Abstract
The one-pot dehydration/hydrolysis of mono- and disaccharides to 5-hydroxymethylfurfural (HMF) in the presence of several imidazolium ionic liquids was efficiently performed. The study aims to make a mechanistic insights on the direct transformation of sugars to HMF, including glucose, fructose, galactose, sucrose, maltose and lactose. With the catalyst of [NMP][HSO4], a HMF yield of 87 % was achieved from sucrose. In addition, [AMIM]Cl revealed a remarkable catalytic activity for the transformation of fructose to HMF without other catalyst or co-solvent and the yield of HMF was 91.1 %. Theoretical calculation results showed that [AMIM]Cl expressed a more efficient catalytic activity than [BMIM]Cl.
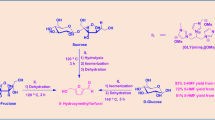
Graphical Abstract
Fructose could directly convert into HMF in [AMIM]Cl without catalyst. Behaviors of disaccharides are largely determined by their basic units. The mechanism to explain the activity of [AMIM]Cl has been confirmed by using DFT method.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, biomass is regarded as a potentially abundant and renewable source to produce energy, chemicals and materials, which are promising alternatives for the sustainable supply of liquid fuels and valuable intermediates (such as alcohols, aldehydes, ketones and carboxylic acids) to the chemical industry [1, 2]. Carbohydrates comprise the main class of biomass compounds and it requires efficient methods to convert them into a variety of chemical products [3]. Therefore, numerous of efforts have been devoted to the degradation of mono- and disaccharides into 5-hydroxymethylfurfural (HMF) or 5-ethoxymethylfurfural (EMF), the versatile and key intermediate both in biofuel chemistry and the petroleum industry [4, 5]. This bifunctional, six-carbon molecule can be easily converted into many useful derivatives, including a variety of polymers and fuels.
Various acidic catalysts have been employed for the formation of HMF from carbohydrates, such as mineral acids [6–8], metal salts [9–12], strong acid exchange resins [13, 14] and Amberlyst-15 [15]. These literatures mainly study the monosaccharide, including fructose and glucose. However, the catalysts used in these studies have a lot of defects: negative effect on the environment, excessive use of heavy metals or low yield of HMF. In 2007, a novel work reported by Zhang et al. revealed that glucose and fructose could convert into HMF in good yields by using a catalysis system based on CrCl2 in ionic liquids (ILs), particularly 1-ethyl-3-methylimidazoliumchloride ([EMIM]Cl) [16]. ILs are reported to be promising solvents for dissolving various carbohydrates, offering environmentally friendly benefits in comparison with hazardous volatile organic counter parts [17, 18]. Recently, He et al. [19] reported that the zero-valent Cr(CO)6-based catalyst system proved more effective for the conversion of glucose to HMF in [EMIM]Cl under 120 °C than the divalent CrCl2-based benchmark catalyst system, especially at low catalyst loadings. Kim et al. [20] reported that acidity modified silver exchanged silicotungstic acid (AgSTA) catalyst was excellent for the dehydration of fructose and sucrose in superheated water. As a result, 98 % conversion of fructose with 85.7 % HMF yield and 87.4 % HMF selectivity was achieved in 2 h reaction time at 120 °C.
However, despite these attractive advances in the synthesis of HMF from fructose [18, 21] and glucose, the conversion of some other mono- and disaccharides such as galactose, maltose and lactose, has not been studied extensively (Fig. 1). What’s more important, the mechanisms of fructose and galactose degradation to HMF in IL solvents are still not fully understood. Because of the interest on preparation of HMF from sugars, we choose to study the transformation of these mono- and disaccharides into HMF in ionic liquid of 1-allyl-3-methylimidazolium chloride ([AMIM]Cl, A is allyl). The ionic liquid of [AMIM]Cl has been demonstrated as an excellent solvent in dissolution of lignocellulosic biomass materials [22, 23]. Because [AMIM]Cl itself can be easily synthesized and recycled, and has good thermal stability when used as solvent. Besides, the allyl group in [AMIM]Cl makes it different from the rest of others, such as [EMIM]Cl, [BMIM]Cl and [OMIM]Cl, which is also beneficial on the conversion of carbohydrates [21].
In this work, we initially attempt to build several different catalytic systems by using 1-alkyl-3-methylimidazolium chloride or bromide as a solvent class. Various acidic ILs, including 1-methylimidazolium hydrogen sulfate ([MIM][HSO4]), N-methyl-2-pyrrolidonium hydrogen sulfate ([NMP][HSO4]), 1-(4-sulfonic acid)-propyl-3-methylimidazolium hydrogen sulfate ([C3SO3HMIM][HSO4]) were synthesized and employed as catalysts for the reaction. Our method is distinguished from previous reports in that we observe high yields of HMF from sucrose and fructose without adding any metal salts. In addition, the combination of [AMIM]Cl, acidic ILs and metal chloride (CoCl2) performed well in conversion of other sugars. Since Brønsted-acidic ILs [NMP][HSO4] and [C3SO3HMIM][HSO4] are efficient catalysts for the conversion of fructose or cellulose into HMF [24, 25], we hypothesize that these catalysts would also provide high yields of HMF from other mono- and disaccharides (Fig. 1). Our study showed that some, but not all mono- and disaccharides, can be transformed efficiently into HMF. Finally, we provide mechanistic insights into the role of [AMIM]Cl in HMF formation from fructose. We believe these efforts will provide a molecular level understanding of the reaction process for the IL-promoted conversion of fructose to HMF, and help us to design and develop a new catalytic system for conversion of sugars to HMF.
2 Experimental Section
2.1 Materials
Sucrose and other sugars used in this study are obtained commercially from Shanghai Chemical Factory (Shanghai, China). N-methylimidazole is obtained from Changzhou Chemical Factory (Jiangsu, China) and further purified by distillation. The ILs are obtained commercially from J&K Chemical Company and dried prior to use. Others are prepared according to the published reports [23–26]. Sulfuric acid (98 %) and other chemicals (AR) are commercially available and used without further purification unless otherwise stated.
2.2 Typical Procedure for Synthesis of Ionic Liquids
The preparation procedures of the ILs used in this study and characterization data were presented in supplementary data file.
2.3 Representative Procedure for Synthesis of HMF from Carbohydrates
The catalytic conversion of sucrose to HMF is carried out in a stainless steel autoclave with glass liner tube that is heated in the oil-bath. Typically, 0.1712 g sucrose (0.5 mmol) is added into 2 mL [AMIM]Cl solvent, followed by the addition of the desired catalyst and H2O at the reaction temperature. After the appointed reaction time, 0.1 mL samples are pipetted, quenched immediately with NaOH solution and diluted with deionized water (×100). The solution is centrifuged at 10,000 rpm for 10 min and the upper clear liquid is pipetted off and diluted with deionized water (×10). Any humins are removed prior to HPLC analysis. Only low levels of colored products, other than HMF, are detected by HPLC.
More experimental sections are presented in supplementary data file.
3 Results and Discussion
3.1 Examine Various Catalysts
The activity of various catalysts for the sucrose conversion in [AMIM]Cl was investigated and the results were presented in Table 1. From the results, we can see that [NMP][HSO4] and [C3SO3HMIM][HSO4] exhibited excellent activity in [AMIM]Cl, with the HMF yields of 82.3 % (entry 5) and 78.5 % (entry 6) at 120 °C for 1 h, respectively. These results were more competitive when compared to GeCl4 [27] and Amberlyst-15 [28], which the solvent was [BMIM]Cl and TEAB, respectively. Moreover, the Brønsted-acidic ILs commonly had more efficient activity than mineral acids and heteropolyacids, except [MIM][HSO4], which presented much lower acidity among these IL catalysts (Supplementary data, Table S1), only 36.6 % (entry 4) of HMF was obtained. The different yields of HMF revealed that both catalyst and solvent had great influence on sucrose conversion, especially the acidity and structure of catalyst, obviously, the catalytic system of [NMP][HSO4]–[AMIM]Cl met the requirements.
3.2 Degradation of Sucrose to HMF
We initially attempt to prepare HMF from sucrose directly and characterize the reactivity of sucrose with different conditions. It was found that the degradation of sucrose was performed well in [AMIM]Cl and [BMIM]Cl (Table 2). In the absence of H2O, the yield of HMF declined at 120 °C. For example, the addition of 0.1 mL H2O enabled HMF yields of 87.0 % (entry 3) in [AMIM]Cl for 1 h catalyzed by [NMP][HSO4], but it was only 82.2 % (entry 1) at the same conditions without addition of water. It is not difficult to understand, because sucrose is a typical disaccharide and a small amount of water can improve the hydrolysis process in the early stages of the degradation reaction. The kind of solvent seem to have a great impact on the yield of HMF, higher HMF yields were obtained in either [AMIM]Cl or [BMIM]Cl when compared to 1-alkyl-3-methylimidazolium bromide solvents (entries 5–6). In addition, when it came to test about the reactivity of [EMIM]Ac (entry 7), we further discovered that there was no HMF formed and nearly all sucrose changed to a kind of intractable gel. When reactions were carried out with different catalysts, HMF yields obtained from the same solvent did not change too much. It indicated that both the two catalysts used in this work were appropriate for the present reaction system.
3.3 Degradation of Maltose and Lactose to HMF
Maltose is another important disaccharide, we found the transformation of it into HMF to be challenging (Table 3). Only [AMIM]Cl acted as an efficient solvent resulting in the yield of HMF about 30 % (entry 6), which was far higher than that obtained in other solvents catalyzed by [NMP][HSO4] with the additive of CoCl2 at 140 °C for 1 h. Maltose almost can not convert into HMF in [BMIM]Cl (entry 2) according to our study. Slightly higher yields of HMF were obtained in [AMIM]Cl with the additive of MnCl2 (entries 4, 9), but CoCl2 enabled the better yields, the highest yield was 55.7 % (entry 12) catalyzed by [C3SO3HMIM][HSO4] at 140 °C for 0.5 h, indicating that [C3SO3HMIM][HSO4] performed better compared to [NMP][HSO4] catalyst, which revealed only 30.4 % (entry 6) yield of HMF in the same reaction conditions.
For further study of lactose in Table 3, this disaccharide showed a lower activity compared to sucrose and maltose. It was hard to use the catalyst of [NMP][HSO4] to promote the conversion of lactose into HMF, with a maximum yield of 19.5 % (entry 15) achieved at 140 °C. However, [C3SO3HMIM][HSO4] was more suitable (entries 18–21) for the present system according to Table 3, the highest yield of HMF was 36.1 % (entry 21) with adding CoCl2 as a co-catalyst in [AMIM]Cl at 140 °C.
The results obtained from the three disaccharides reactions revealed two conclusions. On the one hand, strong acidity catalysts may improve the yield of HMF from some but not all disaccharides, and sucrose gave a higher yield when using a lower acidity catalyst, such as [NMP][HSO4]. Sucrose contains fructose units, too strong acidic environment may go against the degradation process of fructose, which will lead to degradation excessively to give byproducts. On the other hand, the similar route to HMF from disaccharides provided a possible rationale for the results. Sucrose can be cleaved into glucose and fructose units through acid catalysis, analogously, maltose contains two glucose units and lactose can be cleaved into glucose and galactose. What’s more important, the acidic catalyst was supposed to aid in this process. It is known that glucose can be converted efficiently into HMF through acid ILs or metal ions, so another half unit plays as a significant influence factor on the conversion process. Perhaps galactose unit forms HMF less readily compared to fructose and glucose.
3.4 Degradation of Monosaccharide to HMF
In order to confirm our hypothesis about poor galactose reactivity in IL solvents and make mechanistic insights of the regularity and relevance on the conversion of mono- and disaccharides into HMF, we then examined the reactivity of fructose, glucose and galactose in [AMIM]Cl. Firstly, we tested the degradation of fructose without metal salts or even acidic IL catalysts, the results were summarized in Table 4 (entries 1–6). Both [NMP][HSO4] and [C3SO3HMIM][HSO4] gave good yields (98.0 %, entry 1 and 97.2 %, entry 5) with significant selectivity in [AMIM]Cl at 100 °C for 10 min (entries 1, 5), higher than the yields obtained in [BMIM]Cl (entries 2, 6). However, it is impressive that fructose can convert into HMF in [AMIM]Cl without catalyst efficiently, the yield of HMF was 91.1 % (entry 3) at 100 °C. This yield was remarkable when compared to [BMIM]Cl, which only 12.5 % (entry 4) HMF was obtained at 100 °C for 0.5 h. Accordingly, we can reach an important conclusion, [AMIM]Cl is not only a good solvent but also an excellent and significant catalyst in the conversion of fructose into HMF. The reaction conditions are more moderate and rapid caopared to previous results that obtained by Shi et al. [21].
Quite different from fructose, it was hard for glucose degradation into HMF in [AMIM]Cl alone, which [AMIM]Cl acted as both solvent and catalyst, only 1.1 % (entry 7) yield was obtained in Table 4 and truly inappreciable. Even 9 mol% of catalyst was added into the reaction system, the yield was still at a low level (entry 8). It indicated that the reactivity of glucose was poor in the present system without adding any metal salts, because glucose can not convert (the isomerization step, Fig. 1) into fructose efficiently according to Zhang et al. [16]. Then we tested the combination of MnCl2 or CoCl2 with acidic ILs talked above in [AMIM]Cl at 120 °C for 2 h, the results are also displayed in Table 4 (entries 9–12). Notably, the combination of CoCl2 with [C3SO3HMIM][HSO4] showed the best efficiency, 62.2 % (entry 12) of HMF was obtained. Moreover, both CoCl2 and [C3SO3HMIM][HSO4] revealed the more competitive effect on the conversion of glucose. Interestingly, the reaction using higher temperature resulted in lower yield shown as entry 13, possibly due to decomposition of HMF and other side reactions, like the formation of degradation products or humins under such conditions.
Finally, we focused our attention on galactose and additional experiments substantiated our hypothesis about poor galactose reactivity. No HMF was detected in [AMIM]Cl catalyzed by [NMP][HSO4] without co-catalyst (entry 14). Furthermore, [NMP][HSO4] showed a relatively weak reactivity on the conversion of galactose, even worked with CoCl2 which acted as the additive (entries 14–16). Hence, we tested another catalyst, the results were displayed in Table 4 (entries 17–18). Remarkably, in the cases studied, the yield increased and reached 19.7 % (entry 18) at 120 °C for 1 h. From the yields obtained above, we may easily come to the conclusion, metal salts play as critical factor on the conversion of galactose to HMF in solvent. The stereochemical configuration of glucose and galactose provided a possible explanation for the difficulty of galactose conversion to HMF. The isomerization product of galactose is tagatose, one of the ketoses and the C-4 epimer of fructose (Fig. 2). This ketose then dehydrates into HMF, likely via a furanosyl oxocarbeniumion [29]. Thus, inefficient degradation of tagatose into HMF could prevent high HMF yields from galactose. But the transformation of galactose into HMF was affected advantageously by metal ions, such as chromium, and cobalt that used in this work, as a result of it, galactose is less likely to undergo degradation to produce side products and more facilely to convert into HMF.
3.5 Relationship Insights
Further study of the yields of HMF obtained from mono- and disaccharides, we found these results have a meaningful relationship to some extent. For example, considering about the HMF yields from sucrose, which parallel roughly the average of the yields from glucose and fructose under the similar reaction conditions. Coincidentally, we have mentioned that sucrose can be cleaved into glucose and fructose units through acid catalysis in previous discussion. This conclusion also applies to maltose and lactose, the former one can convert into two copies of glucose units, and the later one will cleave into glucose and galactose units. The same consequences reveal the important information that acid-catalyzed behaviors of disaccharides and polysaccharides were affected significantly by their component units, especially those units that are different from glucose. The more facile of these monosaccharide units degradation, the easier those corresponding disaccharides or polysaccharides conversion into HMF. We think the discovery will help us to design and improve the catalytic system on the conversion of carbohydrates to HMF.
3.6 Mechanism Insights on the Conversion of Fructose in [AMIM]Cl
Efficient catalytic chemical transformation of fructose to HMF is one of the key steps for attaining industrial level conversion of biomass to useful chemicals. The reaction mechanisms for the decomposition of fructose to HMF in both neutral and acidic environments have been investigated recently [30]. In this study, fructose can convert to HMF efficiently with high yield catalyzed by [AMIM]Cl, the novel character of [AMIM]Cl will be able to make it become a strong competitor comparing to other catalysts in future biochemical industry. The mechanism of [AMIM]Cl-promoted conversion of fructose to HMF is further investigated by employing DFT study. Besides, this work will also gain insight into why [AMIM]Cl performed better than [BMIM]Cl without co-catalyst or co-solvent.
Thus, a molecular level understanding of the reaction mechanism presented in Fig. 3 was investigated to expound the results obtained from the experimental work through a DFT study on the B3LYP/6-311G (d, p) levels. Particularly, we want to confirm that how [AMIM]Cl affected the reaction pathways of fructose. We initially studied the reaction process in the presence of [AMIM]Cl which was characterized by three elementary steps with three transition states. As shown in Figs. 3, 4, halide ion played an important role on the conversion process. Firstly, a molecule of water is lost when compound 1 formed via the attachment of Cl− to the two OH groups on C1 and C2, the energy barrier is 61.87 kcal mol−1 calculated by TS1, then an enediol intermediate 2 is formed and rapidly isomerizes into its isomers aldehyde 3. Next, with the formation of another molecule of water which releases from 3, the product 4 is formed via the intermediate TS2 with the energy barrier of 31.37 kcal mol−1. Then following the similar route above, the formation of 5 upon the coordination of the oxygen atom at C4 to the [AMIM]Cl, removes a third water molecule with the barrier of 48.05 kcal mol−1. Finally, both the [AMIM]Cl and H2O dissociated from 5 with the broken of the coordination between hydroxy group and Cl− to give the target product HMF.
Furthermore, owing to the attempt to investigate the different reactivity between [AMIM]Cl and [BMIM]Cl, we studied the energy barriers via the transition states and reactants which displayed in Fig. 4 and Table 5. The results indicated that the ILs with different side chain groups have great influence on the energy barriers (or activation energies). The catalytic activity with alkyl group is lower compared to the allyl group, we proposed that in salt solutions with small, strong polarizing cations and large polarizable anions, there exists intensive interactions between them and OH. In comparison with [BMIM]Cl, the cation [AMIM]+ is conducive to the attack on OH groups due to the smaller ion size and a double bond in the cation of [AMIM]Cl. Moreover, the less electronic chemical structure caused by allyl group also enhances the interaction between cations in [AMIM]Cl and hydroxyl.
4 Conclusions
The success of one-step conversion of these carbohydrates to HMF is attributed to the excellent solvent performance of [AMIM]Cl and the appropriate acidity of [NMP][HSO4] and [C3SO3HMIM][HSO4]. The metal salt cobalt chloride (CoCl2) is confirmed as an effictive co-catalyst on the degradation of several mono- and disaccharides, including glucose, maltose and lactose. Galactose, an aldohexose that was proposed can isomerize to tagatose, was converted only poorly into HMF, providing further mechanistic insight. [AMIM]Cl was found to play a remarkable catalytic activity for the transformation of fructose to HMF without other catalyst. In particular, the mechanisms to explain the activity of [AMIM]Cl and [BMIM]Cl are also proposed, and the roles are further investigated by employing DFT study. We believe these efforts will provide a molecular level understanding of the reaction process for the IL-promoted conversion of fructose to HMF, and help us to design and develop a new catalytic system for conversion of sugars to HMF.
References
Mascal M, Nikitin EB (2008) Angew Chem Int Ed Engl 47:7924
Zhang Z, Zhao ZK (2010) Bioresour Technol 101:1111
Hu S, Zhang Z, Zhou Y, Han B, Fan H, Li W, Song J, Xie Y (2008) Green Chem 10:1280
Bredihhin A, Mäeorg U, Vares L (2013) Carbohydr Res 375:63
Gorbanev YY, Kegnæs S, Riisager A (2011) Catal Lett 141:1752
Sievers C, Musin I, Marzialetti T, Valenzuela Olarte MB, Agrawal PK, Jones CW (2009) ChemSusChem 2:665
Dee SJ, Bell AT (2011) ChemSusChem 4:1166
Guo H, Qi X, Li L, Smith RL Jr (2012) Bioresour Technol 116:355
Wei Z, Liu Y, Thushara D, Ren Q (2012) Green Chem 14:1220
Bali S, Tofanelli MA, Ernst RD, Eyring EM (2012) Biomass Bioenergy 42:224
Lima S, Neves P, Antunes MM, Pillinger M, Ignatyev N, Valente AA (2009) Appl Cata 363:93
Wang C, Fu L, Tong X, Yang Q, Zhang W (2012) Carbohydr Res 347:182
Richter FH, Pupovac K, Palkovits R, Schüth F (2012) ACS Cata 3:123
Qi X, Watanabe M, Aida TM, Smith JRL (2008) Green Chem 10:799
Choudhary V, Burnett RI, Vlachos DG, Sandler SI (2012) J Phys Chem C 116:5116
Zhao H, Holladay JE, Brown H, Zhang ZC (2007) Science 316:1597
Bose S, Armstrong DW, Petrich JW (2010) J Phys Chem B 114:8221
Li YN, Wang JQ, He LN, Yang ZZ, Liu AH, Yu B, Luan CR (2012) Green Chem 14:2752
He J, Zhang Y, Chen EY (2013) ChemSusChem 6:61
Jadhav AH, Kim H, Hwang IT (2013) Bioresour Technol 132:342
Shi C, Zhao Y, Xin J, Wang J, Lu X, Zhang X, Zhang S (2012) Chem Commun 48:4103
Zhu S, Wu Y, Chen Q, Yu Z, Wang C, Jin S, Ding Y, Wu G (2006) Green Chem 8:325
Zhang H, Wu J, Zhang J, He J (2005) Macromolecules 38:8272
Tong X, Li Y (2010) ChemSusChem 3:350
Tao F, Song H, Chou L (2011) Bioresour Technol 102:9000
Shi J, Gao H, Xia Y, Li W, Wang H, Zheng C (2013) RSC Adv 3:7782
Zhang Z, Wang Q, Xie H, Liu W, Zhao ZK (2011) ChemSusChem 4:131
Simeonov SP, Coelho JA, Afonso CA (2012) ChemSusChem 5:1388
Binder JB, Cefali AV, Blank JJ, Raines RT (2010) Energy Environ Sci 3:765
Assary RS, Redfern PC, Greeley J, Curtiss LA (2011) J Phys Chem B 115:4341
Acknowledgments
The authors are grateful to the Fundamental Research Funds for the Central Universities (JUSRP211A12) and Natural Science Foundation of Jiangsu Province (SBK201222312) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, J., Liu, W., Wang, N. et al. Production of 5-Hydroxymethylfurfural from Mono- and Disaccharides in the Presence of Ionic Liquids. Catal Lett 144, 252–260 (2014). https://doi.org/10.1007/s10562-013-1148-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-013-1148-6