Abstract
Glycerol carbonate was synthesized by transesterification of glycerol with dimethyl carbonate using KF supported catalyst. KF was impregnated on various oxides and non-oxide supports like Al2O3, SiO2, ZnO, ZrO2, H-beta, and carbon to study the influence of the support on the catalytic performance. The preparation procedure of supported KF catalysts was modified to remove weakly adsorbed KF from the catalyst surface. The generation of basic sites by KF depends upon the extent of KF interaction with the support. KF/Al2O3 catalyst with optimized amount of 3.8 mmol KF, gave the highest activity with 95.8 % glycerol conversion and almost 100 % selectivity for glycerol carbonate. It was truly heterogeneous without any leaching of active sites and also showed good reusability. The catalyst exhibited better performance when compared to conventional solid base catalysts such as MgO, CaO and hydrotalcite.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Depleting crude oil availability all over the world has motivated researchers to look for alternate fuels. Biodiesel is one such fuel synthesized by using vegetable oils or animal fats by transesterification reaction. Glycerol is formed as a byproduct which accounts for one-tenth of every gallon of biodiesel that is produced. Rapidly increasing production of biodiesel has made glycerol an inexpensive byproduct. To improve the economics of biodiesel synthesis, a lot of research has been carried out to convert glycerol into value-added products like acrolein, propanediols, glycerol carbonate, acrylic acid and glyceric acid [1].
Glycerol carbonate, one such important derivative of glycerol, has high potential applications in various fields. For example, it can be used as a raw material for the synthesis of glycidol, polymers, surfactants, lubricating oils as emulsifiers, moisturizers in cosmetic preparations, lustering agents, washing aids and detergents [2, 3]. Recently, the synthesis of glycerol carbonate from glycerol has gained importance as evidenced by the increasing number of reports in literature. Glycerol carbonate can be synthesized from glycerol using different reactants like carbon dioxide [4], urea [5, 6] and alkyl carbonates [6–8] in the presence of either acid or base catalyst. Aresta et al. [9] reported that glycerol carbonate could be produced from the reaction of glycerol with CO2 in the presence of n-butyl tin methoxide as a catalyst. However, the reaction was carried out at elevated temperatures and pressures and the yield of glycerol carbonate was too low to be used for practical purposes. The carbonylation of glycerol by urea has also been studied using catalysts such as zinc sulfate, hydrotalcite and magnesium sulfate, but the reaction is found to be feasible only under vacuum [5, 6, 10]. Liquid alkyl carbonates such as dimethyl carbonate and ethylene carbonate have gained much attention as carbonate sources for the synthesis of glycerol carbonate from glycerol. Recent studies indicate that the conventional solid base catalysts like CaO [11] and K2CO3/MgO [12] showed good conversion for transesterification of glycerol with dimethyl carbonate but suffered from poor reusability. Ebitani and co-workers [13] reported hydrotalcite as an active catalyst using dimethyl formamide as a solvent. Mg/Al/Zr mixed oxide catalyst has also been used as a base catalyst with high glycerol carbonate yield but excess of dimethyl carbonate has been utilized [14]. KF/hydroxyapatite has been reported as a catalyst for transesterification of glycerol with dimethyl carbonate [15]. Recently, NaOH/γ-Al2O3 has also been used for the same reaction but the leaching of ionic Na species was observed with successive reuse of the catalyst [16].
KF/Al2O3 was first reported as a solid base catalyst by Ando et al. [17, 18]. This catalyst has been successfully applied to a variety of organic transformations such as aldol condensation [19], transesterification [20], Michael addition [21] and Tishchenko reaction [22]. Hattori co-workers [23] reported the formation of K3AlF6 and AlO4 2− type of phases by the interaction of KF on alumina support with the formation of OH− species on the catalyst surface.
In the current work, the transesterification reaction of glycerol with dimethyl carbonate has been investigated using KF impregnated on different oxide and non-oxide supports like α- and γ-Al2O3, SiO2, zeolite beta, ZnO, ZrO2 and carbon to understand the KF and support interaction. All the synthesized catalysts have been characterized by XRD, AAS, N2 adsorption and CO2-TPD measurements. The physicochemical properties of the catalyst has been correlated with the activity and selectivity of catalysts. The catalytic activity of KF/Al2O3 catalyst has also been compared with conventional solid base catalysts such as MgO, CaO and hydrotalcite.
2 Experimental
2.1 Materials
Glycerol, dimethyl formamide (DMF), potassium fluoride, zinc nitrate, zirconium oxy chloride, activated charcoal (carbon source), aluminum nitrate, urea, calcium oxide (CaO), magnesium oxide (MgO), magnesium nitrate, sodium hydroxide pellets and sodium carbonate were purchased from Merck India Ltd. Pseudoboehmite (alumina source) and H-beta zeolite (SAR = 25) were kindly donated by Süd-Chemie India Pvt Ltd. Fumed silica as a silica source was purchased from Alfa Aeser. Dimethyl carbonate (DMC) was purchased from SRL Chemicals Pvt Ltd. All the chemicals were of research grade and used without any further purification.
2.2 Catalyst Preparation
KF was loaded on different supports by the wet impregnation method. Supports used in this study were α-Al2O3 (synthesized by combustion method using aluminum nitrate and urea) and γ-Al2O3, SiO2, H-beta zeolite, ZnO (zinc nitrate was decomposed at high temperature), ZrO2 (synthesized by zirconium oxy chloride) and carbon. Moreover, the catalysts with different KF content were prepared by loading KF (in the range 1–24 mmol) on γ-alumina. In a typical procedure, 10 ml of aqueous solution containing 17 mmol KF was mixed with 1 g of support under vigorous stirring at room temperature for 2 h followed by the evaporation of water at 80 °C in a water bath. Then the catalyst was dried in an oven at 120 °C overnight and calcined under air at 600 °C for 4 h. After calcination, the catalyst was placed in 50 ml distilled water, stirred well and filtered to remove any physisorbed KF present on the support, and dried at 120 °C. Different KF loaded Al2O3 catalysts are designated as XKF/Al2O3, where X is the mmol loading of KF (measured by AAS). The alumina used was in gamma form unless otherwise stated. Other solid base catalysts like CaO and MgO were calcined at 850 °C before use and MgAl hydrotalcite (HTc) was synthesized by literature method [24].
2.3 Catalyst Characterization
The powder X-ray diffraction (XRD) patterns were recorded for all the catalysts on a Bruker D2 phaser X-ray diffractometer using Cu Kα radiation with high resolution Lynxeye detector. All the samples were scanned in the 2θ range of 5°–60° with step size of 0.02°/min.
The specific surface areas of the catalysts were measured by N2 physisorption at liquid nitrogen temperature on Quantachrome Nova 1000 at 77 K and determined by using the standard BET method on the basis of adsorption data.
Basicity of the samples was measured by the temperature programmed desorption of CO2 (CO2-TPD) using Quantachrome Autosorb iQ (automated gas sorption analyser). The sample was preheated at 300 °C under the flow of He for 3 h, then cooling to 80 °C. Later, the CO2 from a stream of He (10 % CO2) was fed into the sample for 30 min. Then the sample was purged with He at 100 °C for 1 h in order to eliminate physisorbed CO2. A TPD analysis was carried out from ambient temperature to 600 °C at a heating rate of 10 °C/min. CO2 concentration in the effluent stream was monitored by using a thermal conductivity detector (TCD) and the areas under the peaks were integrated to determine the amount of CO2 desorbed. TCD calibration was performed by passing known volumes of CO2.
The KF concentration on alumina support was determined by analyzing potassium content (sample dissolved in aqua regia) from Perkin Elmer AAnalyst 200 atomic absorption spectrophotometer (AAS) using potassium nitrate as a reference standard. Surface density (SD) of potassium (K) (which indirectly represents the active sites) on the catalyst surface was calculated using the formula,
2.4 Catalytic Activity Studies
Transesterification reaction of glycerol with DMC was carried out in a 100 ml two-necked glass reactor equipped with a magnetic stirring bar, a Liebig condenser, and a thermometer. The required amounts of glycerol and DMC were taken in the reactor and 5 wt% of preactivated catalyst was added into it along with the diluent DMF. The reaction was performed under stirring at a desired temperature. After the reaction, the mixture was taken out and centrifuged for 10 min to separate the catalyst from the liquid phase. The thus obtained product was analyzed in gas chromatography (Shimadzu, GC-2014) with flame ionization detector (FID) equipped with a capillary column (0.25 mm I.D and 30 m length, Stabilwax, Restek). All the products were confirmed by gas chromatography with mass spectroscopy (Shimadzu, GCMS QP 2010). The product yield was calculated by the GC analysis using the formula,
3 Results and Discussion
The synthesized catalysts were characterized by various techniques and the properties were correlated to the catalyst performance based on the glycerol conversion and product yield.
3.1 Catalyst Characterization
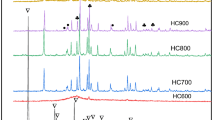
The KF impregnated catalysts were prepared by the commonly used procedure for the preparation of KF/Al2O3 except for the additional washing step after calcination. This additional step removes the weakly adsorbed KF from the catalyst. The remaining KF on the catalyst after water wash can be strongly adsorbed by the interaction with the support. The XRD patterns of different KF loadings on γ-alumina and KF on various supports are shown in Figs. 1 and 2 respectively. The characteristic crystalline phases of supported KF catalysts were identified with Inorganic Crystal Structure Database (ICSD). The peaks at 2θ = 17.4°, 27.5° corresponds to KF phase and peaks at 2θ = 29.7°, 36.4°, 42.4° and 52.3° correspond to K3AlF6 phase [18, 19]. It is seen that the intensity of the peaks including major peak (29.7°) increased with increase in KF loading up to 3.8 mmol and decreased thereafter (Fig. 1). Intensity of a broad peak at 46° corresponds to the γ-alumina phase decreased with increase in the KF loading from 0.5 to 3.2 mmol, whereas at higher loadings of KF (3.8 and 4.3 mmol), the alumina phase was completely replaced with the K3AlF6 phase. The crystalline phases of KF were formed on the amorphous SiO2 and carbon supports whereas the crystallinity of ZrO2 and ZnO improved after impregnating with KF. In case of alumina supports, the phase formation on γ-alumina due to the KF impregnation was different from that of α-alumina (Fig. 2). Interestingly, the XRD pattern of KF/H-beta was similar to that of KF/γ-alumina. This indicates that KF preferentially interacts with Al sites of the zeolite forming KF and K3AlF6 phases.
The BET specific surface areas of different KF supported catalysts are represented in Table 1. The KF/SiO2 and KF/ZrO2 catalysts showed low surface areas of 7.32 and 12.4 m2/g respectively whereas other KF impregnated catalysts showed similar surface areas in the range 20–33 m2/g. The BET surface area of pure γ-alumina was 250 m2/g which decreased with increase in KF loading as shown in Table 2. This decrease in surface area could be due to the blockage of alumina pores by KF resulting in the inaccessibility of N2 molecules to the internal surface.
Surface density of potassium on Al2O3 (in K nm−2) was calculated using mmol of K and surface area of the catalyst (Table 2). Surface density (SD) of K indirectly represents the active sites on the alumina surface. It is found that SD of K increased with increase in KF loading up to 3.8 mmol (SD = 11.8 K nm−2) and remained almost constant at higher loadings. This indicates that most of the Al sites of Al2O3 interacted with KF at 3.8 mmol loading and further loading of KF leads to a weak or no interaction with the support, and hence removed by water wash.
The amount of KF on the support was determined by analyzing the concentration of potassium from AAS. KF content in the final catalyst depends on the nature of support because the interaction of KF varies with the type of support. It is observed that, even though different supports are treated with same amount of KF (17 mmol/g), KF content after water wash is different for different supports (Table 1). Among all, inert carbon, contained the lowest amount of KF (0.3 mmol/g) whereas γ-alumina consisted of the highest KF (3.8 mmol) among different supports taken for this study.
The basicity of the catalysts was measured by CO2-TPD and the graphs are depicted in Fig. 3. The total basicity data of all the KF supported catalysts expressed in mmol/g of desorbed CO2 are listed in Table 1. In order to evaluate the amount of different types of basic sites, the desorption curve was differentiated into 3 peaks corresponding to <200 °C (weak), 200–350 °C (moderate), >350 °C (strong) as represented in Table 3. Comparison of TPD curves of different supported KF catalysts showed an increase in total basicity in the order, KF/carbon < KF/ZrO2 < KF/SiO2 < KF/H-beta < KF/ZnO < KF/Al2O3. The basicity found to have a linear correlation with the amount of KF on the support except for KF/ZnO which was more basic compared to SiO2 and H-beta supported catalysts. KF/Al2O3 catalyst with total basicity = 2.17 mmol/g, showed high amounts of moderate (1.2 mmol/g) and strong (0.88 mmol/g) basic sites compared to all other supported KF catalysts (Table 3). The amount of strong basic sites increased in the order KF/carbon < KF/ZrO2 < KF/SiO2 < KF/ZnO < KF/H-beta < KF/Al2O3. The KF/carbon and KF/ZrO2 catalysts exhibited negligible basicity. This indicates that the nature and strength of basic sites depends mainly on the type of support.
3.2 Transesterification of Glycerol with Dimethyl Carbonate
3.2.1 Comparison of KF/γ-Al2O3 with Different Catalysts
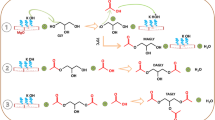
Glycerol undergoes transesterification with a cyclic carbonate ester, DMC to give glycerol carbonate and methanol (Scheme 1). Different metal oxides, non-metal oxide (silica) and non-oxide (activated carbon) supports modified by KF were studied for the transesterification of glycerol with DMC in the liquid phase (Table 1). The reaction was conducted at 75 °C with glycerol: DMC: DMF mole ratio of 1:2:0.5. The 5 wt% catalyst with respect to the total reactant weight was taken for this study. Among the catalysts, 3.8KF/Al2O3 showed the highest activity, producing glycerol carbonate with 95.8 % yield. The activities of KF/H-beta, KF/ZnO and KF/SiO2 were lower than 3.8KF/Al2O3, producing 64.2, 58.2 and 50 % of glycerol carbonate respectively whereas, KF/carbon and KF/ZrO2 exhibited no activity. The basicity measurement indicated that the impregnation of KF on carbon and ZrO2 led to a negligible generation of basic sites. In case of alumina support, it is shown by Hattori and co-workers [23] through 19F solid state NMR that K3AlF6 phase forms by the interaction of KF with alumina which leads to the formation of basic sites like F- and OH- species. The similar interaction is also possible with the Al sites of H-beta zeolite. KF supported on non-metal oxide like silica also showed basic sites as evident by CO2-TPD (Fig. 3). However, the amount of basicity was much lower compared with 3.8KF/Al2O3 which resulted in low activity. KF on metal oxide support such as ZnO showed higher activity compared to silica support but lower than alumina support. Among two types of alumina supports, γ-alumina exhibited maximum yield (95.8 %) of glycerol carbonate compared to α-alumina (52 %). The catalytic activity was best correlated to the strong basic sites of different supported KF catalysts as shown in Fig. 4. The glycerol conversion linearly increased with increase in strong basic sites indicating that strong basicity generated by KF was necessary for glycerol transesterification with DMC. The activity of 3.8KF/Al2O3 was compared with well-known conventional solid base catalysts such as MgO, CaO and HTc. Among these catalysts, 3.8KF/Al2O3 exhibited highest activity (95.8 % yield) followed by HTc (94 %), CaO (70.5 %) and MgO (68 %) (Table 4).
3.2.2 Influence of KF Loading on γ-Alumina Support
The different amounts of KF loaded on alumina support in the mmol range of 0.5 to 4.3 were tested for glycerol transesterification and the performance was compared (Table 2). As the loading increased from 0.5 to 3.8 mmol, the glycerol conversion increased to the maximum (95.8 %) whereas further increase in the KF loading on alumina (4.3 mmol) resulted in the decrease of glycerol conversion. Surface density of K was maximum for 3.8 KF/Al2O3 which resulted in highest activity. These results were supported by XRD where the peak intensity of the major peak at 2θ = 29.7° increased with increase in KF content up to 3.8 mmol and decreased thereafter. Also, the basicity increased as the KF loading increased up to 3.8 mmol KF loading (basicity = 2.17 mmol/g) and decreased at higher loadings. It is interesting to note that KF/Al2O3 uncalcined catalyst showed poor activity with just 2 % conversion indicating the importance of calcination step. The basicity is created on the alumina support by the interaction of KF during calcination at 600 °C.
3.2.3 Influence of Reaction Parameters on Transesterification Reaction
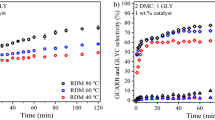
Influence of reaction conditions like temperature, mole ratio and catalyst concentration was studied for 3.8KF/Al2O3 and depicted in Fig. 5. The effect of temperature on the transesterification was investigated in the range of 55–85 °C with catalyst concentration of 5 wt% and glycerol: DMC: DMF mole ratio of 1:2:0.5. The conversion of glycerol carbonate increased with increase in the temperature from 55 to 75 °C and then remained almost constant with further increase to 85 °C. Selectivity for the transesterification product was 99.9 % at all temperatures indicating high efficiency of the catalyst for this reaction.
Influence of reaction conditions. a Effect of temperature: conditions: catalyst = 3.8 KF/Al2O3, catalyst amount = 5 wt%, gly: DMC: DMF mole ratio = 1:2:0.5, time = 1 h. b Effect of glycerol to DMC mole ratio: conditions: catalyst = 3.8KF/Al2O3, catalyst amount = 5 wt%, DMF = 0.5 mol, time = 1 h, temperature = 75 °C. c Effect of catalyst concentration: conditions: catalyst = 3.8KF/Al2O3, gly: DMC: DMF mole ratio = 1:2:0.5, time = 1 h, temperature = 75 °C
The effect of molar ratio of glycerol to DMC was also investigated at 75 °C as shown in Fig. 5. The glycerol conversion was low (25 % in 1 h) for 1:1 mol ratio and it increased to 75 % upon increasing the mole ratio to 1:2. Further increase in DMC concentration did not affect the catalytic activity. Glycerol, due to its high polarity, preferentially adsorbs on to the active sites and hence more DMC is needed for obtaining high conversion.
The effect of catalyst concentration on the conversion of glycerol was studied on 3.8 KF/Al2O3 catalyst. The conversion of glycerol increased as the catalyst concentration increased from 1 to 5 wt% with respect to the total weight of the reactants. However, further increase of active sites with the increase in catalyst concentration from 5 to 10 wt% improved the activity only marginally. Based on the above optimization studies, the best suitable conditions viz. reaction temperature of 75 °C, glycerol: DMC mole ratio of 1:2 and 5 wt% catalyst were employed to get high catalytic performance.
3.2.4 Leaching and Reusability
Leaching test was carried out to investigate the leaching of any KF species from the catalyst into reaction media. The reaction was carried out using KF/Al2O3 catalyst at a reaction temperature of 75 °C with glycerol to dimethyl carbonate mole ratio of 1:2 with DMF as a solvent. Reaction was stopped after 30 min (40 % conversion) and the hot reaction mixture containing catalyst was centrifuged. Supernated liquid was taken and reaction was continued for next 5 h. The conversion remained constant even after 5 h of reaction time, indicating no leaching of active sites into the reaction media (Fig. 6). Even the analysis of reaction mixture with AAS for potassium showed a negligible K content (<0.002 mmol/g catalyst) proving that the water wash during catalyst synthesis has an effective role in removing the weakly adsorbed KF species.
For a recycling test after the first run, the catalyst was filtered, washed thoroughly with methanol, dried at 120 °C and reused with fresh reactants under the same reaction conditions (Table 5). The catalyst showed good reusability with a marginal decrease in activity (3 %) after 3 cycles. It is important to note that catalyst was not calcined before each recycle. Furthermore, XRD patterns of both fresh and recycled catalysts indicated that there was no change in phase purity after the two recycles (Fig. 7).
4 Conclusions
Transesterification of glycerol with dimethyl carbonate to form glycerol carbonate has been carried out using various solid base catalysts. Different supported KF catalysts showed an increase in total basicity in the order, KF/carbon < KF/ZrO2 < KF/SiO2 < KF/H-beta < KF/ZnO < KF/Al2O3. The generation of basicity and the total basic sites depend on the type of the support and its interaction with KF. The additional step of water wash of the supported KF catalyst helps in removing the weakly adsorbed KF from the catalyst surface. The KF/Al2O3 catalyst with optimized amount of 3.8 mmol KF, gave the highest activity with 95.8 % glycerol conversion and almost 100 % selectivity for glycerol carbonate. The catalyst showed better performance compared to conventional solid base catalysts such as MgO, CaO and HTc. In conclusion, KF/Al2O3 was found to be an efficient and reusable solid base catalyst for the transesterification of glycerol to glycerol carbonate.
References
Guerrero-Perez MO, Rosas JM, Bedia J, Rodriguez-Mirasol J, Cordero T (2009) Green Chem 11:21
Uno M, Okutsu M (2011) US patent 6495703 B2
Herault D, Boutty B, Zander L, Strube A (2005) US patent 2005/0038266 A1
George J, Patel Y, Pillai M, Munshi P (2009) J Mol Catal A 304:1
Jean-Luc Dubois, Aresta M, Dibenedetto A, Ferragina C, Nocito F (2011) US patent 2011/0245513 A1
Climent MJ, Corma A, Frutos PD, Iborra S, Noy M, Velty A, Concepcion P (2010) J Catal 269:140
Ochoa-Gomez JR, Gomez-Jimenez-Aberasturi O, Madurga BM, Rodriguez AP, Ramirez-Lopez C, Ibarreta LL, Torrecilla-Soria J, Velasco MCV (2009) Appl Catal A Gen 366:315
Alvarez MG, Sagarra AM, Contreras S, Sueiras JE, Medina F, Figueras F (2010) J Chem Eng 161:340
Aresta M, Dibenedetto A, Nocito F, Ferragina C (2006) J Mol Catal A 257:149
Aresta M et al (2011) US patent 2011/0245513 A1
Simanjuntak FSH, Kim TK, Lee SD, Ahn BS, Kim HS, Lee H (2011) Appl Catal A Gen 401:220
Du M, Li Q, Dong W, Geng T, Jiang Y (2011) Res Chem Intermediat 38:1069
Takagaki A, Iwatani K, Nishimura S, Ebitani K (2010) Green Chem 12:578
Malyaadri M, Jagadeeswaraiah K, Sai Prasad PS, Lingaiah N (2011) Appl Catal A Gen 401:153
Bai R, Wang S, Mei F, Li T, Li G (2011) J Ind Eng Chem 17:777
Bai R, Wang Y, Wang S, Mei F, Li T, Li G (2013) Fuel process. Technology 106:209
Yamawaki J, Ando T (1979) Chem Lett 8:755
Ando T, Yamawaki J (1979) Chem Lett 45:755
Raju V, Radhakrishnan R, Jaenicke S, Chuah GK (2011) Catal Today 164:139
Murugan C, Bajaj HC (2011) Fuel Proc Tech 92:77
Clacens JM, Genuit D, Delmotte L, Ruiz AG, Bergeret G, Montiel R, Lopez J, Figueras F (2004) J Catal 221:483
Baba T (2000) Catal Survey Jpn 4:17
Kabashima H, Tsuji H, Nakata S, Tanaka Y, Hattori H (2000) Appl Catal A Gen 195:227
Reichle WT (1985) J Catal 94:547
Acknowledgments
Swetha S acknowledges Admar Mutt Education Foundation (AMEF) for providing the fellowship and also thankful to Manipal University for permitting this research as a part of the Ph.D programme. The authors thank Bangalore Institute of Technology, India and Indian Institute of Chemical Technology (IICT), Hyderabad, India for providing surface area measurements and CO2-TPD respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sandesh, S., Shanbhag, G.V. & Halgeri, A.B. Transesterification of Glycerol to Glycerol Carbonate Using KF/Al2O3 Catalyst: The Role of Support and Basicity. Catal Lett 143, 1226–1234 (2013). https://doi.org/10.1007/s10562-013-1043-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-013-1043-1