Abstract
The studies on the mechanochemical synthesis and electrochemical characterization of orthorhombic calcium ferrite (CaFe2O4) are reported in this paper. Stoichiometric mixtures of α-Fe2O3 and granulated Ca metal were used as the starting materials for the synthesis process. The synthesized calcium ferrite was characterized by room-temperature Mössbauer spectra, XRD and TEM. The electrochemical characterisation was carried out using cyclic voltammetry studies. Mössbauer spectra provide the yield of the reaction, information on the charge status, the local symmetry and the magnetic state of the iron ions in the mechanosynthesized ferrite material. XRD analysis of the CaFe2O4 compound reveals the orthorhombic crystal structure with an average crystalline size of about 28 nm. TEM micrographs reveal the nanoparticles with irregular crystal morphology ranging from 8 to 30 nm. The electrochemical studies clearly show that the calcium ferrite compound can act as an electrocatalyst for Oxygen Evolution Reaction (OER).
Graphical Abstract
The studies on the mechanochemical synthesis and electrochemical characterization of orthorhombic calcium ferrite (CaFe2O4) are reported in this paper. The electrochemical studies clearly show that the calcium ferrite compound can act as an electrocatalyst for Oxygen Evolution Reaction (OER).

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In industrial electrochemical processes such as water electrolysis for hydrogen production, metal electro winning processes and in energy producing systems a stable anode material with low over voltage for the oxygen evolution is desirable. The oxygen evolution and reduction reactions are also important in fuel cells, batteries and in various aqueous electrolysis [1]. It is reported that even the dimensionally stable anode (DSA) which is the most superior anode material for chlorine evolution is not so good as regard to long term stability for OER [2, 3]. The main requisites for an electrode material for technological applications for the OER are high surface area, high electrical conduction, good electrocatalytic behaviour, minimisation of the gas bubble problem, low cost, and safety as regard to health [4].
A number of metals and alloys have been tested as electrocatalysts for the above applications. Nickel and nobel metals are considered to be very good anode materials in alkaline and acid solutions. It is reported that an oxide layer is always formed at the surface in the potential region at which oxygen evolves even if an inert metal electrode is used as the anode. Therefore, the oxide formed on the metal substrate always affects the reaction mechanism and the OER properties. It is generally believed that the oxide is more stable than the metal in such environments, since an oxide cannot be easily further oxidised. This is true for almost all oxide electrodes and extensive research has been made about the OER concerning oxide electrodes [5–8]. The electrocatalytic behaviour of these anode materials is important which directly influences the overvoltage of the OER. Therefore, a lot of research work is focused on these oxide materials and their applications in electro catalysis [9]. Many oxide materials such as nickel ferrite, cobalt ferrite and manganese ferrite have been synthesized and tested for their OER properties [10–12]. It is reported that the effectiveness of various oxide electrodes towards oxygen evolution decreased in the order Ru > Ir ~ Pt ~ Rh ~ Pd ~ Ni ~ Os > > Co > > Fe. Poor activities were observed on V, Cr, Mo, W, Mn and Re oxide electrodes [1]. Co-based perovskite oxides with molecular formula La 1−x Sr x CoO3 (0 < x < 0.5) and La 0.7Sr0.3 Co1−y B y O3 where B = Cu, Fe, Ni, Cr or Mn; (0.05 < y < 0.2), showed that the partial substitution of La by Sr in the LaCoO3 matrix increased the electrochemically active area (R F), as well as the apparent electrocatalytic activity (i a) but it decreased the true catalytic efficiency (i t) [13]. The substitution of Al by Ni in Ni x Al1−x Mn2O4 influences the rate of O2 reduction and oxygen evolution reactions [14]. The partial substitution of Cr for Fe in the CoFe2O4 greatly enhances the electrocatalytic activity of the oxide [15]. Correlations between solid state chemistry, surface properties and electrocatalytic reactivities towards the reaction of oxygen are investigated on powder electrodes of cobalt and manganese spinel type oxides Mn x Co3−x O4 (0 ≤ x ≤ 1) [16]. A comparative study on the electrochemical and physicochemical behaviour of binary cobalt oxides with spinel structure M x Co3−x O4 (M = Li, Ni, Cu) was performed in alkaline media [17]. NiFe2O4 nanorods synthesized using an emulsion method have shown higher discharge than that of the sample with bigger building blocks [18]. Nano materials behave indeed differently from their macroscopic counterparts if their characteristic sizes are smaller than the characteristic length scales of the physical phenomena occurring in bulk materials [19]. The enhanced properties are achieved from their large number of atoms residing in defect environments such as grain boundaries, interfaces and triple junctions compared to coarse-grained polycrystalline materials [20, 21].
Recently studies have been focused on nanoferrites as electrocatalysts for many organic and inorganic reactions. Nano ferrites have been prepared by mechanical activation process and their structural and magnetic properties have been elucidated [22–25]. Ca–Fe–O is one of the interesting systems, which finds application as oxidation catalysts, high-temperature sensors, gas absorbers, electrodes for solid oxide fuel cells etc. [26–28]. CaFe2O4 is used as pigment [29], as an anode material in lithium batteries [30] and employed as catalytic material [31]. CaFe2O4 has been synthesized using various methods such as ceramic method [31], Pechini process [32] and co-precipitation method [33]. A novel synthesis route for both calcium ferrites (CaFe2O4 and Ca2Fe2O5) was proposed [34], using mechanically activated mixtures of organic precursors. It is prepared by mechanical activation using a mixture of Ca(OH)2/α-FeOOH [35] and by thermal decomposition of Ca[Fe(CN)5NO]·4H2O [36]. A single-step mechanosynthesis of nanocrystalline CaFe2O4 particles has been reported in our previous work [37]. To the best of our knowledge, studies on the electro catalytic behaviour of Ca-ferrite have not been made in aqueous solutions.
In the present investigation, we report the mechanochemical synthesis of nanocrystalline CaFe2O4, using Ca/α-Fe2O3 as the precursor and its electro catalytic behaviour in aqueous environments.
2 Experimental
2.1 Synthesis of CaFe2O4 Powders
For the synthesis of CaFe2O4, stoichiometric mixtures of α-Fe2O3 and granulated Ca metal (Merck, Darmstadt, Germany) were used as starting materials. The mixture was milled using a Pulverisette 6 planetary ball mill (Fritsch, Idar-Oberstein, Germany) at room temperature. A grinding chamber (250 cm3 in volume) and balls (10 mm in diameter) made of tungsten carbide were used for the milling process. The ball-to-powder weight ratio was 40:1. Milling experiments were performed in air at 600 rpm.
2.2 Characterization of CaFe2O4 Powders
The synthesized powders were characterized using Mössbauer spectra, XRD, TEM and Electrochemical studies. Room-temperature Mössbauer spectra were taken in transmission geometry using a 57Co/Rh γ-ray source. The velocity scale was calibrated relative to Fe. Recoil spectral analysis software [38] was used for the quantitative evaluation of the Mössbauer spectra. The X-ray diffraction (XRD) patterns were collected using a PW1820 Philips powder diffractometer (Philips, Eindhoven, Netherlands) with Cu Kα radiation. The microstructural features were obtained from the Rietveld analysis of the XRD data using the Powder Cell program. The JCPDS PDF database was utilized for the phase identification of the compound. The morphology of the powders and the sizes of individual crystallites were examined using a combined field-emission (scanning) transmission electron microscope (S) TEM (JEOL JEM-2100F) with an ultrahigh-resolution pole piece that provides a point resolution better than 0.19 nm at 200 kV. Prior to TEM investigations, powders were crushed in a mortar, dispersed in ethanol, and fixed on a copper-supported carbon grid.
2.3 Electrode Preparation for Electrochemical Studies
CaFe2O4 modified electrode was prepared by brush coating on a nickel foil (Ni) support and stainless steel (SS) support (typically 1 cm2) with a suspension of oxide powder, acetylene black and colloidal poly vinylidene fluoride (PVDF) binder (Kureha Chemical Industry Co., Ltd., Japan) in the weight ratio of 70:20:10. While mixing,
1-Methyl-2-pyrrolidone (CH3NCOCH2CH2CH2, Wako Pure Chemical Industries, Ltd., Japan) was used as the solvent. The composite was coated on nickel foil and stainless steel thin plates. The coated materials were heated in vacuum at 150 °C for 24 h to obtain a smooth adherent coating before they were used for electrochemical studies.
2.4 Electrochemical Measurements
All the electrochemical measurements were performed in a three-compartment glass cell, at room temperature, in 1 mol dm−3 KOH solution (Merck pro analysis). A platinum foil was used as the counter electrode and an Hg/HgO electrode was used as the reference electrode. The working electrode was the Ni/Ca Fe2O4 film and SS/Ca Fe2O4 film (geometric area 1 cm2). Electrical contact was provided with a Cu rod fitted into a Teflon rod. Cyclic voltammograms were recorded by the use of an electrochemical system Autolab PGSTAT 30. The cyclic voltammetric data were processed by the software GPES 4.9. The morphology of the thin film of CaFe2O4 coated electrodes before and after the electrochemical studies were examined using a Scanning Electron Microscope (JEOL-JSM-3.5 CF-Japan).
3 Results and Discussion
3.1 Charge State Characterization
The mechanically induced evolution of the Ca/α-Fe2O3 mixture subjected to high-energy milling was followed by 57Fe Mössbauer spectroscopy is shown in Fig. 1. The spectrum of the starting mixture Ca/α-Fe2O3 shows a sextet with a magnetic hyperfine field of 51.6(4) T corresponding to α-Fe2O3. With increasing milling time, the sextet becomes asymmetric toward the inside of each line, slowly collapses, and is gradually replaced by a central doublet. This spectral component can be assigned to the mechanosynthesized CaFe2O4 product [22]. It is interesting to note that the Mössbauer spectrum of α-Fe2O3 exhibits only a broadened sextet even if the particle size of the material reaches the nanoscale range; i.e., it does not display a super paramagnetic doublet. The fact that the spectral components, corresponding to the initial reactant (α-Fe2O3) and the product (CaFe2O4) phases, are clearly resolved in the spectra. It is evident that 57Fe Mössbauer spectroscopy provides a very sensitive probe for the estimation of the yield of the reaction.
The above results are complemented by the analysis of the room-temperature 57Fe Mössbauer data of paramagnetic CaFe2O4. In addition to the Mössbauer spectra showing the mechanosynthesis route to CaFe2O4 (Fig. 1), we present the discussion of the hyperfine parameters obtained by fitting the spectrum of the mechanosynthesized material. Taking into account the orthorhombic structure of CaFe2O4 with two nonequivalent iron positions, the spectrum of the mechanosynthesized material is fitted by using two quadrupole doublets (Fig. 2). The estimated isomer shifts values (IS1 = 0.216(7) mm/s, IS2 = 0.210(1) mm/s) of the doublet components are both typical for Fe3+ ions in sites octahedrally coordinated by oxygen [39]. The quadrupole splittings of the spectral components (QS1 = 0.613(7) mm/s, QS2 = 0.924(2) mm/s) reflect different values of the electric field gradients acting on Fe3+ nuclei in the two nonequivalent octahedral positions of the mechanosynthesized material. These values are larger than those reported for the conventionally synthesized (bulk) CaFe2O4 (QS1 = 0.30 mm/s, QS2 = 0.75 mm/s) [28, 40]. Note, however, that larger electric field gradients are typically observed for mechanosynthesized complex oxides [41]; they are produced by an asymmetric electronic charge distribution around the iron ions due to the distortion of polyhedra. It is found that for both octahedrally coordinated sites, the relative intensities of the spectral components are almost equal, reflecting the same occupation factor of iron cations within these structural units. Thus, the crystal chemical formula of the mechanosynthesized material can be written as Ca[Fe]oct1[Fe]oct2O4, where brackets enclose Fe3+ cations in nonequivalent distorted oxygen octahedra.
3.2 Powder Phase Characterization
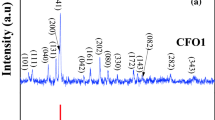
Figure 3 shows the XRD pattern of the mechanosynthesized CaFe2O4 product obtained after 1 h of milling of the Ca/α-Fe2O3 mixture. The Rietveld analysis of the XRD data of the mechanosynthesized material has revealed both an average crystallite size of about 18 nm and the presence of mean strains of 3 × 10−3 in the produced ferrite. Based on the analysis, the crystal structure of the mechanosynthesized product is found to be orthorhombic with the unit cell parameters a = 9.214(3) Å, b = 10.686(3) Å and c = 3.004(4) Å. It should be noted that these unit cell parameters are smaller than those reported for bulk CaFe2O4, JCPDS PDF 32-0168 (a = 9.230 Å, b = 10.705 Å, c = 3.024 Å) [42].
3.3 Powder Morphology Analysis
The high resolution Transmission Electron Micrographs of mechanosynthesized CaFe2O4 are shown in Fig. 4. The TEM images reveal the presence of nanoparticles with irregular shape. The particles have relatively broad size distribution ranging from 8 to 30 nm. The nanoparticle has the core–shell structure consisting of an ordered inner core surrounded by a disordered surface shell region. The thickness of the surface shell is found to be about 1.9 nm (Fig. 4b). The high-resolution TEM images show lattice fringes corresponding to the crystallographic plane (220) (d = 3.5 Å) of the CaFe2O4 phase. The lattice fringes cross the whole particle core demonstrating its single-crystalline character.
a High-resolution TEM image demonstrating the presence of CaFe2O4 nanoparticles of irregular shape with a relatively broad size distribution ranging from about 8–30 nm. b The core–shell configuration of mechanosynthesized nanoparticles with the thickness of the surface shell of about 1.9 nm is evident. The lattice fringes correspond to the crystallographic plane (220) (d = 3.5 Å) of the CaFe2O4 phase (JCPDS PDF 32-0168)
3.4 Cyclic Voltammetric Studies
Figure 5 represents the cyclic voltammograms corresponding to the CaFe2O4 film coated on stainless Steel (SS) substrate and that of the bare Stainless Steel (SS) substrate in 1 M KOH solution at 50 mV/s. No redox features are observed in the voltammogram. The onset of oxygen evolution occurs at the same potential in both the cases. However, the current density for oxygen evolution is found to be lower in the case of the ferrite coated SS substrate. For e.g. at 0.8 V, the current densities corresponding to oxygen evolution are 0.02 for the ferrite coated SS and 0.04 A/cm2 for the SS substrate. It is noted that the current density is two times lower on the CaFe2O4 coated electrode than the bare SS substrate.
Figure 6 represents the cyclic voltammograms corresponding to the CaFe2O4 film coated Ni substrate and bare Ni substrate in 1 M KOH at 50 mV/s. In the case of the bare Ni substrate a small anodic peak corresponding to the oxidation of Ni (OH) 2 is seen at around 0.57 V. On CaFe2O4 coating, the peak corresponding to the oxidation of Ni(OH)2 decreases to a great extent indicating that most of the Ni substrate is covered by the ferrite film. In this case also oxygen evolution occurs at the same potential as in the case of the coated and uncoated substrates. However, the current density for oxygen evolution is higher in the case of the ferrite coated electrode. At 0.8 V, the observed current densities for oxygen evolution turn out to be 0.015 and 0.04 A/cm2 respectively for the bare and ferrite coated Ni substrate respectively.
Figure 7 corresponds to the Tafel polarization curves recorded for the four substrates mentioned above (SS, CaFe2O4 coated SS, Ni and CaFe2O4 coated Ni).The observed Tafel-slopes and the over potentials corresponding to the fixed current densities are presented in Table 1. The catalytic behavior could not be compared based on the exchange current densities as the Tafel slopes are different for all the cases. This indicates that α (Transfer Coefficient) will be different for all the four cases and the path and mechanism will not be the same for them. Hence, a comparison of the electrocatalyic behavior is made at certain fixed current densities and at two low over potentials (Table 1) [13, 43]. The Tafel slope values are quite different in all the four cases under investigation. It is also observed from the literature that the Tafel slopes vary widely in the range 0.038–0.110 V/decade in the case of the ferrites Ni x Fe3−x O4, NiFe2O4, MgFe2O4, MnFe2O4 [9].
At a lower current density (say 1 mA/cm2) the over potentials are lower only for the bare substrates. At a higher current density (say 10 mA/cm2) the over potential is found to be lower for the ferrite coated Ni substrate compared to the bare substrate, while in the case of ferrite coated SS substrate the over potential is higher compared to bare SS substrate. Similarly a comparison is made at two different over potentials namely 625 and 675 mV. In this case also, it is observed that the current densities corresponding to ferrite coated SS substrate are lower compared to bare substrate, while in the case of ferrite coated Ni substrate, higher current density is observed for oxygen evolution compared to bare substrate. These results clearly reveal that the ferrite films on the Ni substrates exhibit better electro catalytic behavior compared to SS substrates. The experiments were repeated to check the reproducibility of the activities of the catalyst materials. The catalyst coated electrode substrates were found to be stable for nearly 20 cycles.
3.5 Morphological Analysis of Coated CaFe2O4
Figure 8a and b corresponds to the SEM images of the SS/CaFe2O4 electrodes before and after electrochemical investigations. No major change is observed in these two images. Morphological characteristics remain the same before and after electrochemical studies. Figure 8c and d corresponds to Ni/CaFe2O4 electrodes before and after electrochemical studies. It is noticed that the sizes of the granules on the electrode surface are reduced after electrochemical studies.
4 Conclusions
The possibility of preparing calcium ferrite through chemical transformation by mechanical energy has been studied using Ca/α-Fe2O3 as the reactants. Mechanochemical activation with high-energy ball milling promotes the synthesis process. The XRD data reveal the crystal structure of the product conforms to orthorhombic structure. Mössbauer spectra provide the yield of the reaction, information of the charge status, the local symmetry and the magnetic state of the iron ions in the mechanosynthesized ferrite material. The synthesized nano crystalline calcium ferrite can act as an electro catalyst for oxygen evolution in alkaline medium. In industrial electrochemical processes such as water electrolysis for hydrogen production, metal electro winning processes and in energy producing systems a stable anode material with low over voltage for the oxygen evolution is desirable. Based on the experimental results, CaFe2O4 can be used as a suitable anode material for such electrochemical applications.
References
Miles MH, Haung YH, Srinivasan S (1978) J Electrochem Soc 125:1931
Loucka A (1977) J Appl Electrochem 7:211
Iwakura C, Morita M, Manabe M, Tamura H (1980) Denki kagaku 48:91
Trasatti S (1984) Electrochim Acta 29:1503
Hoare JP (1968) The Electrochemistry of oxygen. Wiley-Interscience, New york
Hoare JP (1974) In: Bard AJ (ed) Encyclopedia of electrochemistry of the elements, vol 2. Marcel Dekker, New york, p 191
Damjanovic A (1969) In Bockris JO’M, Conway BE (eds) Morden aspects of electrochemistry, vol 5. Butterworths, London, p 369
Trasatti S, Lodi G (1981) In Trasatti S (ed) Electrodes of conductive metallic oxides part B, Elsevier, Amsterdan, p 521
Matsumoto Y, Sato E (1986) Mater Chem Phys 14:397
Singh RN, Singh JP, Lal B, Thomas MJK, Bera S (2006) Electrochim Acta 51:5515
Singh RN, Singh NK, Singh JP, Balaji G, Gajbhiye NS (2006) Int J Hydrog Energy 31:701
Singh RN, Singh JP, Nguyen Cong H, Chartier P (2006) Int J Hydrog Energy 31:1372
Singh RN, Lal B (2002) Int J Hydrog Energy 27:45
Ponce J, Rehspringer JL, Poillerat G, Gautier JL (2001) Electrochim Acta 46:3373
Singh RN, Singh NK, Singh JP (2002) Electrochim Acta 47:3873
Rios E, Gautier JL, Poillerat G, Chartier P (1998) Electrochim Acta 44:1491
Nikolov I, Darkaoui R, Zhecheva E, Stoyanova R, Dimitrov N, Vitanov T (1997) J Electroanal Chem 429:157
Zhang D, Tong Z, Xu G, Li S, Ma J (2009) Solid State Sci 11:113
Kubias B, Fait MJG, Schlogl R (2008) Mechanochemical methods. In: Ertl G, Knozinger H, Schuth F, Weitkamp J (eds) Handbook of heterogeneous catalysis. Wiley-VCH, Weinheim, p 571
Delogu F, Mulas G (2010) Experimental and Theroritical Studies in Modern Mechanochemistry. Transworld Research Network, Kerala
Šepelák V, Feldhoff A, Heitjans P, Krumeich F, Menzel D, Litterst FJ, Bergmann I, Becker KD (2006) Chem Mater 18:3057
Šepelák V, Becker KD (2004) Mater Sci Eng A 375–377:861
Šepelák V, Bergmann I, Feldhoff A, Heitjans P, Krumeich F, Menzel D, Litterst FJ, Campbell SJ, Becker KD (2007) J Phys Chem C 111:5026
Sivakumar N, Narayanasamy A, Ponpandian N, Govindaraj G (2007) J Appl Phys 101:84116
Boldyrev VV (2006) Russ Chem Rev 75:177
Ikenaga N, Ohgaito Y, Suzuki T (2005) Energy Fuels 19:170
Hirabayashi D, Sakai Y, Yoshikawa T, Mochizuki K, Kojima Y, Suzuki K, Ohshita K, Watanabe Y (2006) Hyperfine Interact 167:809
Tsipis EV, Pivak YV, Waerenborgh JC, Kolotygin VA, Viskup AP, Kharton VV (2007) Solid State Ionics 178:1428
Hana SB, Abdel-Mohsen FF, Emira HS (2005) Interceram 54:106
Sharma N, Shaju KM, Subba Rao GV, Chowdari BVR (2003) J Power Sources 124:204
Hirabayashi D, Yoshikawa T, Mochizuki K, Suzuki K, Sakai Y (2006) Catal Lett 110:269
Candeia RA, Bernardi MIB, Longo E, Santos IMG, Souza AG (2004) Mater Lett 58:569
Ma X, Zheng M, Liu W, Qian Y, Zhang B, Liu W (2005) J Hazard Mater 127:156
Berbenni V, Marini A, Bruni G, Milanese C (2008) J Anal Appl Pyrol 82:255
Isupova L A, Tsibulya SV, Kryukova GN, Budneva AA, Paukshtis EA, Litvak GS, Ivanov VP, Kolomiichuk VN, Pavlyukhin YT, Sadykov VA (2002) Kinet Catal 43:122
Gomez MI, de Moran JA, Carbonio RE, Aymonino PJ (1999) J Solid State Chem 142:138
Berchmans LJ, Myndyk M, Da Silva KL, Feldhoff A, Ŝubrt J, Heitjans P, Becker KD, Ŝepelak V (2010) J Alloys Compd 500:68
Lagarec K, Rancourt DC (1998) Recoil Mössbauer spectral analysis software for Windows. University of Ottawa, Ottawa, ON
Menil F (1985) J Phys Chem Solids 46:763
Hudson A, Whitfield HJ (1967) J Chem Soc A 376
Šepelák V, Becker (2000) J Mater Synth Process 8:155
Joint Committee on Powder Diffraction Standards (JCPDS) (2004) Powder diffraction file (PDF). International Center for Diffraction Data, Newton Square, PA, 2004
Mendonça MH, Godinho MI, Catarino MA, Da Silva Pereira MI, Costa FM (2002) Solid State Sci 4:175
Acknowledgements
The authors sincerely thank for the financial support given by German Academic Exchange Service-DAAD—Germany, Department of Science and Technology (DST), New Delhi, India and Council of Scientific and Industrial Research (CSIR), New Delhi, India to carry out this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berchmans, L.J., Karthikeyan, R., Helan, M. et al. Mechanochemical Synthesis and Electrochemical Characterization of Nano Crystalline Calcium Ferrite. Catal Lett 141, 1451–1457 (2011). https://doi.org/10.1007/s10562-011-0636-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-011-0636-9