Abstract
Various epoxides and aziridines undergo smooth ring-opening with water in presence of 10 mol% of bismuth triflate in acetonitrile/water (8:2) under mild reaction conditions to provide the corresponding vic-diols and β-amino alcohols in excellent yields with high regioselectivity.
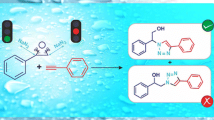
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aziridines and epoxides are versatile intermediates in organic synthesis and their reactions with different nucleophiles have been the subject of current importance [1–5]. They are well-known carbon electrophiles capable of reacting with various nucleophiles and their ability to undergo regioselective ring-opening reactions contributes largely to their synthetic value [6, 7]. In particular, vic-diols and β-amino alcohols are important classes of compounds in the synthesis of biologically active natural products, unnatural amino acids and chiral auxiliaries [8–12]. Some of these β-amino alcohols are being used as starting materials for the preparation of oxazolines, which have been widely explored as protecting groups for carboxylic acids [13]. The classical method for the nucleophilic ring opening of epoxides with water generally requires heating at elevated temperatures for long reaction times. The high temperature reaction conditions are not only detrimental to certain functional groups, but also to the control of regioselectivity [14, 15]. Subsequently, a variety of activators or promoters have been developed to perform the epoxide and aziridine ring opening reactions under mild conditions [16, 17]. Recently, metal triflates have been utilized for the ring-opening of the epoxides with alcohols, amines and phenols [18–22]. In particular, ring-opening of epoxides with phenols has been reported using bismuth triflate [23]. The aminolysis of epoxides has also been reported using bismuth triflate in aqueous media [24]. However, many of these methods suffer from one or more disadvantages such as the use of strongly acidic conditions, expensive/moisture sensitive reagents, stoichiometric amounts of catalysts, poor regioselectivity and low yields of desired products [18, 19]. Since, β-amino alcohols are very useful targets in medicinal chemistry, the development of simple, convenient and efficient approaches are highly desirable.
Lanthanide triflates are unique Lewis acids that are currently of great interest [25, 26]. The high catalytic activity, low toxicity, moisture and air tolerance and their recyclability, make the use of lanthanide triflates attractive alternatives to conventional Lewis acids [27–29]. However, lanthanide triflates are rather expensive and their use in large-scale synthesis is limited. Therefore, cheaper and more efficient catalysts are desirable. In this direction, bismuth triflate has evolved as remarkable Lewis acid catalyst for effecting various organic transformations [30, 31]. Compared to lanthanide triflates, bismuth triflate is cheap, non-toxic and is easy to prepare even on a multi-gram scale, from commercially available bismuth oxide and triflic acid [32]. However, there have been no reports on the use of bismuth triflate for synthesis of and β-amino alcohols through the hydrolysis of aziridines.
2 Results and Discussions
In view of the emerging importance of vic-diols and β-amino alcohols in organic synthesis and as part of our on going programme in developing new synthetic methodologies, we herein report an efficient ring-opening of epoxides and aziridines with water catalyzed by bismuth triflate in acetonitrile/water (8:2) system. Thus, treatment of styrene oxide (1a) with water in the presence of 10 mol% of Bi(OTf)3 in acetonitrile afforded styrene 1,2-diols (2) in 87% yield (Scheme 1).
This remarkable reactivity of bismuth triflate in water provided the incentive to further study of reactions with other epoxides. Interestingly, various terminal as well as cyclic epoxides underwent smooth cleavage with water to afford the respective vic-diols (entries a–m). Like epoxides, aziridines also reacted readily with water in the presence of bismuth triflate in acetonitrile to give the corresponding β-amino alcohols (entries n–r) in excellent yields (Scheme 2).
In the case of cycloalkyl aziridines, the stereochemistry of the ring-opened products was found to be trans from the coupling constants of the ring hydrogens at δ 3.35 (ddd, J = 4.5, 9.8, 9.8 Hz, 1H) for (CH-OH) in 1H NMR spectrum. Similarly the peak at δ 3.15 ppm for (CH-NHTs) showed the similar splitting pattern (ddd, J = 4.0, 9.5, 10.5 Hz, 1H). The reaction was highly regioselective in case of styrene aziridine (entry r, Table 1). All the reactions proceeded efficiently at room temperature and completed with in 3–5 h. The reactions were clean and no rearrangement of epoxide was observed even in case of styrene oxide under these conditions. The results are presented in Table 1. Unlike previously reported methods, the present protocol does not require either strongly acidic or harsh conditions to produce vic-diols or β-amino alcohols.
2.1 The Influence of Various Metal Triflates
The effects of different metal triflates such as In(OTf)3, Cu(OTf)2, Zn(OTf)2, Yb(OTf)3, and Sm(OTf)3 were tested for the hydrolysis of styrene oxide (1a) with water to produce styrene diol (2) and the comparative results are summarized in Table 2. As seen from Table 2, the products were obtained comparatively in low yields when Cu(OTf)2, Zn(OTf)2, and Sm(OTf)3 were used. Of these catalysts, bismuth(III) triflate was found to be the most effective catalyst in water in terms of conversion and reaction rates. Alternatively, 10 mol% of Sc(OTf)3 was found to be equally effective for this conversion. In absence of catalyst, the reaction was rather sluggish in water and resulted in very low yields (15–25%) of diols even under heating conditions.
2.2 The Catalytic Activity
Compared to conventional Lewis acid such as BF3·OEt2 and BiCl3, Bi(OTf)3 is particularly attractive because it can be used in water. Since a water solution of Bi(OTf)3 is acidic [24, 33, 34] it may be possible that the true catalyst is TfOH produced from hydrolysis of Bi(OTf)3. However, TfOH is not as effective as Bi(OTf)3 to catalyze the hydrolysis of styrene oxide (10% TfOH at 25 °C, 3 h, 65%) suggests that a Lewis acid is likely involved in activating the epoxide. Also, the practical use of Bi(OTf)3 is high valuable as TfOH is very corrosive and difficult to handle. Moreover, another Lewis acid like Bi(O2CF3)3 did not give good yield and selectivity. Thus, Bi(OTf)3 proved to be a better catalyst for the hydrolysis of epoxides and aziridines.
2.3 Catalysts Characterization
The catalyst Bi(OTf)3 was characterized by X-ray diffraction (XRD) and FT-IR and the spectra are shown in Figs. 1 and 2. The catalyst is highly crystalline and exhibiting the characteristic patterns of Bismuth. The FT-IR spectrum of Bi(OTf)3 contains strong absorption bands at 1,600 and 1,259 cm−1 which represents –SO2 and CF3, respectively.
2.4 Reusability of the Catalyst
The recovered aqueous solution of Bi(OTf)3 could be reused in further reactions with gradual decrease in activity. For example, treatment of styrene oxide with recovered aqueous solution of Bi(OTf)3 over 3 h gave 88, 80, and 75% yield, respectively, over three cycles. This observation clearly shows the reusability of the catalyst.
2.5 Selectivity of the Catalyst
Various bismuth(III) derivatives as BiCl3, Bi(OCOCF3)3, and Bi(OSO2Me)3 were found to be inefficient for the these reactions. For example, treatment of styrene oxide with Bi(OTf)3 in aqueous acetonitrile gave vic-diol exclusively whereas the same reaction using BiCl3 gave a mixture of vic-diol and chlorohydrin in 1:1 ratio. This experiment clearly shows the high selectivity of this procedure.
3 Conclusion
In summary, we have developed an efficient method for the ring opening of epoxides and aziridines with water using 10 mol% of Bi(OTf)3 in aqueous media. In addition to its simplicity and mild reaction conditions, this method provides high yields of products in short reaction times. The use of bismuth triflate makes it simple, convenient and practical method to prepare biologically relevant β-amino alcohols and vic-diols. Advantages of this method include the highly catalytic nature of the reagent, low toxicity and low cost of the Lewis acid catalyst, fast reaction rates and insensitivity of the Lewis acid to air and moisture.
4 Experimental
Melting points were recorded on Buchi R-535 apparatus and are uncorrected. IR spectra were recorded on a Perkin–Elmer FT-IR 240-c spectrophotometer using KBr optics. 1H-NMR and 13C spectra were recorded on Gemini-200 and Varian Bruker-300 spectrometer in CDCl3 using TMS as internal standard. Mass spectra were recorded on a Finnigan MAT 1020 mass spectrometer operating at 70 eV.
4.1 Typical Procedure
Styrene oxide (1 mmol), was dissolved in acetonitrile/water (8:2) in the presence of bismuth triflate (0.1 mmol) and was stirred at room temperature for appropriate time (see Table 1). The progress of the reaction was monitored by thin layer chromatography. After the complete conversion as indicated by TLC, the reaction mixture was extracted with ethyl acetate (2 × 10 mL). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure to obtain the crude product, which was purified by column chromatography using silica gel (60–120 mesh) to give pure styrene diol.
4.2 Spectral Data for Selected Products
3-(4-2-Methoxyethyl) Phenoxy-1, 2-propanediol (2f). Colourless oil, IR (KBr): υ 3,525, 3,330, 3,255, 2,970, 2,850, 1,605, 1,555, 1,420, 1,275, 1,130, 1,015, 930, 827, 730 cm−1. 1H NMR (200 MHz, CDCl3): δ 2.75 (t, 2H, J = 7.0 Hz), 3.30 (s, 3H), 3.52 (t, 2H, J = 7.0 Hz), 3.60–4.45 (m, 5H), 6.75 (d, 2H, J = 8.2 Hz), 7.05 (d, 2H, J = 8.2 Hz). EIMS: m/z (%): 226 (m+ 20), 152 (100), 94 (70), 77 (40), 43 (15).
Trans-2-(N-tosylamino)-1-cyclopentanol (4n). IR (KBr): υ 3,465, 3,186, 3,067, 2,951, 2,843, 1,612, 1,599, 1,510, 1,493, 1,357, 1,261, 1,127, 1,043, 971, 852, 736 cm−1. 1H NMR (200 MHz, CDCl3): δ 1.30–1.42 (m, 1H), 1.52–2.08 (m, 6H), 2.45 (s, 3H), 3.20–3.30 (m, 1H), 3.98–4.10 (m, 1H), 4.85 (d, 1H, J = 6.5 Hz), 7.36 (d, 2H, J = 8.0 Hz), 7.85 (d, 2H, J = 8.0 Hz). EIMS: m/z (%): 255 (m+ 15), 238 (22), 155 (32), 100 (100), 91 (12), 76 (28), 51 (35).
Trans-2-(N-tosylamino)-1-cyclooctanol (4q).IR (KBr): υ 3,540, 3,280, 3,155, 2,940, 1,580, 1,445, 1,415, 1,250, 1,120, 931, 820, 735 cm−1. 1H NMR (200 MHz, CDCl3): δ1.95–1.34 (m, 12H), 2.42 (s, 3H), 2.65–2.72 (m, 1H), 2.91–3.1 (m, 1H), 3.35–3.44 (m, 1H), 5.10 (d, 1H, J = 8.0 Hz), 7.42 (d, 2H, J = 8.0 Hz), 7.83 (d, 2H, J = 8.0 Hz). EIMS: m/z (%): 297 (m+ 20), 155 (30), 91 (100), 76 (20), 51 (25).
References
Katritzky AR, Rees CW (1984) Comprehensive heterocylic chemistry, vol 7. Pergamon Press, Oxford, p 47
Kump JEG (1991) In: Trost BM, Fleming I (eds) Comprehensive organic synthesis, vol 7. Pergamon Press, Oxford, p 469
Bonini C, Right G (1994) Synthesis 225
Paknikar SK, Kirtane JG (1983) Tetrahedron 39:2323
Smith JG (1984) Synthesis 629
Tanner D (1994) Angew Chem Int Ed Engl 33:599
McCoull M, Davis FA (2000) Synthesis 1347
Corey EJ, Zhang F (1999) Angew Chem Int Ed Engl 38:1931
Li G, Chang HT, Sharpless KB (1996) Angew Chem Int Ed Engl 35:451
Johannes CW, Visser MS, Weatherhead GS, Hoveyda A (1998) J Am Chem Soc 120:8340
Chang BL, Ganesan A (1997) Bioorg Med Chem Lett 7:1511
Ager DJ, Prakash I, Schaad DR (1996) Chem Rev 96:835
Jnaneshwara GK, Deshpande VH, Lalithambika M, Ravindranathan T, Bedekar AV (1998) Tetrahedron Lett 39:459
Deyrup JA, Moher CL (1969) J Org Chem 34:175
Crooks PA, Szyudler R (1973) Chem Ind (London) 1111
Meguro M, Asao N, Yamamoto Y (1994) J Chem Soc Perkin Trans 1:2597
Auge J, Leroy F (1996) Tetrahedron Lett 37:7715
Curini M, Epifano F, Marcotullio MC, Rosati O (2001) Eur J Org Chem 4149
HarrakY PujolMD (2002) Tetrahedron Lett 43:819
Likhar PR, Kumar MP, Bandyopadhyay AK (2001) Synlett 836
Andrews PC, Blair M, Fraser BH, Junk PC, Massi M, Tuck KL (2006) Tetrahedron Asym 17:2833
Chandrasekhar S, Ramachander T, Prakash SJ (2000) Synthesis 1817
Kamal A, Ahmed SK, Sandbhor M, Khan MNA, Arifuddin M (2005) Chem Lett 34:1142
Ollevier T, Compin GL (2004) Tetrahedron Lett 45:49
Imamoto T (1994) Lanthanides in organic synthesis. Academic Press, London
Molander GA (1992) Chem Rev 92:29
Kobayashi S (1994) Synlett 689
Kobayashi SJ (1995) Synth Org Chem Jpn 53:370
Kobayashi S (1999) Eur J Org Chem 15
Leonard MN, Wieland LC, Mohan RS (2002) Tetrahedron 58:8373
Iloughmane HG, Roux CL (2004) Eur J Org Chem 2517
Repichet S, Zwick A, Vendier L, Roux CL, Dubac J (2002) Tetrahedron Lett 43:993
Ollevier T, Nadeau E, Bégin AAG (2006) Tetrahedron Lett 47:8351
Frank W, Reiss GJ, Schneider J (1995) Angew Chem Int Ed Engl 34:2416
Acknowledgments
KP thanks to CSIR New Delhi, for the award of fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkat Narsaiah, A., Reddy, B.V.S., Premalatha, K. et al. Bismuth(III)-Catalyzed Hydrolysis of Epoxides and Aziridines: an Efficient Synthesis of vic-diols and β-Amino Alcohols. Catal Lett 131, 480–484 (2009). https://doi.org/10.1007/s10562-009-9927-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-009-9927-9