Abstract
Perovskite-like oxides (i.e., LaSrNiO4) prepared by combustion method with various complexes and amounts are compared and their catalytic performances for CO oxidation are tested. Results indicate that sample prepared with oxalic acid as the complex and 100% in excess of cations shows the best and stable activity for oxidation reaction, suggesting that oxalic acid is a preferential complex in the synthesis of perovskite-like catalyst by combustion method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Oxides with ABO3 and/or A2BO4 structure have been received great attention in catalysis since the report of Liby [1] and Voorhoeve et al. [2]. They possess lots of interesting properties, such as high thermal stability, controllable oxygen vacancies, changeable A- or B-site cations, etc. [3], which offer the possibility of studying the relationship between structure properties and catalytic performances [4], and hence comprehending the reaction nature. As a result, these oxides are widely applied in catalysis as catalyst of, such as, CO oxidation [5, 6], NO reduction [7, 8], CH4 combustion [9–11], etc.
Numerous methods have been adopted to prepare these mixed oxides, for example, spray pyrolysis [12], freeze-drying [13], ceramic [14], sol–gel combustion [15], etc. Among them, the so-called citric acid combustion method (i.e., using citric acid as the complex) is popularly applied due to its easy operation conditions. On the other hand, in order to tune the catalytic performance of catalyst, the way used in the literatures mostly is through the substitution of A- or B-site cation with a foreign one [16–20] or by a different preparation methods [21]. Few works [5] regarding the effect of complex (in combustion method) on the catalytic performance has been reported, especially no reason and sound explanation for (1) why citric acid is used as the complex and (2) how much citric acid should be used, is normally given. Hence, the objective of this work is to investigate the effect of complex on the catalytic performance of perovskite-type catalyst synthesized by combustion method, and subsequently to optimize the complex and its amount that are best for the synthesis of highly active perovskite-type catalyst.
Therefore, synthesized here are the mixed oxides (LaSrNiO4) by a combustion method with different types (basic and acidic) and different (carbon) amount of complex (i.e., blank, oxalic acid with different amounts, citric acid and ammonia). The catalytic performances of LaSrNiO4 prepared under different conditions are tested by CO oxidation reaction, since this reaction not only has an interest to eliminate CO—a poisonous compound from exhaust, but also is a test reaction for surface structure-activity correlation studies [22]. Results indicate that sample prepared with oxalic acid as the complex shows far higher activity than that prepared by citric acid, suggesting that oxalic acid would be a more preferential complex in the preparation of highly active perovskite- type catalyst by combustion method.
2 Experimental
The procedure for the preparation of LaSrNiO4 is similar to that reported elsewhere [23]. Namely, the stoichiometric La3+, Sr2+, Ni2+ nitrates were first dissolved in de-ion water, then a solution of oxalic acid, citric acid or ammonia 200% in excess of cations was added (for oxalic acid, 50 and 100% were also used; for blank experiment, the same procedure was done although no complex was added). The resulting solution was evaporated to dryness and subsequently, the precursors were thermal decomposed, calcined at 500 °C for 2 h, and finally pelletized and calcined in air at 900 °C for 5 h. According to the complex and its amount (in case of oxalic acid), the samples were defined as: LSN(B), LSN(O)_50, LSN(O)_100, LSN(O)_200, LSN(C), and LSN(A), respectively. Note: The letter in the bracket is the initial of the complex, e.g., symbol LSN(O)_100 means that LaSrNiO4 is prepared with oxalic acid as the complex and its amount is 100% in excess of cations.
Powder X-ray diffraction (XRD) was obtained from an X-ray diffractometer (type D/max-IIB, Rigaku) operated at 40 kV and 10 mA at room temperature, using Cu Kα radiation combined with nickel filter.
Catalytic reactions were tested using a single-pass flow micro-reactor made of quartz, with an internal diameter of 6 mm. The reactant gas (1.0% CO–0.5%O2/He) was passed through 0.2 g catalysts at a rate of 30 mL/min. The gas composition was analyzed before and after the reaction by an online gas chromatography equipped with a TCD detector. CO conversion is defined as:
where [CO]inlet and [CO]outlet is the initial and end CO concentration, respectively.
3 Results and Discussion
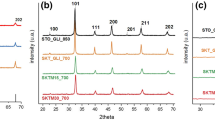
Figure 1 is the XRD patterns of LaSrNiO4 prepared by combustion method under different conditions. All of them show the two characteristic diffraction peaks indexed to oxides with A2BO4 structure at 30° < 2θ < 35°, indicating that the LaSrNiO4 phase is formed in all the samples. The formation of LaSrNiO4 in the blank experiment indicates that perovskite-type oxides could be synthesized by calcining the corresponding mixed oxides, without the assistance of complex. For samples prepared with the assistance of oxalic acid, the intensity of the diffraction lines weakens with increasing the amount of oxalic acid, indicating that high concentration of oxalic acid leads to the formation of poorly crystallized LaSrNiO4 phase. A possible reason is that the oxygen (in air) round the solution samples is largely consumed during the combustion process, leading to the deficiency of oxygen in the formation of metal oxides, which subsequently affects on the formation of perovskite-type oxides during the calcination step. The deficiency of oxygen becomes urgent at high concentration of oxalic acid according to the reaction: H2C2O4 + 1/2O2 = H2O + 2CO2. As a result, the formation of perovskite-type oxides (i.e., La + Sr + Ni + 2O2 = LaSrNiO4; the electron transfer is not marked here for convenience) is difficult with increasing the amount of oxalic acid, that is, the formation of a poorly crystallized LaSrNiO4 phase.
Although the diffraction peaks indexed to LaSrNiO4 are also observed when using citric acid as the complex, lots of impurities appear at this time, indicating that high concentration of citric acid solution are more disadvantageous for the formation of pure LaSrNiO4. The reason is similar as that discussed above. However, more oxygen will be consumed in the combustion process (H8C6O7 + 9/2O2 = 4H2O + 6CO2) at this time due to the more carbon atoms of citric acid. The deficiency of oxygen thus is more urgent and consequently, some of metal ions even can not enter the A2BO4 frame. These metal ions are the source of the “impurities” observed in XRD patterns.
To our surprise, the diffraction lines detected from sample prepared by ammonia solution (LSN(A)) is almost the same as those detected from sample prepared by oxalic acid solution (LSN(O)_200), suggesting that ammonia and oxalic acid may have similar effect on the formation of LaSrNiO4 at this time. But it should be noted that for ammonia with basic property, it has the possibility of producing OH− anion and its oxidation state can not be increased. Therefore, the metal ions in this case have the possibility of forming basic compound, e.g., Ni(OH)2, and the corresponding oxides are obtained not from the combustion process, but from the decomposition of the compound, e.g., Ni(OH)2 → NiO + H2O.
Figure 2 shows the catalytic performance of the samples for CO oxidation reaction. Overall, the best CO conversion is obtained from sample LSN(O)_100; while the worse one is from sample LSN(C), indicating that oxalic acid is a better complex than citric acid in the preparation of perovskite-type catalyst for CO oxidation. One may think that the worst activity of LSN(C) is due to the appearance of the impurities in this sample (see XRD patterns). However, even for a relatively pure LaSrNiO4 catalyst prepared by combustion method with 100% in excess of citric acid as the complex, we found that its activity for CO oxidation is also very low (~20% at T = 300 °C). This indicates that LaSrNiO4 prepared with citric acid as the complex can not show high activity for CO oxidation.
The activity of samples LSN(A) and LSN(O)_200 is similar at the beginning, since they have the similar XRD patterns. But an interesting change in their activity is observed: the activity of LSN(A) is a little higher at T ≤ 275 °C, but is a little lower at T ≥ 300 °C than that of LSN(O)_200. This indicates that the nature of them maybe different. The catalytic performance of them will be further discussed in Fig. 3 below. While for LSN(B), its activity for CO oxidation is moderate in all the temperature range.
Obviously, for samples prepared with oxalic acid as the complex but with different amounts, LSN(O)_100 shows the best activity while LSN(O)_50 shows the worst, indicating that the amount of complex has a great effect on the catalytic performance of the sample prepared. Hence, attention on the amount of complex should be paid when synthesizing perovskite-type mixed oxides by combustion method.
Finally, in order to see if there have any differences in the nature of samples LSN(A) and LSN(O)_200, we also tested their activities at different reaction time (T = 325 °C). The results in Fig. 3 indicate that the nature of them is different, that is, sample LSN(O)_200 shows relatively higher and stable activity than LSN(A). Based on this fact, the slight change in the activity of LSN(A) and LSN(O)_200 (see Fig. 2) could be explained as follows: although LSN(A) shows higher activity than LSN(O)_200 at T ≤ 275 °C, its activity will decrease substantially due to its instability, especially at t < 200 min., while LSN(O)_200 could show a relatively stable activity even at t = 30 min. As a result, LSN(A) shows a slightly lower activity than LSN(O)_200 with the increase of reaction time and at T ≥ 300 °C. The exact reason for the stability of activity is not clear yet, but it should be pointed out that for LSN(A), the complex is basic ammonia, which offers the possibility of forming basic compound and the starting oxide materials are obtained from the decomposition process, as discussed above; while for LSN(O)_200, the complex is acidic oxalic acid and the starting oxide materials are obtained through the burning of the complex.
4 Conclusion
In summary, this work studies the effect of preparation conditions on the crystal formation and catalytic performance of perovskite-like oxides in catalytic reaction. Results indicate that oxalic acid is a preferential complex for preparing highly active perovskite-type catalyst, and the optimum value is at 100% in excess of cations in the present case. Hence, we suggest that future efforts on this field should pay more attention, not to citric acid, but to oxalic acid, when choosing the complex.
References
Libby WF (1971) Science 171:499
Voorhoev RJ, Remeika JP, Matthias BT, Freeland PE (1972) Science 177:353
Voorhoeve RJH (1977) Advanced materials in catalysis. Academic Press, New York
Nitadori T, Misono M (1985) J Catal 93:459
Taguchi H, Yamasaki S, Itadani A, Yosinaga M, Hirota K (2008) Catal Commun 9:1913
Zhu JJ, Zhao Z, Xiao DH, Li J, Yang XG, Wu Y (2005) Ind Eng Chem Res 44:4227
Giannakas AE, Ladavos AK, Pomonis PJ (2004) Appl Catal B-environ 49:147
Liu J, Zhao Z, Xu CM, Duan AJ, Jiang GY (2008) J Phys Chem C 112:5930
Leontiou AA, Ladavos AK, Giannakas AE, Bakas TV, Pomonis PJ (2007) J Catal 251:103
Taguchi H, Nakade K, Yosinaga M, Kato M, Hirota K (2008) J Am Ceram Soc 91:308
Kharton VV, Sobyanin VA, Belyaev VD, Semin GL, Veniaminov SA, Tsipis EV, Yaremchenko AA, Valente AA, Marozau IP, Frade JR, Rocha J (2004) Catal Commun 5:311
Yokoi Y, Uchida H (1998) Catal Today 42:167
Traina K, Steil MC, Pirard JP, Henrist C, Rulmont A, Cloots R, Vertruyen B (2007) J Eur Ceram Soc 27:3469
Belessi VC, Trikalitis PN, Ladavos AK, Bakas TV, Pomonis PJ (1999) Appl Catal A-gen 177:53
Wang H, Zhao Z, Liang P, Xu CM, Duan AJ, Jiang GY, Xu J, Liu J (2008) Catal Lett 124:91
Zhu JJ, Xiao DH, Li J, Yang XG, Wu Y (2005) J Mol Catal A-chem 234:99
Knizek K, Daturi M, Busca G, Michel C (1998) J Mater Chem 8:1815
Teraoka Y, Nii H, Kagawa S, Jansson K, Nygren M (1996) J Mater Chem 6:97
Natile MM, Poletto F, Galenda A, Glisenti A, Montini T, De Rogatis L, Fornasiero P (2008) Chem Mater 20:2314
Li J, Singh UG, Schladt TD, Stalick JK, Scott SL, Seshadri R (2008) Chem Mater 20:6567
Natile MM, Ugel E, Maccato C, Glisenti A (2007) Appl Catal B-environ 72:351
Ciambelli P, Cimino S, Lasorella G, Lisi L, De Rossi S, Faticanti M, Minelli G, Porta P (2002) Appl Catal B-environ 37:231
Zhao Z, Yang XG, Wu Y (1996) Appl Catal B-environ 8:281
Acknowledgments
This work was financially supported by the program for New Century Excellent Talents in University (NCET-06-0577), the National Natural Science Foundation of China (NNSFC-20861001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, K., Zhong, P. & Zhu, J.J. Preparation of Highly Active and Stable Perovskite-like Catalyst by Combustion Method: Effect of Complex. Catal Lett 131, 672–675 (2009). https://doi.org/10.1007/s10562-009-9999-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-009-9999-6