by processing inulin and sucrose to HMF with yield of 71.9 and 52.1 % under certain conditions, respectively. PEG400-DAILs possess modified physicochemical properties such as low viscosity, strong hydrophilicity and hydrogen bonding with sugars so as to improve activity for the dehydration of fructose into HMF. The used IL catalyst could be separated and recycled repeatedly without significant loss of catalytic activity. The research emphasizes preparation of the novel catalysts combining polymers with functionalized ILs for conversion of renewable biomass.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Under the dual pressure of the energy crisis and environmental protection, development and utilization of renewable clean energy have been extensively concerned. In this respect, biomass, which is affluent, pollution-free and widespread, provides an ideal substitute to fossil resources for the production of fine chemicals and fuels [1–4]. Recently, an increasing efforts have been devoted towards transforming biomass into 5-hydroxymethylfurfural (HMF), which is one of vitally important versatile intermediates for preparing a broad of valuable chemicals [5–11], polymers [12], pharmaceutical [13].
As a sustainable precursor for monomers and fuel components, HMF can be synthesized from all types of C6 carbohydrates, including monomeric and polymeric saccharides, among which conversion of fructose to HMF is of significant interest with acidic catalysts. Over the past few years, all sorts of mineral or organic acids catalysts for direct production of HMF from fructose have been extensively studied in water [14] and various aprotic organic solvents [15, 16]. However, the homogenous acid catalysts show innate drawbacks in corrosion and non-recyclability. Recently, there were several novel heterogeneous solid acid catalysts which were employed for the conversion of fructose to HMF under different conditions, such as silica materials [17, 18], acid-functionalized carbons [19, 20], acidic resins [21], zeolites [22, 23], functionalized metal–organic frameworks (MOFs) [24], graphene oxide (GO) [25], polyoxometalates (POMs) [26]. Generally, heterogeneous catalysis is preferred over homogeneous catalysis because of the ease of catalyst separation and its reusability. However, soluble polymers and insoluble humins formed can deposit in the catalyst pores, leading to the partial deactivation of the catalysts. This suggests a need to explore efficient homogeneous catalytic processes for HMF production from fructose.
Interestingly, ionic liquids (ILs) have been defined as a sort of powerful reaction medium as well as catalysts for the dehydration of hexose to HMF [27–34], while the use of innovative catalysts containing ionic liquids have been scarce and subject to intense research. Lee et al. [35] reported that functionalized mesoporous silica nanoparticles with both sulfonic acid and ionic liquid were applied as effective and recyclable catalysts for generating HMF from fructose. Shi et al. [36] first developed a novel class of fiber supported ionic liquid to catalyze fructose dehydration into HMF and showed excellent catalytic activity. Recently, Jadhav et al. [37, 38] prepared a series of homogeneous supported dicationic ionic liquids, in which each IL contains short oligo (ethylene glycol) linkers. These ionic liquids showed high catalytic activity for selective dehydration of fructose into HMF. At present, the functionalized ionic liquid catalysts still exist some deficiencies, thus we need to make further constant efforts to modify the kind of catalysts to improve catalyst activity for fructose conversion into HMF.
The abundance of differently functionalized ionic liquids are incorporated PEG chain into cationic units, generating an attractive group of solvents or catalysts that find applications across a range of disciplines, including extractions, gas separations, carbohydrate dissolution, organic synthesis and catalysis [39]. Recent studies have demonstrated that ether-functionalized ILs tend to present lower viscosity and reduced toxicity than their aliphatic-substituted counterparts [40, 41]. Considering the above factors, we attempted to prepare polyethylene glycol-400-functionalized dicationic acidic ionic liquids (PEG400-DAILs) to broaden homogeneous supported ionic liquids catalysts applications in the dehydration of fructose into HMF. In the present study, a variety of PEG400-DAILs were synthesized and used as catalysts for dehydration of fructose into HMF. Moreover, the influence of different anion moieties on the catalytic activity of ionic liquids were discussed and the effects of various process parameters were investigated in detail.
2 Experimental
2.1 Materials and Equipment (General Remark)
1,3-Propanesultone, phosphotungstic acid (H3PW12O40) and phosphomolybdic acid (H3PMo12O40) were purchased from shanghai Aladdin Industrial Inc.; HMF used in the study was obtained from Sigma-Aldrich Co. LLC.; inulin was obtained from Alfa Aesar; fructose, sucrose, glucose, cellobiose, polyethylene glycol-400, imidazole, sodium ethoxide, potassium carbonate, sodium hydroxide, were purchased from Sinopharm Chemical Reagent Co. Ltd.; All other reagents and solvents were of analytical grade and used without further purification unless otherwise stated. Deionized water was produced by a laboratory water-purification system (RO DI Digital plus). FT-IR spectra were recorded on a Nicolet 360 FT-IR instrument (KBr discs) in the 4,000–500 cm−1 region. 1H NMR spectra were measured with a Bruker DPX 300 (400 MHz) spectrometer and tetramethylsilane (TMS) was used as internal standard. UV–Vis spectra were performed on TU-1901 spectrophotometer in water.
2.2 Catalysts Preparation
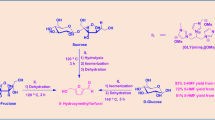
The IL 4 was prepared according to literature procedure with slight modifications (Fig. 1) (see the Supporting Information for details).
The PEG400-DAILs used in the study were synthesized by the treatment of IL 4 or 5 with acid in water. Typically, the ionic liquid of [4·2H][HSO4]2 was prepared as follows: the H2SO4 (0.7 mL, 12 mmol) was added to the IL 4 (4.46 g, 6 mmol) in water (10 mL) followed by stirring of the mixture at 50 °C for 12 h. On completion, water was removed in vacuum and the residue was washed with petroleum ether three times then evaporated under reduced pressure, and the final product, [4·2H][HSO4]2 was obtained as brown viscous liquid and characterized by FI-IR spectroscopy. The other PEG400-DAILs were prepared following the above same procedures.
2.3 General Procedure for the Dehydration of Fructose to HMF
All the dehydration reaction experiments were performed in a 5 mL reaction vial equipped with magnetic stirrer. A typical procedure for dehydration of fructose was as follow: fructose (50 mg), PEG400-DAIL (7.5 mol%) and DMSO (2 mL) were added into the reaction vial. The mixture was stirred vigorously and heated with a thermostatically controlled oil bath for a specific time. After completion of the reaction, the mixture was decanted into a volumetric flask using ultrapure water as diluents, and analyzed by high-performance liquid chromatography (HPLC) system. HMF: 1H NMR (400 MHz, D2O, TMS): δ 4.68 (s, 2H), 6.66 (d, J = 4 Hz, 1H), 7.51 (d, J = 4 Hz, 1H), 9.44 (s, 1H).
2.4 Analyses
The liquid solutions were analyzed with HPLC using Agilent 1100 series with a refractive index detector and a Shodex SURGER SP-0810 column (300 × 8.0 mm). Ultrapure water was used as the mobile phase at a flow rate of 0.7 mL/min, and the column temperature was maintained at 70 °C. The amount of HMF and fructose were determined using an external standard. The conversion of fructose and the yield of HMF were evaluated as follows:
3 Results and Discussion
3.1 Preparation and Characterizations of PEG400-DAILs
Seeking to efficient and environment-friendly ionic liquids for conversion of fructose into HMF, polyethylene glycol (PEG) is grafted to the cation to lead to variation in viscosity and hydrophilicity of the resulting ILs. Figure 1 illustrates method for synthesis of PEG400-DAILs including several synthetic steps. These ILs were characterized by 1H NMR, FT-IR analysis. In this method, PEG-400 is first converted to an alkyl chloride, followed by reaction with a nucleophilic imidazole anion. Then, quaternization of the PEG-400-substituted imidazole with 1,3-propane sultone is performed to produce functionalized dicationic ionic liquid. Finally, functionalized dicationic ionic liquids are protonated to achieve ultimate PEG-400-functionalized dicationic imidazolium acidic ionic liquids. A similar approach has also been used in the preparation of PEG-400-functionalized dicationic quaternary ammonium acidic ionic liquids. The detailed synthesis conditions are described in the Supporting Information.
3.2 Hammett Acidity of PEG400-DAILs (HSO4)
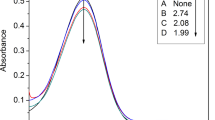
It has been suggested that the dehydration of fructose to HMF is proportional to the acidity of catalyst [42], the Hammett acidity function H 0 value of [4·2H][HSO4]2 and [5·2H][HSO4]2 were determined using UV–Visible spectrophotometer with p-nitroaniline as the indicator, which was similar to the method utilized in the previous literature [43]. Therein, the IL and p-nitroaniline were dissolved in water with concentrations of 30 and 0.1 mmol/L, respectively, and then the solution UV spectra were recorded, the obtained data were listed in Table 1. The H 0 value was calculated by the equation H 0 = pK(I)aq + log([I]/[IH+]), where pK(I)aq is the pKa value (about 0.99) of the indicator solution, [IH+] and [I] are respectively the molar concentrations of the protonated and unprotonated forms of the indicator. The maximum absorbance of the unprotonated forms of the indicator was obtained at 380 nm in water. Furthermore, the corresponding H 0 values of [4·2H][HSO4]2 and [5·2H][HSO4]2 were 1.42 and 1.72, respectively. The results demonstrated that the acidity of [4·2H][HSO4]2 was stronger than [5·2H][HSO4]2.
3.3 Conversion of Fructose to HMF by Various PEG400-DAILs Catalysts
HMF is a triple dehydration product of fructose (Fig. 2) in the presence of acidic catalyst. As shown in Fig. 3a, b, the reaction time and temperature were optimized to achieve maximum quantity of HMF from fructose. Temperature ranged from 90 to 120 °C were discussed at different time of 30, 45, 60, 75, 90 and 120 min in the presence of the equimolar amounts of catalyst (7.5 mol% to fructose) both for [4·2H][HSO4]2 and [5·2H][HSO4]2. For [4·2H][HSO4]2, it can be seen that the yield of HMF increased to 91.2 % after 120 min at 90 °C, while it could reach as high as 96.5 % with a full fructose conversion (Table 2, entry 1) at 110 °C for 1 h in DMSO, confirming that increasing the reaction temperature facilitates the formation of HMF from fructose. However, with the further increasing reaction temperature and time, the main by-products of insoluble humins were created and HMF decomposed to levulinic acid and formic acid which coherently leading to reduced HMF yield. Similarly, for optimization of the reaction conditions, two parameters such as reaction time and reaction temperature were investigated using [5·2H][HSO4]2 as a catalyst. These results reflected that the best HMF yield of 92.7 % (Table 2, entry 2) was achieved at 110 °C for 60 min. It was noted that both [4·2H][HSO4]2 and [5·2H][HSO4]2 showed close catalytic activity toward the dehydration of fructose into HMF. These results are consistent with the Hammett acidity function order of the ILs.
Moreover, we repeated the reaction with different dosages of anion-based PEG400-DAILs (5 and 10 mol% to fructose) under 110 °C for 60 min. As a result (Table 2, entries 3–6), the slight decrease of HMF yield was observed with 5 or 10 mol% of catalyst, which might be attributed to a difference in acidic sites. Thus, the optimum amount of catalyst is 7.5 mol% to fructose.
In addition, the effect of PEG-400-functionalized heteropolyanion based ionic liquids on the dehydration of fructose was examined and the results were summarized in Table 2 (entries 7–14). The reactions were carried out at 110 °C for 60 min using 0.9 or 1.1 mol% of catalyst in 2 mL DMSO. Generally, phosphotungstic anion based ILs showed excellent to good activity toward the fructose dehydration. With an increasing catalyst dosage, HMF gradually increases to a relatively high yield of 94.8 % or 91.8 % with 1.1 mol% of [4·2H]3[PW12O40]2 or [5·2H]3[PW12O40]2. Nevertheless, the low HMF yields (Table 2, entries 11–14) with phosphomolybdic anion based ILs catalyst may be explained by taking into account lower acid strength and thermal stability [44].
On the basis of the above results, we discover that various PEG400-DAILs exhibit excellent catalytic performance which can be attributed to the following aspects. On the one hand, the wettability of catalyst has great impact on the HMF stabilization [37, 38].
Literature reported that the addition of alkoxy groups to the cation remarkably increased the hydrophilicity of ILs [39]. Thus, PEG400-DAILs have aroused the enormous attention in our study. When prolonging the reaction time, the hydrophilic groups of PEG400-DAILs have a close interaction with water molecule, improving the formation of HMF from fructose. When extending optimized reaction time, the decrease of HMF yield could be caused, which indicated by-product formation and product instability at this stage. What’s more, we speculated that the intermolecular hydrogen bonding between ether chain oxygen atom and sugar molecules facilitated the sugar dissolution. To a certain extent, the strong intermolecular interaction is one factor that contributes to promote the dehydration of fructose. Besides, HSO4 anion-based PEG400-DAILs display relative lower viscosity which can accelerate the rate of mass transport, enhancing the yield of HMF. According to the above understanding, we can rationally deduce that PEG400-DAILs show unique physicochemical properties, which are favorable for conversion of fructose to HMF.
To compare the applicability and the efficiency of PEG400-DAILs with the reported catalysts for the dehydration of fructose, we have summarized the results in Table 3. It can be seen that PEG400-DAILs showed better catalytic performance than other catalysts.
3.4 Effect of Solvent on Fructose Dehydration
Similarly, A series of solvents, including DMA, DMF, NMP, isopropanol, MIBK and acetone were also used to investigate the effect of solvents with respect to the fructose conversion and HMF yield by using [4·2H][HSO4]2 as catalyst. 100 % fructose conversion and 96.5 % HMF yield were obtained in DMSO. Clearly, DMA, DMF, NMP and isopropanol were less effective reaction media in the study. When NMP was used as solvent, the yield of HMF reached 48.7 % with 72.8 % fructose conversion. HMF yield of 37.5 % were achieved in isopropanol. In the polar aprotic solvent, such as DMA and DMF, 42.7 and 54.8 % HMF yield were obtained, respectively. It is noteworthy that DMSO is the most efficient solvent for facilitating the dehydration of fructose to HMF, which is probably due to DMSO being as an electron acceptor to improve the dehydration of fructose [45]. However, the approach make it unable to separate HMF by distillation owning to the high boiling point of DMSO. In addition, the catalytic conversion of fructose into HMF in a biphasic system including water and organic solvents as well as the [4·2H][HSO4]2 to further verify the catalytic activity of the PEG400-DAILs. It was demonstrated that acetone with the co-solvent of water gave the good yields of 60.5–62.8 % (Table 4, entries 8–9). Besides, the results show that water and MIBK mixtures (1:9) under 110 °C for 60 min of reaction time obtained the highest yield of 82.5 % (Table 4, entry 6).
3.5 Recyclability of the Catalysts
From the point of view of economizing resource and preventing pollution, recyclability of the catalysts seems to be pretty important. Therefore, we chose [4·2H][HSO4]2 as catalyst to research the recyclability for the dehydration reaction of fructose over five times. All reactions were conducted at 110 °C for a reaction time of 60 min. The resulting mixture after reaction was first distilled under reduced pressure to obtain DMSO and the remaining mixture was extracted with ethyl acetate. It had been demonstrated fructose and [4·2H][HSO4]2 were insoluble in ethyl acetate and could be easily separated, while HMF was the sole product in ethyl acetate phase. The obtained mixture containing IL was dried in vacuum at 60 °C for 12 h to remove water and residual ethyl acetate. It was then reused for further reaction by adding the same amount of fructose. As shown in Fig. 4, the yield of HMF was maintained at 96 % after the first recycle. Furthermore, no significant loss of HMF yield was observed till the sixth run, meaning that [4·2H][HSO4]2 has excellent stability and catalytic activity.
3.6 Conversion of Other Feedstock to HMF
Apart from the discussion above, fructose-based saccharides such as inulin and sucrose were selected as feedstock to evaluate catalytic performance using PEG400-DAILs as catalysts. It can been seen in Table 5, HMF was produced with 71.9 and 66.6 % yield in the presence of [4·2H][HSO4]2 and [5·2H][HSO4]2, respectively. Then we also further researched the heteropolyanion based ILs catalyzed inulin transformation to HMF. Among these catalysts, phosphotungstic acid anion based ILs still show the higher catalytic performance in HMF formation. The tendency is in agreement with the results achieved by fructose to HMF.
Furthermore, the dehydration of sucrose was also investigated with PEG400-DAILs as catalyst, the results are summarized in Table 6. It can be seen that 52.1 % yield of HMF was obtained with [4·2H][HSO4]2 as catalyst (Table 6, entry 1), in keeping with results obtained using mineral acid catalysts (Table 6, entry 9). Then, catalytic transformation of sucrose to HMF was researched in the presence of other PEG400-DAILs. It is well known that sucrose is a disaccharide composed of one molecule glucose and one molecule fructose. These data show that the ketose is more easily dehydrated to HMF than aldose using current conditions. When glucose and cellobiose were subjected to our reaction system, the HMF yield was remarkably inferior (Table 6, entries 10–13). This result clearly indicates that PEG400-DAILs as Brønsted acid catalysts can hardly promote the isomerization of glucose to fructose.
But these excellent catalytic properties, together with its nontoxic nature, make the PEG400-DAILs a very promising catalyst for the dehydration of carbohydrates.
4 Conclusions
In conclusion, we have shown that a series of new PEG400-DAILs can act as a novel, efficient and homogenous catalysts for one-pot conversion of fructose into HMF. The kinds of PEG400-DAILs were used for the first time in conversion of fructose, inulin, and sucrose into HMF and displayed high catalytic activity. Moreover, in comparison with commonly used acidic ILs, PEG400-DAILs in this study grant greater variety and regulate the physicochemical properties. PEG400-DAIL combines PEG-400 moieties with the cationic units of acidic IL, generating an appealing catalyst which changes viscosity, hydrophilicity, and intermolecular hydrogen bonding in reaction process. These unique properties are beneficial to fructose dehydration. These catalysts also show high recyclability with a minor loss of performance. The novel catalysts integrating polymers and functionalized ionic liquids for biomass conversion open up a wide avenue of green biomass refinery processes toward the production of HMF.
References
Corma A, Iborra S, Velty A (2007) Chem Rev 107:2411
Binder JB, Raines RT (2009) J Am Chem Soc 131:1979
Serrano-Ruiz JC, Dumesic JA (2011) Energy Environ Sci 4:83
Alonso DM, Bond JQ, Dumesic JA (2010) Green Chem 12:1493
Saha B, Gupta D, Abu-Omar MM, Modak A, Bhaumik A (2013) J Catal 299:316
Román-Leshkov Y, Barrett CJ, Liu ZY, Dumesic JA (2007) Nature 447:982
Lew CM, Rajabbeigi N, Tsapatsis M (2012) Ind Eng Chem Res 51:5364
Wang H, Deng T, Wang Y, Qi Y, Hou X, Zhu Y (2013) Bioresour Technol 136:394
Szabolcs Á, Molnár M, Dibó G, Mika LT (2013) Green Chem 15:439
Weingarten R, Kim YT, Tompsett GA, Fernández A, Han HS, Hagaman EW, Conner CC, Dumesic JA, Huber GW (2013) J Catal 304:123
Upare PP, Yoo J-W, Kim MY, Kang H-Y, Hwang DW, Hwang YK, Kung HH, Chang J-S (2013) Green Chem 15:2935
Eerhart A, Faaij APC, Patel MK (2012) Energy Environ Sci 5:6407
Lukevics E, Ignatovich L, Shestakova I (2003) Appl Organomet Chem 17:898
Asghari FS, Yoshida H (2007) Ind Eng Chem Res 46:7703
Gallezot P (2012) Chem Soc Rev 41:1538
Chheda JN, Román-Leshkov Y, Dumesic JA (2007) Green Chem 9:342
Graaff WNP, Olvera KG, Pidko EA, Pidko EA, Hensen E (2014) J Mol Catal A 388:81
Huang Z, Pan W, Zhou H, Qin F, Xu H, Shen W (2013) ChemSusChem 6:1063
Qi X, Guo H, Li L, Smith RL (2012) ChemSusChem 5:2215
Liu R, Chen J, Huang X, Chen L, Ma L, Li X (2013) Green Chem 15:2895
Li Y, Liu H, Song C, Gu X, Li H, Zhu W, Yin S, Han C (2013) Bioresour Technol 133:347
Dornath P, Fan W (2014) Microporous Mesoporous Mater 191:10
Ordomsky VV, Schaaf J, Schouten JC, Nijhuis TA (2012) J Catal 287:68
Chen J, Li K, Chen L, Liu R, Huang X, Ye D (2014) Green Chem 16:2490
Mondal D, Chaudhary JP, Sharma M, Prasad K (2014) RSC Adv 4:29834
Chen J, Zhao G, Chen L (2014) RSC Adv 4:4194
Moreau C, Finiels A, Vanoye L (2006) J Mol Catal A 253:165
Bao Q, Qiao K, Tomida D, Yokoyama C (2008) Catal Commun 9:1383
Qi X, Watanabe M, Aida TM, Smith RL (2009) Green Chem 11:1327
Tong X, Li Y (2010) ChemSusChem 3:350
Ryu J, Choi JW, Suh DJ, Ahn DJ, Suh YW (2012) Catal Commun 24:11
Zhang Z, Liu B, Zhao ZK (2012) Carohydr Polym 88:891
Okano T, Qiao K, Bao Q, Tomida D, Hagiwara H, Yokoyama C (2013) Appl Catal A Gen 451:1
Kotadia DA, Soni SS (2013) Catal Sci Technol 3:469
Lee YY, Wu KCW (2012) Phys Chem Chem Phys 14:13914
Shi XL, Zhang M, Li Y (2013) Green Chem 15:3438
Jadhav AH, Kim H, Hwang IT (2012) Catal Commun 21:96
Jadhav AH, Chinnappan A, Patil RH, Kostjuk SV, Kim H (2014) Chem Eng J 243:92
Cecchini MM, Charnay C, De Angelis F, Lamaty F, Martinez J, Colacino E (2014) ChemSusChem 7:45
Zhao H, Baker GA, Song Z, Olubajo O, Crittle T, Peters D (2008) Green Chem 10:696
Bara JE, Gabriel CJ, Lessmann S, Carlisle TK, Finotello A, Gin DL, Nobl RD (2007) Ind Eng Chem Res 46:5380
Watanabe M, Aizawa Y, Iida T, Nishimura R, Inomata H (2005) Appl Catal A Gen 295:150
Thomazeau C, Olivier-Bourbigou H, Magna L, Luts S, Gilbert B (2003) J Am Chem Soc 125:5264
Timofeeva MN (2003) Appl Catal A Gen 256:19
Amarasekara AS, Williams LD, Ebede CC (2008) Carbohydr Res 343:3021
Acknowledgments
This work was financial supported by National Natural Science Foundation of China (21206057), and the Natural Science Foundation of Jiangsu Province, China (BK2012118), (BK2012547), and MOE & SAFEA for the 111 Project (B13025).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, W., Wang, Y., Li, W. et al. Polyethylene Glycol-400-Functionalized Dicationic Acidic Ionic Liquids for Highly Efficient Conversion of Fructose into 5-Hydroxymethylfurfural. Catal Lett 145, 1080–1088 (2015). https://doi.org/10.1007/s10562-015-1485-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1485-8