Abstract
Systems prepared in situ by addition of n equivalents of triphenylphosphine to palladium dichloride in the presence of m equivalents of para-toluenesulfonic acid (TSA), PdCl2/nPPh3/mTSA (n and m varying between 2 and 10), were used as precatalysts for the olefin carbonylation (1-hexene, cyclohexene and styrene) with alcohols (MeOH, EtOH, n-PrOH and i-PrOH) to generate the corresponding esters (hydroalcoxycarbonylation), under mild reaction conditions. For 1-hexene carbonylation in presence of methanol (1-hexene hydromethoxycarbonylation), the most active system was PdCl2/6PPh3/5TSA at P(CO) = 50 atm and T = 125 °C, which was also active for the hydromethoxycarbonylation of other olefins (1-hexene > styrene > cyclohexene). This system was regioselective towards the linear product for 1-hexene and towards the branched product for styrene. A kinetic study of 1-hexene hydromethoxycarbonylation catalyzed by PdCl2/6PPh3/5TSA showed that the initial reaction rate (r o) was first order on Pd and MeOH concentrations and fractional order with respect to CO concentration; for olefin concentration was found a saturation curve. These kinetic results, together with coordination chemistry and computational DFT studies, allow us to propose a catalytic cycle involving species of the type [Pd(H)(L)(PPh3)2]+n (L = Cl, n = 0; L = CO, MeOH, olefin and PPh3, n = 1) as the catalytically active species and three sequential reactions: (1) olefin insertion into the Pd–H bond to yield Pd–alkyl species, (2) CO insertion into the Pd–C bond to generate Pd–acyl intermediates, and (3) the methanolysis of Pd–acyl species to produce the corresponding methyl esters, regenerate the active species and restart the cycle; the last reaction is considered the rate-determining step (rds) of the mechanism.
Graphical Abstract
Systems prepared in situ by addition of triphenylphosphine to palladium dichloride in the presence of para-toluenesulfonic acid (TSA), PdCl2/nPPh3/mTSA were used as precatalysts for the olefin carbonylation with alcohols (MeOH, EtOH, n-PrOH and i-PrOH) to generate the corresponding esters (hydroalcoxycarbonylation), under mild reaction conditions. For 1-hexene hydromethoxycarbonylation, the most active system was PdCl2/6PPh3/5TSA at P(CO) = 50 atm and T = 125 ºC. Kinetic, coordination chemistry and computational DFT studies, allow us to propose a catalytic cycle involving species of the type [Pd(H)(L)(PPh3)2]+n (L = Cl, n = 0; L = CO, MeOH, olefin, n = 1) or [Pd(H)(PPh3)3]+ as the catalytically active species.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbonylation of unsaturated substrates in presence of alcohols (hydroalcoxycarbonylation), one of the Reppe reactions, is a promising clean method for esters synthesis, which are valuable products as solvents and for fine chemistry, mainly as flavorings and perfumes; when methanol is used as the nucleophile, the reaction is called hydromethoxycarbonylation [1]. Among the precatalysts employed for this reaction, palladium/triphenylphosphine systems promoted with strong acids are of special interest; the protonic acid stabilizes the catalyst and besides, accelerates the reaction by forming the active metal-hydride species [2]. Kiss [3], Toniolo and coauthors [4] and Beller et al. [5] have published comprehensive reviews dealing with recent advances in palladium-catalyzed olefin hydroalcoxycarbonylation, including some aspect of the reaction kinetics. In other studies, the use of alternative sources for the formation of metal-hydride complexes (water, hydrogen and acids) [6, 7], the effect of the oxidation state of the palladium center on the catalyst activity [8], as well as on the kinetic and mechanistic studies of these reactions [9–16] have been performed. Most of research works initially reported have involved the influence of mono- and bidentatephosphines on the activity and regioselectivity of palladium systems of this reaction [17–21], including modified precatalysts containing phosphorous ligands with the metallocene fragment [22–29]; the effect of the nature of diphosphine on the formation of either esters or polyketones in the palladium-ethylene carbonylation have been also reported [4, 30, 31].
Recently reported papers involve the use of new mono- and bidentate phosphines for the hydroalcoxycarbonylation reactions. Aguirre et al. [32] reported the use of palladium precatalysts with P,N ligands [2-(diphenylphosphinoamino)pyridine (Ph2PNHpy), 2-(diphenylphosphinomethyl)pyridine (Ph2PCH2py) and 2-(diphenylphosphino)quinoline], in the presence of para-toluenesulfonic acid (TSA) for the hydromethoxycarbonylation of cyclohexene, 1-hexene and styrene; the complex [PdCl(PPh3)(Ph2NHpy)]Cl and the equimolar mixture of PdCl2(Ph2NHpy) and PPh3 demonstrated to be the most active systems for these reactions. Some of these authors also reported that palladium complexes containing the 1,1-bis(diphenylphosphino)ferrocene (dppf) ligand showed higher catalytic activity than those having the naphthyridine ligand in its structure for the olefin hydromethoxycarbonylation; again, the addition of PPh3 to these systems increased the activity and selectivity of these precatalysts [33]. Subsequently, Klahn and coauthors [34] used a diphenylphosphinocyrhetrene palladium complex in the styrene hydromethoxycarbonylation; trans-[(η5-C5H4PPh2)Re(CO)3]PdCl2(NCMe) in the presence of PPh3 (ratio = 1:2) and HCl as acidic promoter, showed good catalytic activity and excellent regioselectivity towards the branched products.
Claver and coauthors [35] reported the use of palladium complexes containing novel cis-1,2-bis(di-t-butyl-phosphinomethyl) carbocyclic ligands as catalytic precursors for the ethylene hydromethoxycarbonylation; some aspect of catalytic cycle have been elucidated from HP NMR spectroscopic study.
On the other hand, Pringle and coauthors [36, 37] reported the efficient and chemoselective ethylene hydromethoxycarbonylation by using of a series of Pd-complexes containing heterodiphosphines of the type o-Ph(CH2PR2)(CH2PR′2); the complexes containing the bulkiest ligands showed the highest activity.
Finally, Toniolo and coauthors [38–40] have recently published a series of new triphenylphosphine modified palladium precatalysts for the ethylene hydromethoxycarbonylation as well as the characterization, reactivity and roles of some intermediates in the catalytic cycle.
In spite of the importance of the olefin hydroalcoxycarbonylation, only a few studies on the kinetics of these reactions have been reported [3, 7, 9–15], from which only two are related to hydromethoxycarbonylation reactions [7, 14]. In the present work, we report an exhaustive study of the olefin hydroalcoxycarbonylation (olefin = 1-hexene, cyclohexene and styrene; alcohol = MeOH, EtOH, n-PrOH, i-PrOH) catalyzed by palladium systems containing triphenylphosphine, including detailed kinetic, coordination chemistry and computational DFT studies on the 1-hexene hydrometoxycarbonylation.
2 Experimental
All manipulations and reactions were conducted with rigorous exclusion of air. TSA was purified by recrystallization and dried under vacuo, whereas 1-hexene, styrene, cyclohexene and solvents were dried over the corresponding reagents and distilled at reduced pressure prior to use [41]. The IR spectra of the complexes (in KBr disks) were recorded on a Shimadzu 8300 FT-IR instrument. 1H-, 13C{1H}- and 31P{1H}-NMR spectra were recorded on a Bruker AM-300 spectrometer; chemical shift are expressed in ppm upfield from Me4Si and H3PO4, respectively.
2.1 Catalytic Reactions and Kinetic Study
The catalytic reactions including those corresponding to the kinetic study were carried out in a high pressure reactor (125 mL), supplied by Parr Instruments (model 4561), which was provided with arrangements for sampling of liquid contents, automatic temperature and pressure control as well as variable stirring speed. In a typical run, a solution of PdCl2, the corresponding equivalents of triphenylphosphine and TSA, the substrate, the alcohol, isooctane (7.5 mmol) as the internal standard and toluene were added to the reactor. The solution was carefully deoxygenated with argon, and the reactor charged with CO (5–50 atm) and heated to a pre-set temperature. At the end of the reaction, the catalytic mixtures were analyzed by gas chromatography, using a 3300 Series VARIAN instrument fitted with a flame ionization detector (FID) and a 2 m 20 % SP-2100 on a 0.1 % carbowax 100/120 Supelcoport column, using N2 as carrier gas. Products were quantified with a microcomputer coupled to Measurement Computing interphase using the Cromat 1.2 software; the organic products were additionally identified by GC/MS HP 5890/5971 coupled system using a Quadrex PONA 5 % phenyl methyl silicone, 25 m, 320 μm column. Each reaction was repeated at least twice in order to assure there producibility of the results. Statistical studies were performed using analysis of variance (ANOVA) to test the difference between means. Significant differences were considered when P < 0.05.
2.2 Kinetic Calculations
The percentage of conversion was restricted to 5–10 % in order to calculate the initial rate (r o) of the reaction [42]. The data were plotted as molar concentration of the products versus time yielding straight lines, which were fitted by conventional linear regression programs (r2 > 0.95); r o values were obtained from the corresponding slopes. The dissolved CO concentrations in the reaction medium were calculated from solubility data reported elsewhere [43].
2.3 Coordination Chemistry Related with Olefin Hydromethoxycarbonylation
The coordination chemistry studies related with the olefin hydromethoxycarbonylation reactions were performed by the reaction of PdCl2/6PPh3 or a palladium triphenylphosphine complex with one or several of the reaction components (TSA, olefin, methanol and/or carbon monoxide), either in a high pressure reactor or in an NMR tube. The reaction was monitored by 1H, 31P{1H} and/or 13C{1H} NMR.
2.3.1 Interaction of PdCl2/6PPh3 with Carbon Monoxide
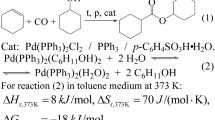
In a reactor, PdCl2 (89 mg, 0.5 mmol) and an excess of triphenylphosphine (0.8 g, 3 mmol) were placed in toluene (10 mL) under a carbon monoxide pressure of 10 atm; the reaction mixture was heated at 125 °C. After 4 h, the solution was transferred to a Schlenk tube and the solvent was evaporated at reduced pressure. The obtained yellow solid was thereafter characterized as PdCl2(PPh3)2 [44]. Yield = 50 %. 1H NMR (CDCl3, 300 MHz, ppm): 7.7 and 7.4 (series of m); 31P{1H} NMR (CDCl3, 121 MHz, ppm), 24.5 (s).
2.3.2 Interaction of PdCl2/6PPh3/5TSA with Olefins in Presence of Carbon monoxide
These reactions were carried out through the procedure described in the prior section adding five equivalents of TSA (2.5 mmol) and either 1-hexene or cyclohexene (1 mL) obtaining yellow oils, which were characterized by NMR.
2.3.3 Interaction of PdCl2(PPh3)2 with the Other Components of the Catalytic Reactions
In an NMR tube, complex PdCl2(PPh3)2 (10 mg) was dissolved in d 6-benzene (0.5 mL) and the reaction mixture was heated at 100 °C for 30 min; NMR spectra were acquired. In a series of different experiments, to this solution were added the components of the catalytic reaction one by one and consecutively (TSA, substrate and methanol) in different order of addition each time. After the addition of each component, the tube was heated at 100 °C by 30 min and NMR spectra were acquired before the addition of the following component.
2.4 Computational Details
The optimized energy calculations for all palladium intermediates involved in olefins hydromethoxycarbonylation process were carried out using simplified PH3 analogues and ethylene as the substrate in order to reduce the computational cost. These calculations were performed with the Gaussian 2003 (G03) computational package [45]. The unconstrained geometry optimizations and frequency calculations were performed at the hybrid functional B3LYP [46–48] combined with the effective core potential (ECP) LanL2DZ for the Pd, P and Cl atoms, and the extended basis set 6-31+G(d,p) for the C, H and O atoms [49–52].
3 Results and Discussion
The carbonylation of olefins with alcohols (hydroalcoxycarbonylation) was studied using palladium systems containing triphenylphosphine as precatalysts (olefin = 1-hexene, styrene and cyclohexene; alcohol = MeOH, EtOH, n-PrOH and i-PrOH); linear and branched esters were obtained in the case of 1-hexene and styrene (Eq. 1, R = n-Bu, Ph), whereas in the cyclohexene carbonylation only one product was obtained (Eq. 2). The catalytic systems were prepared in situ by addition of n equivalents of triphenylphosphine to palladium dichloride in the presence of m equivalents of para-TSA, PdCl2/nPPh3/mTSA, n and m varying in the range of 2–10.


3.1 Hydromethoxycarbonylation of Olefins
Preliminary tests of the carbonylation reactions were carried out using 1-hexene and methanol (1-hexene hydromethoxycarbonylation). As PPh3 and TSA concentrations are important factors in the catalytic processes, reactions were carried out at several [Pd]:[PPh3] and [Pd]:[TSA] ratios. All of these systems were efficient and regioselective precatalysts for the 1-hexene hydromethoxycarbonylation (Eq. 1, R = C4H9, R′ = Me) to give methyl heptanoate (heptanoic acid methyl ester) and methyl 2-methylhexanoate (2-methylhexanoic acid methyl ester); results are shown in Table 1.
The increase of either acid (entries 1–3) or PPh3 concentrations (entries 2 and 4–7) had a positive effect on the activity up to a threshold value above which the inhibition of the reaction was observed; the best [Pd]:[TSA] and [Pd]:[PPh3] ratios were 1:5 and 1:6, respectively (entry 2). Regarding the effect of temperature, the activity of the system PdCl2/6PPh3/5TSA was four times lower at 100 °C (entry 8) than at 125 °C (entry 2), whereas at temperatures above 125 °C, this system was not catalytically active possibly due to the formation of palladium metal. We further examined the effect of acid nature on the activity and selectivity of PdCl2/6PPh3/5HX systems (entries 2, 9 and 10). The order of activity was H2SO4 < HCl < TSA, which is consistent with the strength of the acid; the sulfuric acid has the highest acidity and the system shows the lowest activity whereas the TSA is the weakest acid and therefore the catalytic system presents the best activity; in all cases, the l/b ratio was close to the unit. The higher activity of TSA with regard to HCl and H2SO4 may be also explained due to the formation in the reaction medium of different palladium complexes, such as Pd(TS)2(PPh3)2, Pd(SO4)(PPh3)2 or PdCl2(PPh3)2, and to the presence of an ion-pair with TSA [6, 7, 14, 39].
For comparison, the hydromethoxycarbonylation of other olefins (styrene and cyclohexene) were also studied using the PdCl2/6PPh3/5TSA system as precatalyst, although this ratio may depend on the substrate nature; these carbonylations proceeded even under lower reaction conditions: [Pd] = 1.67 mM; [MeOH] = 1.25 M; [substrate]:[catalyst] ratio = 300:1; P(CO) = 8 atm. This palladium system was an efficient precatalyst for the hydromethoxycarbonylation of all substrates; the linear olefin (1-hexene) was carbonylated three times faster (TOF = 143 h−1) than styrene (TOF = 44 h−1), whereas the cyclic olefin (cyclohexene) showed the lowest activity (TOF = 10 h−1). Under these conditions, the regioselectivity of this system was higher towards the linear product for 1-hexene (l/b = 2.4) and towards the branched product for styrene (l/b = 0.4).
3.2 Kinetics Investigation of the 1-Hexene Hydromethoxycarbonylation
The kinetic study of the 1-hexene hydromethoxycarbonylation catalyzed by PdCl2/6PPh3/5TSA, the most active system, was performed using toluene as the solvent at 398 K. A typical reaction profile is presented in Fig. 1, which shows the decrease of the substrate (1-hexene) concentration and the increase of the corresponding products (methyl heptanoate and methyl 2-methylhexanoate) as a function of time. In all of reactions, there is an induction period (20–30 min) which was necessary for the formation of catalytically active species.
In order to study the effect of each component on the reaction rate, four experiment sets were carried out varying the concentration of only one of the component within each set while keeping constant the concentration of the other components at 398 K. The concentration ranges studied were: [Pd] = 0.75–2.11 mM, [1-hexene] = 0.28–0.60 M, [MeOH] = 0.49–1.54 M; [CO] = 1.9 × 10−2–0.118 M. The results are summarized in Table 2; the standard conditions are depicted in entry 2. Plots of log r o versus the logs of the concentration of each component are shown in Fig. 2. The kinetic results indicate that:
-
1.
The dependence of the reaction rate on the concentration of the catalyst (entries 1–4) is close to one as the graph of the log r o versus log [Pd] yields a straight line with a slope of 0.92. This finding is consistent with a mononuclear complex as the catalytically active species. Aver’yanov et al. [14] reported a fractional order (0.5) on catalyst concentration for cyclohexene hydromethoxycarbonylation catalyzed by a Pd/PPh3 system; however, these authors did not give any explanation about this finding.
-
2.
The initial carbonylation reaction varies according to a saturation curve with increasing 1-hexene concentration. When the 1-hexene concentration is lower than 0.38 (entries 2, 5 and 6), the reaction is close to first order, whereas the reaction rate does not depend on the 1-hexene at concentrations higher than 0.54 (entries 9 and 10). This result is consistent with the substrate involved in a pre-equilibrium prior to the rate-determining step (rds) of the cycle, which is displaced to the right at high substrate concentrations. Benedek et al. [53] reported that the alkyl intermediate formed in the deuteroalkoxycarbonylation of 1-hexene catalyzed by PdCl2(PPh3)2 undergo reversible β-elimination even at low reaction temperature.
-
3.
The dependence of the initial rate with respect to the dissolved carbon monoxide concentration (entries 2 and 10–12) was of fractional order; the graph of log r o versus log [CO] yields a straight line, whose slope was 0.7. This order indicates that CO is also involved in a reversible reaction prior to the rds of the mechanism.
-
4.
The initial reaction rate shows a direct dependence as a function of the methanol concentration (entries 2 and 14–17), thus indicating a first order dependence with respect to this parameter. Consistent with other reports [7–14], this finding may be explained if methanol is directly involved in the rds of the catalytic cycle.
-
5.
A decreasing in reaction rate was observed when the reaction was carried out in d 4-MeOH (1-hexene deuteromethoxycarbonylation) instead of MeOH (entry 17); a primary kinetic isotope effect (KIE) of 2.9 was found, thus confirming that methanol is involved in the rds of the reaction.
-
6.
The linear to branched ratio (l/b) of the esters formed by the use of palladium precursor varied from 1.5 to 3.6. A negative effect on l/b ratios was observed with the increment of dissolved CO and methanol concentrations and a positive effect with the increase of catalyst concentration; no substantial changes in regioselectivity with the variation of substrate concentration were observed. Similar results have been reported previously for the hydroformylation of olefins with Rh/PPh3 [54] and Rh/AsPh3 [55] systems.
In view of the previous observations, the 1-hexene hydromethoxycarbonylation catalyzed by PdCl2/6PPh3/5TSA system proceeds according to the rate law:
Where a, b, c and d are constants, rate constants, equilibrium constants and/or their products.
Our findings considerably differ from kinetic studies performed by other authors. In his review, Kiss [3] described that kinetics of palladium-catalyzed olefin hydrocarboxylation, a reaction related to hydroalcoxycarbonylation, is of first order on palladium catalyst, varies between first and zero order on substrate, while for water (the nucleophile) and CO, the order is greater than one. On the other hand, for the cyclohexene hydromethoxycarbonylation, Toniolo and coauthors [7] reported for the Pd(TS)2(PPh3)2-catalyzed reaction (TS is the anion para-toluenesulfonate), that the reaction rate is of first order on catalyst, substrate and methanol and passes through a maximum with increasing of the CO pressure, whereas Aver’yanov et al. [14] reported for the PdCl2(PPh3)2/PPh3/TSA-catalyzed reaction that it is of fractional order on catalyst (0.5), first order on cyclohexene and passes through maxima for CO and methanol. All of these differences may be explained by the variety of reaction conditions employed as well as the nature of the solvent used in each experimental kinetic study. The simplicity of our findings may stem from the performance of the kinetic studies under the better [PPh3]:[Pd] and [TSA]:[Pd] ratios and the use of toluene as a non-coordinating solvent.
3.3 Hydroalcoxycarbonylation of 1-Hexene
The carbonylation of 1-hexene was carried out using other alcohols (EtOH, n-PrOH and i-PrOH) as nucleophiles (1-hexene hydroalcoxycarbonylation); the results are listed in Table 3. As is depicted (entries 1–3, Table 3), the values of TOF (turnover frequency, moles of products per mole of catalyst in 1 h of reaction) and r o (initial reaction rate) increased with the increment of carbon length of linear aliphatic alcohols such as methanol, ethanol and n-propanol, thus reflecting an electronic effect (inductive effect). Hence, the reaction with n-propanol was the fastest, followed closely by ethanol while methanol was the slowest one. On the other hand, increasing the bulkiness of the alcohol slightly retarded the reaction: the reaction rate with isopropanol (entry 4) was lower than with n-propanol (entry 3). These results are, together with the first order kinetics on MeOH concentration and the values of KIE, in concordance with the alcoholysis as the rds of the hydroalcoxycarbonylation reaction.
3.4 Coordination Chemistry Related with Olefin Hydromethoxycarbonylation
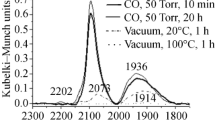
In order to gain further understanding on the mechanism of the olefin hydrometoxycarbonylation catalyzed by the PdCl2/6PPh3/5TSA system, particularly to isolate and/or detect some intermediates of the reaction, the interaction of PdCl2 with each component of the catalytic mixture was carried out separately (triphenylphosphine, TSA, olefin, methanol and carbon monoxide); results are displayed in Fig. 3.
The reaction of PdCl2 with six equivalents of triphenylphosphine in toluene at 125 °C under 10 atm of CO yielded a yellow precipitate. The FT-IR spectrum of this complex showed no bands corresponding to ν(CO). The 1H NMR spectrum showed only two multiplets at 7.7 and 7.3 ppm corresponding to phenyl groups of the triphenylphosphine, whereas the 31P{1H} NMR showed a singlet at 24.5 ppm. These spectroscopic data are consistent with the formula PdCl2(PPh3)2 (1); this complex was also prepared by reaction of PdCl2 with concentrated HCl to generate H2[PdCl4], thereafter followed by the addition of PPh3 [44]. On the other hand, this reaction carried out in the presence of Zn, or the reaction of complex 1 with an excess of four equivalents of PPh3 in presence of Zn, yielded a mixture of Pd(PPh3)4 (2), complex 1, triphenylphosphine oxide and free PPh3, as corroborated by the 31P{1H} NMR spectrum. The reaction of 1 or 2 with excess of TSA did not yield the corresponding metal-hydride complexes, which was indicative of hydride species too unstable to be isolated from the reaction medium.
When the reaction of PdCl2/6PPh3/5TSA (or 1/4PPh3/5TSA) with CO was carried out adding an excess of olefin (1-hexene or cyclohexene), hard-to-handle bright yellow oils were obtained, which could not be isolated as solids. 1H, 31P{1H} and 13C{1H} NMR spectra of these oils indicated the presence of several species, thus precluding their full elucidation. However, the 13C{1H} NMR spectra showed two signals at 179 and 182 ppm, which can be possibly attributed to the presence of two acyl type species, [Pd–C(O)(CH2)5CH3 and Pd–C(O)CH(CH3)(CH2)3CH3] for 1-hexene, and one signal at 179 for the reaction with cyclohexene [Pd–C(O)(c-C6H11)]. Similar types of acyl complexes have been reported to be isolated from the palladium-catalyzed hydroalcoxycarbonylation of olefins. Toniolo and coauthors [56] have reported the isolation of complex trans-PdCl(C(O)(CH2)5CH3)(PPh3)2 in the hydroalcoxycarbonylation of 1-hexene, as well as the reactivity studies of this type of Pd–acyl complexes, which is relevant to the catalytic cycle [57]; more recently, some of them reported the isolation and characterization of trans-PdCl(C(O)CH2CH3)(PPh3)2 [39].
The reactions of complex 1 with each component of the catalytic reaction were also performed in an NMR tube using d 8-toluene as the solvent at 100 °C. The only reaction that yielded a new species was the reaction of 1 with MeOH in the presence of 4 equivalents of PPh3. After 20 h, approximately 50 % of complex 1 was transformed into complex 2; in this reaction, formaldehyde was also detected by GC. This finding may be explained through the Eq. 4 as proposed by Toniolo and coauthors [6, 7] and Aver’yanov et al. [14]. Formaldehyde in the presence of methanol yields hemiacetal or acetal but small amounts of water may hydrolyze these products back to the original compounds (CH2O and MeOH). When isopropanol is used instead of MeOH, small amounts of acetone were also detected [59].
Based on this coordination chemistry results, we think that under the reaction conditions, complex 1 is transformed into a palladium(0) species of the type Pd(L)(PPh3)2, which contains some other neutral ligand (L = methanol, olefin, CO or PPh3), which are protonated by TSA to form the corresponding cationic hydrido-palladium species of the type [Pd(H)(L)(PPh3)2]+. Neutral complexes of the type Pd(L)(PPh3)2 with L = CO, PR3 [17] or olefin [17, 58] and the corresponding cationic hydrido species containing MeOH [35, 59] or a monophosphine ligand [17, 40, 60] have been isolated. This notion was further supported by the fact that when the catalysis was performed in the presence of Zn (conditions as in entry 2 of Table 2), the reaction took place faster (7.85 × 10−6 M s−1) than in its absence (5.56 × 10−6 M s−1), although the TOF values were rather similar (123 and 133 h−1, respectively). Although, we suggest that the active species were cationic hydride complexes, the neutral species PdHCl(PPh3)2, also generated from complex 1, could not be discarded. Another possibility is that under the catalytic conditions, the palladium hydride intermediate could also obtain directly from PdCl2(PPh3)2 and MeOH via β-hydride elimination from a molecule of alcohol coordinate to the palladium center as reported by Clegg et al. [59]; in this case, TSA could act as a stabilizer of the Pd–H species against deprotonation to generate Pd(0) species.
3.5 Proposed Mechanism for Olefin Hydroalcoxycarbonylation
On the basis of our experimental findings (kinetic and coordination chemistry studies), of some studies using HP NMR spectroscopy performed for palladium complexes containing triphenylphosphine [38–40] and diphosphine ligands [35], and the present knowledge on palladium-catalyzed olefin hydroalcoxycarbonylation [3–5], we propose a catalytic cycle depicted in Fig. 4 for the linear ester (branched ester excluded for clarity); experimental evidence is more in favor of the so-called “hydride mechanism”. Complexes of the type [Pd(H)(L)(PPh3)2]+n (A), L = Cl, n = 0; L = CO, MeOH, olefin, n = 1, in a cis configuration or [Pd(H)(PPh3)3]+ are considered the possible catalytically active species (although trans species are also possible, vide infra). Zudin et al. [60] elucidated that [Pd(H)(PPh3)3]+ was the key palladium intermediate for the hydrocarbonylation of ethylene to diethyl ketone, a reaction also related to the hydroalcoxycarbonylation of olefins.
The first step of the cycle is the reversible insertion of the olefin in the Pd–H bond of A (K 1 ) to yield the Pd–alkyl species [Pd(C6H13)(L)(PPh3)2]+n (B). The following step is the CO insertion into the Pd–C bond of B to generate the Pd–acyl intermediate [Pd(C(O)C6H13)(L)(PPh3)2]+n (C) according to the equilibrium K 2 ; intermediates B and C are possibly generated through pentacoordinated intermediates containing olefin and CO ligands, respectively. The cycle is completed by the reaction of Pd–acyl species C with methanol (methanolysis) to yield the methyl esters, regenerate the active species and restart the cycle; this reaction is considered the rds of the mechanism and could occur through an intra- or intermolecular attack of methanol. van Leeuwen et al. [61] and Liu et al. [62] established that the alcoholysis of acetyl intermediates in diphosphine palladium systems is thought to be an intramolecular nucleophilic attack of alcohol at the acyl group; however, Donald et al. [63], using theoretical DFT calculations, proposed that the reaction is intermolecular for diphosphine palladium systems. We believe that the reaction catalyzed by the triphenylphosphine palladium system could proceed through either a pentacoordinated species with the alcohol bonded to the palladium metal center and then an hexacoordinated intermediate resulting from the oxidative addition of the methanol (intramolecular pathway) or an external nucleophilic attack of alcohol on the acyl carbon atom (intermolecular pathway).
According to this mechanism, the rate law for 1-hexene hydromethoxycarbonylation catalyzed by Pd/triphenylphosphine system may be derived by applying the Equilibrium Approximation. As methanolysis of Pd–acyl species is considered the rds, the reaction rate may be expressed as:
Considering the equilibria K 1 ([B] = K 1 [A][olefin]) and K 2 ([C] = K 2 [B][CO]), the concentration of C ([C] = K 1 K 2 [A][olefin][CO]) can be substituted in Eq. 4 and the rate expression may be rewritten as:
Taking into consideration the mass balance of palladium ([Pd]o = A + B + C), Eq. 6 may be transformed in:
This equation is similar to the rate law obtained in the kinetic study, where a = K 1 K 2 k 3 , b = 1, c = K 1 and d = K 1 K 2 [olefin], and explains the fractional order obtained on CO concentration and the saturation curve observed for [olefin].
At low substrate concentration (K 1 [olefin] + K 1 K 2 [olefin][CO] ≪ 1), Eq. 7 is transformed to Eq. 6 (first order on each component), whereas at high [olefin], K 1 [olefin] + K 1 K 2 [olefin][CO] ≫ 1, therefore, Eq. 6 can be rewritten as:
which explains the zero order with respect to olefin when its concentration was higher than 0.54 M; under these conditions the order on CO concentration is fractional and it is first order on catalyst and MeOH concentrations.
Similar mechanisms should operate when the reaction of 1-hexene carbonylation is performed in presence of other alcohols (hydroalcoxycarbonylation), such as EtOH, n-propanol and i-PrOH, instead of methanol.
3.6 DFT-Calculations of the Elemental Steps of the Olefin Hydromethoxycarbonylation Mechanisms
In spite of its industrial importance, the olefin hydromethoxycarbonylation had not received much attention from the molecular modeling point of view. Donald et al. [63] investigated the methanolysis of a Pd–acyl species, an elementary reaction which is considered the rds in our mechanism, through density functional theory; calculations suggest that intermolecular attack of methanol may be important in the methanolysis of simple Pd–acyl systems and that the energetics of this process are strongly dependent on the metal coordination environment.
In order to reinforce the mechanisms of the reaction catalyzed by the PdCl2/6PPh3/5TsOH system which was proposed on the basis of our experimental findings, the Gibbs free energy (∆G°) for the three possible elementary steps (olefin insertion, CO insertion and methanolysis) were calculated via DFT. We use ethylene as a model for the substrate and the simplified PH3 model, in which the phenyl groups of the original ligand were replaced by hydrogen. In order to test our model-methodology, we calculated the structural parameters of the optimized structure of PdCl2(PH3)2 and compared it with experimental studies (Fergunson) of X-ray structure for the complex PdCl2(PPh3)2 [64]. The obtained bond distances and angles for PdCl2(PH3)2 were in good agreement with the experimental findings (Table 4). The agreement between the calculated structural parameters and the experimental values (less than 5 % error) showed that the hybrid method used in this work can provide reliable geometries for the species involved along the entire catalytic cycle.
The calculation were performed starting from five different active species, [Pd(H)(L)(PH3)2]+n (L = Cl, n = 0; L = CO, MeOH or olefin, n = 1) both in a cis or trans configuration and [Pd(H)(PH3)3]+. Calculations were carried out at 398 K and 8 atm in order to simulate the experimental conditions; the results are listed in Table 5.
As may be observed, the three elementary step of the cycle are thermodynamically favored (ΔG is negative) for[Pd(H)(PH3)3]+ and for both configurations (cis and trans) of [Pd(H)(L)(PH3)2]+n (L = Cl, n = 0; L = CO, MeOH or olefin, n = 1), except the step of CO insertion on the Pd–alkyl species containing ethylene ligand in a trans configuration, for which a positive ΔG was obtained. In general, the elementary steps involving species in cis configuration were somewhat more spontaneous than those involving trans ones. The obtained theoretical results indicate that the catalytic cycle may be initiated by any of the [Pd(H)(L)(PH3)2]+n species; however, we believe that the species [Pd(H)(MeOH)(PPh3)2]+and [Pd(H)(PPh3)3]+ may be the most probably catalytically active species participating in the catalytic cycle since [Pd(H)(MeOH)(diphosphine)]+ [59] and [Pd(H)(PPh3)3]+ [60] have been isolated in prior works.
More detailed DFT theoretical calculations are in progress in order to explore the potential energy surfaces (PES) that connect to these step sequences of the catalytic process (transition states), aimed at better understanding each step of the catalytic cycle.
4 Conclusions
The PdCl2/6PPh3/5TSA system (125 °C, 8–50 bar of CO) was an efficient and regioselective precatalyst for the hydromethoxycarbonylation of olefins (1-hexene, cyclohexene and styrene) to generate the corresponding methyl esters, under mild reaction conditions; the order of individual activities was 1-hexene > styrene > cyclohexene. A kinetic study of the 1-hexene hydromethoxycarbonylation catalyzed by PdCl2/6PPh3/5TSA together with coordination chemistry and computational DFT studies allowed us to propose a catalytic cycle involving species of the type [Pd(H)(L)(PPh3)2]+n (L = Cl, n = 0; L = CO, MeOH, olefin or PPh3, n = 1) as the catalytically active species and three sequential reactions: olefin insertion in the Pd–H bond, CO insertion into the Pd–C bond and the methanolysis of Pd–acyl species to yield the products, regenerate the active species and restart the cycle, being the last reaction the rds of the mechanisms.
References
Van Leeuwen PWNM (2004) Homogeneous catalysis. Kluwer Academic Publisher, Dordrecht, p 407
Chepaikin EG, Bezruchenko AP, Suerbaev KA, Shalmagambetov KM (2006) Pet Chem 46:117
Kiss G (2001) Chem Rev 101:3435
Cavinato G, Toniolo L, Vavasori A (2006) Top Organomet Chem 18:125
Brennführer A, Neumann H, Beller M (2009) ChemCatChem 1:28
Vavasori A, Cavinato G, Toniolo L (2001) J Mol Catal A 176:11
Vavasori A, Toniolo L, Cavinato G (2003) J Mol Catal A 191:9
Temkin ON, Bruk LG (2003) Kinet Catal 44:661
Noskov YG, Simonov AI, Petrov ES (2000) Kinet Catal 41:564
Kron TE, Terekhova MI, Noskov YG, Petrov ES (2001) Kinet Catal 42:204
Noskov YG, Petrov ES (2001) Russ Chem Bull (Int Ed) 50:1839
Kron TE, Terekhova YG, Petrov ES (2004) Kinet Catal 45:551
Aver’yanov VA, Sevost’yanova NT, Batashev SA, Nosova NM (2006) Kinet Catal 46:405
Aver’yanov VA, Batashev SA, Sevost’yanova NT, Nosova NM (2006) Kinet Catal 47:375
Averyanov VA, Nosova NM, Astashina EV, Sevostyanova NT (2007) Kinet Catal 47:167
Seayad A, Jayasree S, Damodaran K, Toniolo L, Chaudhari RV (2000) J Organomet Chem 601:100
Pérez PJ, Calabrese JC, Brunel EE (2001) Organometallics 20:337
Del Rio I, Ruiz N, Claver C, van der Veen LA, van Leeuwen PWNM (2000) J Mol Catal A 161:39
Muñoz B, Marinetti A, Ruiz A, Castillon S, Claver C (2005) Inorg Chem Commun 8:1113
Guiu E, Caporali M, Muñoz B, Müller C, Lutz M, Spek AL, Claver C, van Leeuwen PWNM (2006) Organometallics 25:3102
Muñoz BK, Santos García E, Godard C, Zangrando E, Bo C, Ruiz A, Claver C (2008) Eur J Inorg Chem 2008:4625
Gusev OV, Kalsin AM, Peterleitner MG, Akhmedov NG, Bianchini C, Meli A, Oberhauser W (2002) Organometallics 21:3637
Gusev OV, Kalsin AM, Petrovskii PV, Lyssenko KA, Oprunenko YF, Bianchini C, Meli A, Oberhauser W (2003) Organometallics 22:913
Bianchini C, Meli A, Oberhauser W, van Leeuwen PWNM, Zuideveld MA, Freixa Z, Kamer PCJ, Spek AL, Gusev OV, Kalsin AM (2003) Organometallics 22:2409
Bianchini C, Meli A, Oberhauser W, Parisel S, Gusev OV, Kalsin AM, Vologdin NV, Dolgushin FM (2004) J Mol Catal A 224:35
Bianchini C, Meli A, Oberhauser W, Parisel S, Passaglia E, Ciardelli FW, Gusev OV, Kalsin AM, Vologdin NV (2005) Organometallics 24:1018
Kalsin AM, Vologdin NV, Peganova TA, Petrovskii PV, Lyssenko KA, Dogulshin FM, Gusev OV (2006) J Organomet Chem 691:921
Godard C, Ruiz A, Claver C (2006) Helv Chim Acta 89:1610
Diab L, Gouygou M, Manoury E, Kalck P, Urrutigoïty M (2008) Tetrahedron Lett 49:5186
Liu J, Heaton BT, Iggo JA, Whyman R, Bickley JF, Steiner A (2006) Chem Eur J 12:4417
Zuldema E, Bo C, van Leeuwen PWNM (2007) J Am Chem Soc 129:3989
Aguirre PA, Lagos CA, Moya SA, Zuñiga C, Veraoyarce C, Sola E, Peris G, Bayón JC (2007) Dalton Trans 46:5419
Zuñiga C, Moya SA, Aguirre PA (2009) Catal Lett 130:373
Zuñiga C, Sierra D, Oyarzo J, Klahn H (2012) J Chil Chem Soc 57:1101
De la Fuente V, Waugh M, Eastham GR, Iggo JA, Castillón S, Claver C (2010) Chemistry 15:6919
Fanjul T, Eastham G, Floure J, Forrest SJK, Haddow MF, Hamilton A, Pringle PG, Orpen AC, Waugh M (2013) Dalton Trans 42:100
Fanjul T, Eastham G, Haddow MF, Hamilton A, Pringle PG, Orpen AC, Turner TPW, Waugh M (2012) Catal Sci Technol 2:937
Amadio E, Cavinato G, Dolmella A, Rochin L, Toniolo L, Vavasori A (2009) J Mol Catal A 298:103
Cavinato G, Facchetti S, Toniolo L (2010) J Mol Catal A 333:180
Amadio E, Cavinato G, Härter P, Toniolo L (2013) J Mol Catal A 745–746:115
Perrin D, Armarego WLF (1988) Purification of laboratory chemicals, 3rd edn. PergamonPress, Great Britain
Casado J, López-Quintela MA, Lorenzo-Barral FM (1986) J Chem Educ 63:450
Bhanage BM, Divekar S, Deshpande R, Chaudhari R (1997) J Mol Catal A 115:247
Habib M, Trujillo H, Alexander C, Storhoff B (1985) Inorg Chem 24:2344
Frisch MJ, Trucks GW, Schlegel HB (2003) GAUSSIAN 03 Revision B.04. Gaussian Inc., Pittsburgh
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Miehlich B, Savin A, Stoll H, Preuss H (1989) Chem Phys Lett 157:200
Collins JB, Schleyer PVR, Binkley JS, Pople JA (1967) J Chem Phys 64:5142
Dobbs KD, Hehre WJ (1987) J Comput Chem 8:880
Gordon MS (1980) Chem Phys Lett 76:163
Raghavachari K, Trucks GW (1989) J Chem Phys 91:1062
Benedek C, Gömöry A, Heil B, Törös S (2001) J Organomet Chem 622:112
Cavalieri d’Oro P, Raimondi G, Montrasi G, Gragorio G, Andreeta A (1991) J Chem Soc Chem Com 149:1096
Srivastava V, Sharma S, Shukla R, Subrahmanyam N, Jasra R (2005) Ind Eng Res 44:1764
Bardi R, Plazzesi AM, Del Pra A, Cavinato G, Toniolo L (1995) Inorg Chim Acta 102:99
Cavinato G, Toniolo L (1990) J Organomet Chem 398:187
De Peter JJM, Tromp DS, Tooke DM, Spek AL, Deelman B-J, van Koten G, Elsevier CJ (2005) Organometallics 24:6411
Clegg W, Eastham GR, Elsegood MRJ, Heaton BT, Iggo JA, Tooze RP, Whyman R, Zacchini (2002) J Chem Soc Dalton Trans 3300
Zudin VN, Chinakov VD, Nekipelov VM, Rogov VA, Likholobov VA, Yermakov YI (1989) J Mol Catal 52:27
van Leeuwen PWNM, Zuideveld MA, Swennenhuis BHG, Freixa Z, Kamer PCJ, Goubitz K, Fraanje Lutz M, Spek AL (2003) J Am Chem Soc 125:5523
Liu J, Heaton BT, Iggo JA, Whyman R (2004) Chem Commun 1326
Donald SMA, Mcgregor SA, Settels V, Cole-Hamilton DJ, Eastham GR (2007) ChemComm 562
Ferguson G, McCrindle R, McAlees A, Parvez M (1982) Acta Cryst B38:2679
Acknowledgments
Financial supports from ONCTI (Proyecto 2011001188) and CONDES-LUZ are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosales, M., Pacheco, I., Medina, J. et al. Kinetics and Mechanisms of Homogeneous Catalytic Reactions. Part 12. Hydroalcoxycarbonylation of 1-Hexene Using Palladium/Triphenylphosphine Systems as Catalyst Precursors. Catal Lett 144, 1717–1727 (2014). https://doi.org/10.1007/s10562-014-1335-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1335-0