Abstract
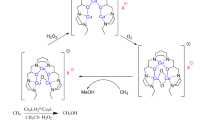
With the catalysis of K2PdCl4 and H5PMo10V2O40 in CF3COOH, methane can be oxidized into CH3COOH and CF3COOCH3 using molecular oxygen as the oxidant at a low temperature. H5PMo10V2O40 is a reversible oxidant that allows to retain Pd(II) in CF3COOH and thus to complete a two-step catalytic cycle of oxidation of methane by molecular oxygen; in addition, it can catalytically oxidize methane into CH3COOH and CF3COOCH3.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The activation of methane has attracted much attention due to its high abundance in natural gas and its low activity. Current technologies for the activation of methane proceed by generation of carbon monoxide and hydrogen (syngas), followed by converting syngas into liquid products through Fischer–Tropsch chemistry. However, the production of syngas is an energy-intensive and cost-intensive process. In contrast, direct, low-temperature, partial oxidation of methane becomes promising.
Although methane can be oxidized into methanol and chloromethane with PtCl6 2− as the oxidant and with PtCl4 2− as the catalyst in an aqueous solution, the yield and selectivity of methanol are very low [1]. Periana et al. [2, 3] have reported that high conversion of methanol derivatives from methane could be obtained with the catalysis of Hg(II) or Pt(II) in concentrated sulfuric acid. The metal ion M(II) (M=Hg, Pt) reacts with methane by an electrophilic displacement mechanism to produce CH3MOSO3H which can readily decompose to CH3OSO3H and the reduced metal species. The catalytic cycle is completed by the reoxidation of the reduced metal species with concentrated sulfuric acid to regenerate M(II) [2, 3]. Pt(II) is more efficient than Hg(II) during the catalysis reaction, however, it will be irreversibly reduced in bulk metal, which results in fast deactivation of catalysts and steep decrease of selectivity [2]. In order to inhibit the irreversible reduction, Periana et al. [3] further developed a stable platinum ligand (bpym)PtCl2, which could directly oxidize methane into a methanol derivative with higher yield and selectivity. About 90 % methane conversion and 81 % selectivity could be obtained at 220 °C for 2.5 h [3]. Although Hg(II) and Pt(II) salts can give high catalytic activity, Hg(II) salt is high toxic, and (bpym)PtCl2 is too expensive, which hinders their industrial application.
In addition, the oxidation of methane to acetic acid catalyzed by Pd2+ in 96 wt% sulfur acid has been described by Periana et al. [4], in which the selectivity of liquid-phase product, acetic acid and methyl bisulfate, is as high as 90 %. However, Pd2+ is also reduced irreversibly to Pd black, which leads to the deactivation of catalyst. In order to regenerate the catalyst, Zerella et al. [5] have suggested to reoxidize palladium black by oxygen and sulfur acid.
Molybdovanadophosphoric acid (HPA) was reported to have good catalytic activity in the partial oxidation of methane by H2O2 or K2S2O8 [6–8]. With the catalysis of H4PVMo11O40, 33.0 % conversion of methane could be obtained at 80 °C in (CF3CO)2O using H2O2 as the oxidant [6, 7].
Besides catalysts, the oxidants are crucial to the partial oxidation process. Strong oxidants, such as concentrated sulfuric acid, H2O2 and K2S2O8, are necessary in transition metal catalytic systems or in HPA catalytic systems. Molecular oxygen is an environmentally friendly, inexpensive oxidant; however, due to its relatively poor oxidation capacity, there are only a few reports on partial oxidation of methane using molecular oxygen as the oxidant. In addition to the above mentioned catalytic system suggested by Bell et al., An et al. developed a catalytic system for the one-pot aerobic oxidation of methane with the combination of the three redox couples Pd(II)/Pd(0), p-benzoquinone (BQ)/hydrobenzoquinone (H2Q), NO2/NO in CF3COOH [9]. However, methane conversion is too low in this catalytic system [9].
Pd(II)/HPA/O2 system is very important in homogeneous catalysis of organic synthesis [10]. In this system, HPA is a reversible oxidant that allows to retain Pd(II) in the solution and thus to complete a two-step catalytic cycle of oxidation of the substrates by molecular oxygen [10]. In order to develop an efficient method for partial oxidation of methane using molecular oxygen as the oxidant, K2PdCl4/H5PMo10V2O40 catalytic system is suggested in this study.
2 Experimental
2.1 Materials
K2PdCl4 and CF3COOH were purchased from Shanghai Jingchun Reagent Co. Ltd., P. R. China. BQ was purchased from Sinopharm Chemical Reagent Co. Ltd., P. R. China. Methane, oxygen and nitrogen were obtained from Beijing Huayuan Gas Co. Ltd., P. R. China. H5PMo10V2O40 was prepared from H3PO4, MoO3, and V2O5 [11]. H3PO4, MoO3 and V2O5 were from Beijing Chemical Factory, P. R. China. All of the chemical reagents are at analytic reagent grade.
2.2 Partial Oxidation of Methane
K2PdCl4 and H5PMo10V2O40 were dissolved in CF3COOH before methane oxidation reaction. In addition, NaCl was also introduced into CF3COOH, and the molar ratio of NaCl to K2PdCl4 is 50.
Methane oxidation in 10 ml of CF3COOH was conducted in a 50 ml cylindrical stainless steel autoclave with a Teflon liner inside. Mild stirring was provided by a magnetic stirring bar coated with Teflon. The reactor was three times purged with 1.0 MPa of methane and then pressurized with methane and oxygen. It was heated to 80 °C in an oil bath and kept under stirring. After the reaction, the reactor was cooled in ice/water mixture to 3 °C, thus the pressure was slowly reduced.
The products were analyzed by gas chromatography- mass spectroscopy (GC–MS) and 1H-NMR (Brucker AV600, Swiss). The gas phase was analyzed by a gas chromatograph (GC 4000A, East & West Analytical, PRC) with TDX-01 column, and the liquid was qualified by a gas chromatograph with a Porapak QS column.
2.3 Preparation of the Nascent Pd(0)
The nascent Pd(0) powders were prepared by the reduction of Pd(OAc)2. pH value of Pd(OAc)2 aqueous solution was adjusted to about ten by adding ammonia solution, then hydrazine hydrate was added into the solution, thus the nascent Pd(0) sediment formed. The black sediment was cleaned by distilled water for times in order to remove the residual Pd2+ ions.
2.4 Oxidation Reaction of the Nascent Pd(0) by H5PMo10V2O40 in CF3COOH
0.0044 g of H5PMo10V2O40 powders was firstly dissolved in 25 ml of CF3COOH, then 20 ml of H5PMo10V2O40 solution were used to oxidize 0.0022 g of the nascent Pd(0). At the same time, 1 ml acetic acid was also added to provide acetate ions. Concentration of Pd2+ in the samples at various intervals was analyzed by colorimetric method using 5-Cl-PADAB as the developer at wavelength of 570 nm.
3 Results and Discussion
3.1 Partial Oxidation of Methane
The results of Gretz et al. [12] implied that Pd(II) could oxidize methane selectively to CF3COOCH3 in CF3COOH at 80 °C. In that process, Pd(II) did not act as a catalyst because it was stoichiometrically converted to Pd(0) [13]. In order to regenerate Pd(II), we chose H5PMo10V2O40 to oxidize Pd(0), and molecular oxygen as the terminal oxidant to regenerate H5PMo10V2O40.
Table 1 lists a series of typical reaction results for partial oxidation of methane in CF3COOH catalyzed by H5PMo10V2O40 or K2PdCl4/H5PMo10V2O40. It shows in Table 1 that Pd2+ can stoichiometrically oxidize methane into CF3COOCH3 in CF3COOH, which is consistent with the previous results [9, 13]. Using molecular oxygen as the oxidant, methane can be oxidized into CH3COOH and CF3COOCH3 with the catalysis of H5PMo10V2O40 or K2PdCl4/H5PMo10V2O40, and CH3COOH is the major product. In addition, the yield in K2PdCl4/H5PMo10V2O40 binary-component system is more than the yield sum in single-component system (Table 1, entry 1, 2 and 3), indicating K2PdCl4/H5PMo10V2O40 binary-component catalyst exhibits good activity. Moreover, higher concentration of H5PMo10V2O40 in K2PdCl4/H5PMo10V2O40 catalysts leads to higher conversion (Table 1, entry 3 and 4). Higher concentration of H5PMo10V2O40 can improve the activity of Pd(II); however, limited by the solubility of H5PMo10V2O40 in CF3COOH, it is difficult to increase its concentration further.
The yield of CH3COOH and CF3COOCH3 increases with increasing partial pressure of oxygen (Table 1, entry 3, 5 and 6), since the regeneration rate of HPA increases with partial pressure of oxygen [12]. In addition, the yield of CH3COOH and CF3COOCH3 increases with increasing partial pressure of methane (Table 1, entry 6, 7 and 8).
In Pd(OAc)2/BQ/NO2/O2 system, CF3COOCH3 is the only product [9]. In contrast, CH3COOH is the major product when using K2PdCl4/H5PMo10V2O40 as catalysts. With the increase of reaction time, the concentration of CH3COOH and CF3COOCH3 increases (Fig. 1). At 8 h, the conversion of methane adds up to 10.85 %, higher than the results in Pd(OAc)2/BQ/NO2/O2 system [9].
3.2 Possible Mechanism
Periana et al. [3] tried to prevent the reduction of Pd(II) by the addition of HPA in concentration sulfuric acid but failed. It is probably because concentration sulfuric acid could not regenerate HPA. Once HPA is reduced, it cannot oxidize Pd(0) to Pd(II), which results in catalyst deactivation.
Although it is well known that HPA can oxidize Pd(0) into Pd(II) in aqueous solution [14, 15], there has been no reports on the oxidization of Pd(0) in CF3COOH. Figure 2 shows oxidation rate of the nascent Pd(0) by H5PMo10V2O40 in CF3COOH at various temperatures. It can be seen from Fig. 2 that H5PV2Mo10O40 can oxidize Pd(0) powders into Pd(II) in CF3COOH, and the increase of reaction temperature can enhance the oxidation rate. That indicates that H5PMo10V2O40/O2 system can inhibit the deactivation of Pd(II).
The partial oxidation of methane with Pd(II)/H5PMo10V2O40 as the catalysts and with molecular oxygen as the oxidant might occur in the following routes: (1) Pd(II) oxidizes methane into CF3COOCH3, then is regenerated with H5PMo10V2O40 and oxygen; (2) H5PMo10V2O40 abstracts H· from CH4 to form a methyl radical (CH3·) which would be oxidized by H5PMo10V2O40 to a methyl cation (CH3 +), then CH3 + combines with COO− from CF3COOH into CH3COOH, and with CF3COO− into CF3COOCH3 [8, 16].
4 Conclusion
Methane can be partially oxidized into CH3COOH and CF3COOCH3 with the catalysis of K2PdCl4 and H5PMo10V2O40 using molecular oxygen as the oxidant at a low temperature. H5PMo10V2O40 is a reversible oxidant that allows to retain Pd(II) in CF3COOH and thus to complete a two-step catalytic cycle of oxidation of methane by molecular oxygen; in addition, it can catalytically oxidize methane into CH3COOH and CF3COOCH3.
References
Shilov AE, Shul’pin GB (1997) Chem Rev 97:2879
Periana RA, Taube DJ, Evitt ER, Löffler DG, Wentrcek PR, Voss G, Masuda T (1993) Science 259:340
Periana RA, Taube DJ, Gamble S, Taube H, Satoh T, Fujii H (1998) Science 280:560
Periana RA, Mironov O, Taube D, Bhalla G, Jones CJ (2003) Science 301:814
Zerella M, Kahros A, Bell AT (2006) J Catal 237:111
Seki Y, Mizuno N, Misono M (2000) Appl Catal A Gen 194–195:13
Seki Y, Min JS, Misono M, Mizuno NJ (2000) Phys Chem B 104:5940
Piao D, Inoue K, Shibasaki H, Taniguchi Y, Kitamura T, Fujiwara Y (1999) J Organomet Chem 574:116
An Z, Pan X, Liu X, Han X, Bao X (2006) J Am Chem Soc 128:16028
Kozhevnikov IV (1998) Chem Rev 98:171
Weinstock IA (1998) Chem Rev 98:113
Grate GH (1996) J Mol Catal A Chem 114:93
Gretz E, Oliver TF, Sen A (1987) J Am Chem Soc 109:8109
Zhizhina EG, Simonova MV, Odyakov VF, Matveev KI (2007) Appl Catal A Gen 319:91
Burton HA, Kozhevnikov IV (2002) J Mol Catal A Chem 185:285
Fujiwara Y, Takaki K, Taniguchi Y (1996) Synlett 7:591
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China (Grant No. 20776013), Beijing Natural Science Foundation (Grant No. 2102034) and the Special Fund of Basic Research in Central Universities (Grant No. JD1107).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, J., Liu, L., Wang, L. et al. Partial Oxidation of Methane with the Catalysis of Palladium(II) and Molybdovanadophosphoric Acid Using Molecular Oxygen as the Oxidant. Catal Lett 143, 126–129 (2013). https://doi.org/10.1007/s10562-012-0931-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-012-0931-0