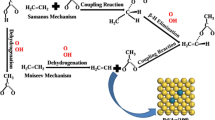
Abstract
Adsorption and dehydrogenation of formic acid, hydrazine and isopropanol have been investigated using periodic density functional theory (DFT). All the intermediates and transition states have been optimized and the preferred reaction pathways have been found. The adsorption energies for the most stable mode of formic acid, hydrazine and isopropanol are 38.6 kJ/mol, 63.9 kJ/mol and 46.1 kJ/mol, respectively. The dehydrogenation mechanisms of formic acid, hydrazine and isopropanol on Pd(111) surface are proposed and calculated. According to the calculation results, dehydrogenation of formate is more favorable than those of other molecules/groups, and that can be an explanation for the high reactivity of formats in Pd catalyzed transfer hydrogenation.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Catalytic transfer hydrogenation (CTH) is a practical alternative milder reduction method, which has been used for facile reduction of nitro-compounds and azides to amines, dehalogenation of mono- and poly-chlorinated aryl compounds [1, 2]. Several compounds, such as formic acid (or formate), hydrazine and isopropanol, have been selected as hydrogen donors [3–7], and Pd is a commonly used catalyst. All the experiments indicated that in Pd catalyzed CTH, formic acid and hydrazine were both effective hydrogen donors, while isopropanol was less active. According to the previous experimental study on CTH [1, 7–9], dehydrogenation of hydrogen donor on Pd surface was a vital process. Though previous reports on CTH experiment revealed macroscopic behavior of the hydrogen donors, the reaction detail remains unknown.
Numerous theoretical and experimental works on the interaction between organic molecules and Pd have been reported. These works mostly focused on the adsorption and dissociation of organic molecules, especially small molecules, on Pd. Periodic slab models and the cluster models have been used in the theoretical studies. If one needs to get insight into dehydrogenation on Pd surface, then periodic slab model is necessary [10]. In this work, Pd(111) model is used to perform comparative investigation on the adsorption and dehydrogenation of different hydrogen donors. Three hydrogen donors, formic acid, hydrazine and isopropanol, will be investigated.
2 Computational Methods
DFT calculations were performed with the program package CASTEP in Materials Studio of Accelrys Inc [11–13]. Plane wave basis functions with spin polarization and the Perdew, Burke, Erzenhof gradient corrected functional (GGA-PBE) were used [14–17]. The transition state search was performed with the linear and quadratic synchronous transit (LST/QST) complete search [18]. Ultrasoft pseudopotential (USP) was used to perform simulation of core electron [19]. Energy cut-off of 400.0 eV was used to improve computational performance.
Pd(111) surface was modeled using three-layer periodic slab model with a (3 × 3) super cell including 10 Å vacuum slab, within which the adsorption and dehydrogenation occurs. The reciprocal space of the (3 × 3) super cell was sampled with the k-points set of (3 × 3×2). Larger k-points sets are needed if more accurate energy value wanted. Study in this work focused on the relative results of different systems, so the k-points set of (3 × 3×2) should be enough. To confirm sufficiency of the selected model (super cell of 3 × 3, k-points set of 3 × 3×2 and 3-layer Pd), we compared three calculated adsorption configurations HCOOHbridge, N2H4bridge and C3H8Oon top with those calculated on a larger model, negligible discrepancy was produced. Similar model was used previously in calculations of reactions on metal surface and proved to be effective and convincing [20]. Geometry optimization was performed for all the relevant adsorbates with Pd atoms constrained except the uppermost layer. The convergence tolerances of energy and displacement were 2 × 10−5 eV/atom and 2 × 10−3 Å, respectively, and the SCF tolerance was 2 × 10−6 eV/atom.
The adsorption energies were calculated using the equation
where E ads represents the adsorption energy of the adsorbate on Pd(111) surface, E adsorbate is the energy of free adsorbate, E Pd is the energy of clean slab and E adsorbate–Pd is the energy of adsorbate-Pd adsorption system.
For a dehydrogenation reaction, which can be represented as: RH → R + H, the energy barrier was calculated as follows:
where E TS is the energy of transition state and E RH–Pd is the energy of RH–Pd adsorption system.
3 Results and Discussion
For clarity, this section is organized as follow. First, the adsorption information of formic acid, hydrazine and isopropanol is presented. Then, all the possible reaction pathways for are presented. Finally, the calculation results are summarized.
3.1 Adsorption of Hydrogen Donors on Pd(111)
In this subsection, adsorbed structures and energies for all the involved species are presented. Table 1 tabulates the information of all the possible adsorption modes.
3.1.1 Formic Acid
For free formic acid in gas phase, the calculated bond lengths are 1.106 (1.097) Å for C–H, 1.357 (1.343) Å for C–O1, 1.218 (1.202) Å for C–O2 and 0.986 (0.972) Å for O1–H, our results are in agreement with previous experimental values [21] displayed in the parentheses. Formic acid has two adsorption modes, “on top” and “bridge” (shown in Fig. 1). The calculated adsorption energies are 38.6 kJ/mol and 25.1 kJ/mol for the “bridge” and “on top” mode, respectively. The “bridge” mode forms via cleavage of the carbonyl π bond and bonding between the C, O2 atoms and two neighbor Pd atoms on the surface. The C–Pd, O2–Pd distance are 2.162 Å and 2.185 Å, and the C–O2–Pd, O2–C–Pd, O2–C–H, O2–C–O1angles are 113.5°, 104.1°, 121.9° and 121.0°, respectively. The four atoms (H, O1, O2 and Pd) that bond with C in formic acid compose a tetrahedron, with C as center. The extension of O2–Pd distance and configuration change of formic acid indicate that the hybridization of C has varied from sp2 in free formic acid to sp3 in adsorbed formic acid. This thus proves the cleavage of carbonyl π bond in formic acid. For “on top” mode, formic acid is adsorbed via the single O–Pd bond. The O–Pd distance is 2.305 Å, the C–O–Pd and O′–O–Pd angles are 156.5° and 129.2°, respectively. The molecule-surface distance in “on top” mode is about 0.2 Å longer than that in “bridge” mode, consist with the lower adsorption energy of “on top” mode. The calculation result indicates that the “on top” mode has a much lower adsorption energy on Pd(111) surface. Previous study gave less discrepancy on adsorption energies for the two adsorption modes on Pd(100) surface [22], probably due to the different Pd atom distributions on Pd(111) and Pd(100) surface.
3.1.2 Hydrazine
For gauche-hydrazine in gas phase, the calculated bond lengths and angles are 1.024 (1.015) Å for N–H, 1.447 (1.447) Å for N1–N2, 112.0°(112°) for N1–N2–H1 angle and 108.3°(106°) for N1–N2–H2 angle, consistent with previous experimental data [23]. For anti-hydrazine in gas phase, the calculated bond lengths and angles are 1.491 Å for N1–N2, 1.034 Å for N1–H, 104.1° for N1–N2–H1 angle and 104.0° for N1–N2–H2 angle. Hydrazine can be adsorbed via three modes, “bridge” for cis-hydrazine, “on top” for gauche- and anti-hydrazine (shown in Fig. 2). For the adsorption mode cis-hydrazinebridge, the bond lengths of N1–N2, N1–Pd and N2–Pd are 1.461 Å, 1.154 Å, 1.155 Å, respectively, and the angles of N1–N2–Pd and N2–N1–Pd are 105.6° and 108.4°. gauche-Hydrazine and anti-hydrazine have similar adsorption configurations of “on top” mode, the N–Pd bond lengths are 2.115 and 2.114, and the N2–N1–Pd angles are 117.3° and 121.8°, respectively. The calculation results indicate that the adsorption energy for “bridge” mode of cis-hydrazine is 63.9 kJ/mol, much more than that for “on top” mode of cis-hydrazine (41.8 kJ/mol) and anti-hydrazine (42.7 kJ/mol). The distance between hydrazine molecule and Pd surface is about 2.05 Å for “bridge” adsorption mode, while the distances are about 2.10 Å for the other two “on top” modes. Thus the shorter distance between hydrazine and Pd surface for “bridge” mode is in accord with the higher binding energy.
View of the adsorption structure of hydrazine on Pd (111) surface. Notation of atom is as same as Fig. 1, and blue ball represents N atom
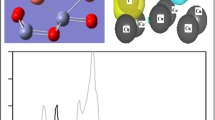
In acidic environment, hydrazine exists mainly as N2H6 2+, in which the N atoms donate the lone-pair electrons to bond with proton. Taking account of the steric hindrance around N atoms, N2H6 2+ may have a weak adsorption mode, and it is proved by the calculated weak adsorption energy (9.2 kJ/mol). We have also compared the density of states (DOS) of “naked” N2H6 2+, Pd slab and N2H6 2+/Pd. It is clear in Fig. 3 that no peak of new bonds appeared in adsorbed N2H6 2+–Pd system. The small peaks between 15 and 25 eV could be attributed to the inductive effect of Pd surface to the N2H6 2+ group. So N2H6 2+ binds onto Pd surface via physisorption. Then it is also difficult for N2H6 2+ to be dehydrogenated on Pd surface. Then we can conclude that basic environment favors the CTH that using hydrazine as hydrogen donor, consistent with previous experiment [7].
3.1.3 Isopropanol
For isopropanol in gas phase, the calculated bond lengths and angles are 1.448 (1.431) Å for C1–O, 0.976 (0.97) Å for O–H, 112.7°(112°) for C2–C1–C3 and 108.1°(105°) for C1–O–H, respectively, consistent with the calculated results [24]. It is generally believed that isopropanol is adsorbed via donation of the lone-pair electrons of O atom to solid surfaces [25, 26] (shown in Fig. 4), which is exhibited as “on top” mode. For isopropanol adsorbed via “on top” mode, the bond lengths of C–O, O–H and O–Pd are 1.487 Å, 0.981 Å and 2.212 Å, the angles C–O–H and C–O–Pd are 107.5° and 119.2°, respectively. The adsorption energy is 46.1 kJ/mol.
View of the adsorption structure of isopropanol on Pd (111) surface. Notation of atom is as same as Fig. 1
3.2 Dehydrogenation of Hydrogen Donors on Pd(111)
In this section, possible dehydrogenation pathways of the three species will be presented. Reaction energy and barrier of every elementary reaction are shown in Table 2.
3.2.1 Dehydrogenation of Formic Acid
Dehydrogenation of formic acid involves several pathways, which initiated by cleavage of C–H bond or O–H bond for both of the two adsorption modes. We performed calculation of the possible dehydrogenation routes, and the preferred pathway is shown in Fig. 5.
3.2.1.1 Initial O–H Bond Cleavage for “bridge” Mode
Three reaction pathways are involved into the dehydrogenation for “bridge” mode initialized by cleavage of O–H bond. In the initial step, the O–H bond is ruptured and Pd–H bond forms. Then the rest group rotates to form “on top” adsorption mode. However, HCOO has three more stable adsorption modes, “1bridge”, “3bridge” and “fcc” (see Fig. 6). The adsorption geometries and energies of HCOO are shown in Table 1. Zhang et al. [27] reported experimental parameters of adsorbed HCOO via “3bridge” mode, the detected bond lengths of O–Pd and C–O are 2.16 ± 0.06 Å (calculated values: 2.121 Å and 2.123 Å) and 1.26 ± 0.05 Å (calculated values: 1.274 Å and 1.274 Å), respectively, and the O–C–O angle is 130 ± 5° (calculated values: 129.6°). Thus our calculation results are in good agreement with the previous experiment. The “on top” mode can be transformed into “fcc” mode easily with no energy barrier, into “1bridge” and “3bridge” modes with barriers of 0.5 kJ/mol and 15.7 kJ/mol, respectively. The “fcc” mode can be transformed into “1bridge” and “3bridge” modes with barriers of 5.8 kJ/mol and 18.5 kJ/mol, respectively. Among the three adsorption modes of HCOO, the “1bridge” mode has the lowest C–H bond cleavage barrier (16.5 kJ/mol).
View of the adsorption structure of HCOO on Pd (111) surface. Notation of atom is as same as Fig. 1
The cleavage of O–H bond for “bridge” mode is endothermic by 11.2 kJ/mol with a energy barrier of 20.2 kJ/mol, which means that it is a slow step. Our result suggests that the “3bridge” mode for HCOO is the most stable adsorption mode with binding energy of 49.2 kJ/mol, consistent with the experiment [28]. Of all the three adsorption modes of HCOO, “1bridge” has the lowest dehydrogenation barrier. Besides, the mode transformation from “fcc” to “1bridge” is also a fast step (with barrier of 5.8 kJ/mol and reaction energy of 3.1 kJ/mol).
3.2.1.2 Initial C–H Bond Cleavage for “bridge” Mode
The cleavage of C–H bond for “bridge” mode is exothermic by 14.4 kJ/mol with energy barrier of 21.0 kJ/mol. After cleavage of C–H bond, a stable adsorption mode of COOH forms. The decomposition of adsorbed COOH may proceed along two pathways, COOH → CO2 + H and COOH → CO + OH, the calculation result indicates that the latter pathway is preferable. However, the dehydrogenation of OH is thermodynamically unfavorable with a high energy barrier of 56.5 kJ/mol. Taking account of that CO can escape from Pd surface, the decomposition of COOH can hardly produce active H.
3.2.1.3 Initial O–H Bond Cleavage for “on top” Mode
Dehydrogenation of formic acid for “on top” adsorption mode on Pd(100) has already been studied by Clarence, Yue and Lim [22]. Their calculation result showed little difference between initial cleavage of O–H bond and C–H bond thermodynamically, so the reaction should proceed along both of the two pathways according to their conclusion. However, our calculation result indicates that for adsorbed HCOOH on Pd(111) surface, the initial O–H bond cleavage is more favorable kinetically by 10.3 kJ/mol. So dehydrogenation of formic acid for “on top” mode is mainly initiated by cleavage of O–H bond. Further discussion will base on this approach.
Cleavage of O–H bond for “on top” mode HCOOH has a barrier of 12.5 kJ/mol and the reaction energy is 8.4 kJ/mol. Then the “on top” adsorption of HCOO forms, subsequent reaction pathways are as same as those discussed in Sect. 3.2.1.1.
3.2.1.4 Summary of Dehydrogenation of Formic Acid
After comparing all the elementary reactions, we can get the most favorable reaction pathway for dehydrogenation of formic acid on Pd(111) surface. Although the pathway HCOOHon top → HCOOon top + H → HCOO1bridge → CO2 + H has the lowest barrier, the “on top” adsorption mode of HCOOH is much less stable than “bridge” mode (by 13.5 kJ/mol). Thus only a few of the HCOOH molecules are adsorbed via “on top” mode, so the dehydrogenation is initiated from the “bridge” mode. Then the preferred pathway for the whole process of dehydrogenation of HCOOH is HCOOH → HCOOHbridge → HCOOon top + H → HCOO1bridge → CO2 + H. Therefore, almost all the product of dehydrogenation of formic acid is CO2, consistent with the CTH experiments [1, 4, 6]. Compared to formic acid, formate has more stable adsorption mode, so dehydrogenation of formate is more favorable than that of formic acid. So according to our calculation, formates are more effective hydrogen donors than formic acid, in agreement with the comparative experiment results [1, 2, 29].
3.2.2 Dehydrogenation of Hydrazine
Considering the significant difference on the adsorption energy between the three adsorption modes of hydrazine, almost all the hydrazine adsorbs via “bridge” mode of cis-hydrazine, so dehydrogenation of hydrazine will be investigated on the “bridge” model. The calculated structures of the reactants, intermediates and transition states for proposed reaction pathways are shown in Fig. 7.
It is clear in Table 2 that dehydrogenation of hydrazine mainly proceeds as H2NNH2 bridge → H2NNHbridge + H → H + HNNHbridge → HNNbridge + H → N2 + H. After dissociation of the first two H atom, the H–N–N–H dihedral angle is unfolded so that the last four atoms share a plane which parallels with Pd surface. Dissociations of the first three H have comparable reaction barriers, while the dissociation of the last H atom is much easier due to the instability of N2H.
We have also tried to perform calculation of dehydrogenation of hydrazine as an alternative sequence, but a high energy barrier (57.1 kJ/mol) has been found for the dissociation H2NNH → H2NN + H. So it is very difficult for the reaction to proceed along this pathway. We also considered cleavage of the N–N bond, but the high barrier and reaction energy indicated that it cannot lead to a preferred reaction pathway. No ammonia was detected in our experiment carried out in basic solution, consistent with the calculation results.
3.2.3 Dehydrogenation of Isopropanol
Three reaction pathways are found for dehydrogenation of isopropanol, involving four elementary reactions. The calculated structures of the reactants, products, intermediates and transition states for preferred pathways are shown as Fig. 8.
For the first pathway, the initial step is the cleavage of C–H bond on the tertiary carbon. The first step has the highest reaction barrier along the pathway (39.3 kJ/mol). Then new adsorption mode forms via formation of the C-Pd bond (tertiary carbon). The second step is the cleavage of O–H bond, with a barrier of 22.7 kJ/mol.
For both of the other two pathways, the first step is the cleavage of O–H bond. This reaction is energy unfavorable by 21.2 kJ/mol. Then the “on top” adsorption mode of C3H7O forms, which is less stable and prone to be transformed into “fcc” mode. No stable “bridge” adsorption mode of C3H7O has been found. Dehydrogenation of C3H7O for “on top” mode has lower energy barrier than that of the “fcc” mode and it is thermodynamically favorable. Dehydrogenation for “fcc” mode is a slow step with a high barrier of 54.8 kJ/mol.
Thus the possible reaction pathways of dehydrogenation of isopropanol have been found, the reaction prefers cleavage of O–H bond initially, following by subsequent cleavage of C–H bond from “on top” mode.
3.2.4 Summary of Dehydrogenation
From Table 2 we can see that in all the preferred pathways, dehydrogenation of formic acid has the lowest energy barrier (16.5 kJ/mol). So dehydrogenation of formic acid is much easier to proceed than the other two species both kinetically. The energy profiles of the preferred reaction pathways are shown in Fig. 9. It is notable that HCOO can be adsorbed more stably than formic acid, so formaets should be the most effective hydrogen donor in Pd catalyzed CTH. As dehydrogenation of isopropanol has a higher energy barrier and lower adsorption energy than hydrazine, isopropanol should be less reactive in Pd catalyzed CTH. Previous experimental study has given a good proof for this approach [7].
4 Conclusions
Adsorption and dehydrogenation of formic acid, hydrazine and isopropanol have been investigated using DFT. We have summarized the main points of this work as follows.
-
(1)
Formic acid adsorbs on Pd(111) surface via two modes, “on top” and “bridge”. Of all the species, hydrazine has the highest adsorption energy (“bridge” mode). There is only one stable adsorption mode for isopropanol, “on top” mode.
-
(2)
N2H6 2+ is found difficult to be adsorbed on Pd(111) surface. So basic condition is favorable for Pd catalyzed CTH reactions that use hydrazine as hydrogen donor.
-
(3)
All the preferred pathways for dehydrogenation of the three species are obtained. For formic acid, the most favorable reaction pathway is HCOOHbridge → HCOOon top + H → HCOO1bridge → CO2 + H, for hydrazine is H2NNH2 → H2NNH + H → HNNH + H → HNN + H → N2 + H, for isopropanol is C3H7OHon top → C3H7Oon top + H→C3H6O + H.
-
(4)
The preferred pathway for dehydrogenation of formic acid is found to be much more favorable than that of hydrazine and isopropanol, and hydrazine is more effective as hydrogen donor than isopropanol. Furthermore, as HCOO has more stable adsorption mode than formic acid, we can also explain why formate is more effective in Pd catalyzed CTH.
In order to get further insight into Pd catalyzed CTH reaction, the hydrogenation of acceptors should be introduced into the calculations, and a lot of work need to be done.
References
Rajagopal S, Spatola AF (1995) J Org Chem 60:1347
Arcadi A, Gerichelli G, Chiarini M, Vico R, Zorzan D (2004) Eur J Org Chem 2004:3404
Cellier PP, Spindler JF, Taillefer M et al (2003) Tetrahedron Lett 44:7191
Ukisu Y (2008) Appl Catal A: Gen 349:229
Sydnes MO, Isobe M (2008) Tetrahedron Lett 49:1199
Wiener H, Blum J, Sasson Y (1991) J Org Chem 56:4481
Kopinke FD, Machenzie K, Koehler R, Georgi A (2004) Appl Catal A: Gen 271:119
Reddy PG, Baskaran S (2002) Tetrahedron Lett 43:1919
Tike MA, Mahajani VV (2006) Chem Eng J 123:31
German ED, Sheintuch M (2008) J Phys Chem C 112:14377
Srivastava GP, Weaire D (1987) Adv Phys 26:463
Marlo M, Milman V (2000) Phys Rev B 62:2899
Payne MC, Teter MP, Allan DC, Arias TA, Joannopoulos JD (1992) Rev Mod Phys 64:1045
Delley B (2000) J Chem Phys 113:7756
Gajdos M, Eichler A, Hafner J (2004) J Phys: Condens Matter 16:1141
Van Santen RA, Neurock M (1995) Catal Rev Sci Eng 37:557
Gil A, Clotet A, Ricart MJ, Kresse G, Garcia-Hernandez M, Roesch N, Sautet P (2003) Surf Sci 530:71
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Halgren TA, Lipscomb WN (1977) Chem Phys Lett 49:225
Vanderbilt D (1990) Phys Rev B 41:7892
Staykov A, Kamachi T, Ishihara T, Yoshizawa K (2008) J Phys Chem C 112:19501
Landolt-Boernstein (1976) Structure data of free polyatomic molecules, vol II/7., New SeriesSpringer, Berlin
Yue C, Lim KH (2009) Catal Lett 128:221
Tarmyshov KB, Mvller-Plathe F (2007) J Chem Phys 126:074702
Harcourt RD, Klapoetke TM, White PS (1998) Inorg Chim Acta 269:1
Feng G, Huo CF, Deng CM, Huang L, Li YW, Wang JG, Jiao HJ (2009) J Mol Catal A: Chem 304:58
Zheng T, Stacchiola D, Saldin DK, James J, Sholl DS, Tysoe WT (2005) Surf Sci 574:166
Rajagopal S, Spatola AF (1997) Appl Catal A: Gen 152:69
Cai S, Sohlberg K (2003) J Mol Catal A: Chem 193:157
Acknowledgments
We acknowledge the funding support by a grant from the National Natural Science Foundation of China (No. 20776127), the National Key Technology R&D Program (No. 2007BAI34B07).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, S., Qian, C. & Chen, X. Comparative Theoretical Study of Adsorption and Dehydrogenation of Formic Acid, Hydrazine and Isopropanol on Pd(111) Surface. Catal Lett 141, 726–734 (2011). https://doi.org/10.1007/s10562-011-0553-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-011-0553-y