Abstract
The cesium salts Cs x H3−x PW12O40 of Cs content x = 2 up to x = 3 were tested as the catalysts in the gas and liquid phase reactions. Dehydration of ethanol and transesterification of triglycerides with methanol were selected as the catalytic reactions. Apart from the standard preparation, the catalysts were prepared by two-stage procedure with methanol or water as a solvent. The Cs-salts were characterized by FT-IR, XRD, scanning electron microscopy and energy dispersive X-ray techniques. In turn, the influence of Cs-salts composition on the pH and conductivity of their aqueous colloidal solutions was investigated. The results obtained by the latter techniques were also characteristic for acidity of surface layer of colloidal particles because of surface layer-solution equilibrium. It has been shown that the secondary structure of acidic cesium salts existing in crystalline samples (solid solution of H3PW12O40 in Cs3PW12O40) changes after contacting with polar medium to the system consisting most probably of Cs3PW12O40 core with epitaxial layer of heteropolyacid. This is result of the protons migration from bulk to surface layer of primary particles enhanced by polar medium. It strongly influences the surface acidity of primary particles as well as the activity of Cs-salts in transesterification of triglycerides with methanol. In such polar medium, Cs2HPW12O40 salt becomes the most active catalyst, more active than Cs2.5H0.5PW12O40. An accumulation of partial glycerides and in particular glycerol on the surface of primary particles of Cs-salts resulted in relatively low maximum conversion of triglycerides, most probably due to partial blockage of the catalytic centers. This effect and the almost constant activity of Cs-salts under recycling use in the transesterification experiments are considered to be experimental evidences that methanolysis over Cs-salts was accomplished with the participation of surface protons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The Keggin-type heteropolyacids are effective catalysts in both the homogeneous and the heterogeneous catalytic reactions [1, 2]. Their catalytic activity can be further improved by a partial neutralization, using e.g. cations like Cs+ to replace a part of acidic protons and/or oxonium ions, without changes of Keggin unit structure. The Cs2.5H0.5PW12O40 and Cs2HPW12O40 are the most investigated cesium salts of 12-tungstophosphoric acid. They both have structure built of Keggin units, but differ in the fraction of protons replaced by cesium cations. Although the Cs2.5H0.5PW12O40 salt has a smaller number of protons than the other Cs-salts it proves to be effective solid acid catalyst and in some cases even much more active than the other Cs-salts of lower number of Cs cations. This high activity of Cs2.5H0.5PW12O40 salt is explained by its high “surface acidity” resulting from extended microporous structure and large surface area [3–6]. The case of Cs2HPW12O40 salt is different because its catalytic and sorption properties are strongly dependent on the way it is being prepared [7].
In the literature it is shown that among cesium salts of tungstophosphoric acid the Cs2.5H0.5PW12O40 salt was the most active catalyst in the gas-phase reactions. However, some experiments showed that also Cs2HPW12O40 salt can demonstrate the highest catalytic activity e.g. in isomerisation reactions [8, 9]. In the case of reactions performed in liquid phase some authors reported that the Cs2.5H0.5PW12O40 salt was much more active than Nafion, HY-zeolite, Al3+-exchanged montmorilonite and SO4 2−/ZrO2 system [10–12] but there was not data for the activity of Cs2HPW12O40 salt. On the other hand, in the liquid phase alkylation of isobutene/butane the Cs1.9H1.1PW12O40 as well as Cs2HPW12O40 salts were found to be the most active [6, 13]. The latter salt exhibited also the highest conversion in the esterification of benzoic acid with n-butanol performed without solvent [14]. In turn, the salts with cesium loading of 2 or of 2–2.3 showed optimum performance in the transesterification of triglycerides with methanol [15–17].
In this paper we compare the catalytic activity of Cs x H3−x PW12O40 salts of Cs content ranging from x = 2 up to x = 3 in the reactions performed in the gas and liquid phase. The dehydration of ethanol in the gas phase and the transesterification of triglycerides with methanol in the liquid phase were involved as the test reactions. It is well known that cesium salts of tungstophosphoric acid during the reactions involving gaseous reagents are in the form of aggregates composed of primary particles. This aggregated state was formed when the colloidal solution of precipitated Cs-salts was slowly evaporated up to dryness. As mentioned before, under such reaction conditions, among Cs-salts the Cs2.5H0.5PW12O40 salt is commonly accepted to be the most active solid acid catalyst. However, it is very interesting to find out how the splitting of Cs-salts aggregates due to the presence of polar solvents would influence their catalytic activity determined in liquid phase reactions. Therefore, present work concentrates on the catalytic properties of Cs-salts for methanolysis of triglycerides accomplished in polar (methanol) medium. This reaction has been chosen because of its importance from environmental, technological and economic points of view. Transesterification of triglycerides derived from vegetable oils or animal fats with methanol yields methyl ester of fatty acids, known as Biodiesel and glycerol (Scheme 1). Biodiesel fuel belongs to ecological fuels and is an alternative and promising diesel fuel regarding the limited resources of fossil fuel and the environmental concerns.
2 Experimental
2.1 Catalysts Preparation
A commercially available 12-tungstophosphoric acid (H3PW12O40, HPW, Merck) was used to prepare the Cs-salts. From the DTG measurements the content of water in commercial HPW corresponding to H3PW12O40·24H2O was determined. The cesium salts Cs x H3−x PW12O40 of Cs contents x = 2, 2.25, 2.5 and 3 (denoted as Cs2, Cs2.25, Cs2.5 and Cs3) were prepared by two different procedures using Cs2CO3 (Aldrich) as the cesium ions source. In standard (one-stage) procedure, described previously [15], the stoichiometric quantities of cesium carbonate (0.04 M) were added to aqueous solutions of the tungstophosphoric acid (0.10 M). In two-stage procedure the Cs2.5 and Cs3 salts were prepared using the Cs2 salt, obtained by means of standard method, as the substrate. The known amount of Cs2 salt after drying at 393 K was dispersed again in water or methanol. To such colloidal solutions appropriate amounts of cesium carbonate dissolved in water or methanol were added to obtain Cs2.5-W, Cs3-W or Cs2.5-M, Cs3-M salts, respectively. In all cases the colloidal dispersions of precipitated Cs-salts were slowly evaporated overnight in the oven at 313 K to dryness.
2.2 Catalysts Characterization
The specific surface areas of samples were calculated from the nitrogen adsorption–desorption isotherms at 77 K in an Autosorb-1, Quantachrome equipment. Prior to the measurements the samples were preheated and degassed under vacuum at 473 K for 2 h.
The X-ray diffraction patterns (XRD) were obtained at 293 K with a Siemens D5005 diffractometer using Cu Kα radiation (55 kV, 30 mA).
The FT-IR spectra were recorded with Bruker-Equinox 55 spectrometer and standard KBr pellets technique. The samples before the XRD and FT-IR measurements were dried at 393 K in order to remove crystallization water.
Scanning electron micrographs (SEM) were recorded using Field Emission Scanning Electron Microscope JEOL JSM-7500 F. Energy dispersive X-ray (EDS) measurements were carried out for selected samples taking into account the Cs and W elements and the ratio of Cs/W contents was calculated.
The pH and conductivity of aqueous colloidal solutions of cesium salts were measured using WTW Inolab 740 apparatus. The concentrations of Cs-salts in aqueous solutions were the same as those applied in the transesterifcation of triacetin (0.00225 mol/dm3) or of castor oil (0.0115 mol/dm3) with methanol.
2.3 Catalytic Tests
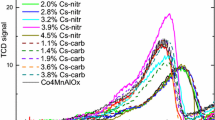
The catalytic experiments in the gas phase were carried out in a quartz flow reactor with the concentration of ethanol in the nitrogen of 1.5 μmol, in the temperature range from 373 to 548 K and GHSV = 10.000 h−1. Prior to the reaction samples were pretreated in the nitrogen at 523 K for 4 h. The products were analyzed using SRI 8610B gas chromatograph equipped with HayeSep D column, TCD and FID detectors.
The transesterification of triacetin (FLUKA) with methanol (molar ratio = 1:29) was performed at 323 K using the concentration of catalysts equal to 0.00225 mol/dm3. Methanolysis of castor oil (Microfarm, Poland) was performed at 333 K (molar ratio = 1:29) and catalyst concentration equal to 0.0115 mol/dm3. Prior to the reactions the Cs-salts were ground into white powders and in order to remove crystallization water were dried at 393 K. In standard procedure, the reactions were performed for 3 h and the content of methyl esters was analysed by GC methods. The detailed analysis procedure was described previously [15].
3 Results and Discussion
The results presented in the literature show that among cesium salts of HPW, the Cs2.5 salt is the most active catalyst in the gas phase reactions whereas Cs2 salt exhibits the highest activity in the reactions accomplished in liquid polar medium. This fundamental difference in the reactivity of acidic cesium salts of HPW suggests that secondary structure of primary particles of acidic cesium salts may change its architecture upon contacting with polar solvents like e.g. water or methanol. In order to prove this hypothesis the one-stage (standard) and two-stage preparations of Cs x H3−x PW12O40 salts were performed. The one-stage procedure was consisted of simple mixing of aqueous solutions of both reagents, Cs2CO3 and HPW. Figure 1 shows electron micrographs of cesium salts prepared by standard method. It can be clearly observable that the morphology of all three Cs2, Cs2.5 and Cs3 salts is similar. In the images of all the salts almost spherical aggregates can be seen, however, they differ relative to the size. Cs2 salt is composed of aggregates of 50–100 nm in size, whereas the aggregates in the case of Cs2.5 and Cs3 salts are remarkably larger, 150–350 nm. It should be stressed that especially well shaped quite spherical aggregates are formed in the case of Cs2.5 salt. The aggregates of all Cs-salts consist of primary particles of quite uniform size 10–20 nm. The size of aggregates is about 5–35 times larger than those of primary particles. Alike, the literature data show that such aggregated state of Cs-salts particles was to high extent split in water medium to form primary particles [15, 18–20].
The Cs2 salt obtained according to the standard procedure was the base to prepare the Cs2.5 and Cs3 salts by two-stage procedures. Before the two-stage preparation, Cs2 salt was dried at 393 K. It is well known from the literature that after annealing process at 373–473 K, as a result of protons/Cs-cations migration a secondary structure in the form of solid solution of H3PW12O40 in neutral cesium salt Cs3 is formed [20].
Next, the known amount of Cs2 salt was dispersed in water again to form colloidal dispersion. Assuming that the protons existing in the obtained primary particles of Cs2 salt can migrate in polar medium to their surfaces the appropriate amounts of aqueous solution of cesium carbonate were added to the colloidal solution of Cs2 salt to obtain Cs2.5-W and Cs3-W samples. During this preparation the protons existing on the surface of primary particles of Cs2 salt were partially (Cs2.5-W) or completely (Cs3-W) neutralized depending on the amount of Cs+ ions added. On the other hand, when the migration of protons in Cs2 particles did not occur it may be thought that the surface of its primary particles will be completely neutralized. It should be reflected by catalytic activity of Cs-salts obtained by two-stage procedure determined in gas-phase dehydration of ethanol.
The two-stage procedure was also applied to prepare Cs2.5-M and Cs3-M samples using methanol (M) as the solvent because methanol is the reactant in transesterification of triglycerides, catalytic reaction tested in the present work. In synthesis, methanol was used to form colloidal dispersion of Cs2 salt and appropriate amounts of methanol solutions of Cs2CO3 were added. The morphology of Cs2.5-W and Cs3-W was studied by SEM technique and the obtained micrographs are shown in Fig. 2. They show almost the same morphology as the samples prepared by standard method.
Energy dispersive X-ray (EDS) analysis was carried out for W and Cs elements in as-received standard Cs2 sample and both samples prepared by two-stage method, Cs2.5-W and Cs3-W. The ratio of W/Cs calculated from the EDS analysis is considered. The W/Cs ratio obtained from EDS data for standard Cs2 salt is equal to ca. 6 being almost the same as the stoichiometric value. In the case of Cs2.5-W and Cs3-W salts the W/Cs ratios from EDS analysis are determined to be 5.3 and 4.4, respectively. These values are close (with ca. 10% accuracy) to the stoichiometric values of 4.8 and 4 for Cs2.5 and Cs3 salts. These data prove that cesium cations were introduced to the standard Cs2 salt during the second stage of salts preparation.
The cesium salts obtained by standard and two-stage procedures in both, water and methanol solvents were characterized by FT-IR and XRD techniques to prove their Keggin structure (Fig. 3). Apart from that the FT-IR spectrum and XRD pattern for H3PW12O40·6H2O were also recorded for comparison.
The same set of four peaks characteristic of the Keggin structure can be seen in the FT-IR spectra of all the samples, parent HPW as well as Cs-salts, however, some of peaks in the spectra of Cs-salts are slightly shifted relative to those of HPW. The peaks characteristic of the Keggin structure in HPW are located: v as(P–O i) at 1,080 cm−1, v(W–O c –W) at 890 cm−1, v(W–O e –W) at 798 cm−1 and v(W=O t) at 982 cm−1. In the spectra of Cs-salts the latter two peaks only slightly shifted to 800 and 986 cm−1, respectively. It is experimental prove that in all the samples the polyanion structure is preserved (Fig. 3a). However, some changes in the micro-structure of Cs-salts may proceed as a result of cesium cations interactions with the host lattice.
The XRD diffraction patterns obtained for all samples are displayed in Fig. 3b. All cesium samples prepared by standard and two-stage procedures exhibit the XRD diffraction peaks characteristic of the cubic structure of Cs3 salt that differ from that of parent H3PW12O40·6H2O which crystallizes also in cubic structure but with a larger lattice parameter. The diffraction pattern recorded for parent HPW is in agreement with the literature data [5]. The most characteristic reflexes of Keggin units are located in the HPW diffraction pattern at 10.3°, 25.4°, 34.6°, 37.7° and 53.2° of 2θ. They are slightly shifted to higher values in the diffraction patterns of Cs-salts what confirm their shorter lattice parameters [5]. On the other hand the lack of peaks originating from crystalline heteropolyacid in the XRD pattern of acidic cesium salts confirmed the formation of solid solution after the samples were annealed at 393 K.
Before the use of Cs-salts as solid acid catalysts in gas phase reaction their specific surface area (BET) were measured. The obtained data are presented in Table 1. It is well known that a lot of preparation parameters (for instance temperature of precipitation and evaporation, duration, aging and grinding conditions) influence the properties of obtained Cs-salts, among them the specific surface area. This implies that the specific surface area of H3PW12O40·6H2O may be observed in the range from 2.2 to 7 m2/g, for Cs2 salt from 1 up to 71 m2/g, for Cs2.5 salt from 119 to 138 m2/g and for Cs3 salt from 92 to 208 m2/g. Literature data also show that the specific surface area of Cs2 salt prepared using cesium carbonate is very low of 0.5–1 m2/g being distinctly lower relative to that of parent HPW [5]. On the other hand, the surface area of HPW increases rapidly when cesium cations of 2.5 are introduced attaining ca. 130 m2/g. The same relationship between the Cs content and surface area was observed for our Cs-salts prepared by standard as well as two-stage procedures (Table 1). The specific surface area of HPW was determined to be 5 m2/g while that of Cs2 salt was distinctly lower and equal to ca. 1 m2/g. The surface area of standard Cs2.5 salt attained 136.7 m2/g and also high surface area was observed for standard Cs3 salt (119.8 m2/g). As shown in Table 1, specific surface areas of cesium salts prepared by two-stage procedures are only slightly lower than those of their standard Cs2.5 and Cs3 analogous. The specific surface areas of Cs2.5-W and Cs2.5-M salts are 99.0 and 96.5 m2/g whereas those of Cs3-W and Cs3-M samples are 99.6 and 100.7 m2/g, respectively.
The salts obtained in standard and two-stage procedures were applied as solid acid catalyst in the dehydration of ethanol performed in gas phase. This reaction was studied at temperatures within the range from 373 to 548 K and as the products ethylene, diethylether and water were formed. As shown in Table 1 practically the same conversions of ethanol were obtained in the presence of standard Cs2.5 salt and both Cs2.5-W, Cs2.5-M analogous prepared by two-stage method. Their similar catalytic activities may be considered as an evidence of their similar secondary structures. Moreover, it is also evidence that the same secondary structure of two-stage Cs2.5-W (Cs2.5-M) salt may be formed due to the migration of protons from bulk to surface of colloidal particles of starting Cs2 salt facilitated by polar medium, water or methanol. Analogical effect was observed for Cs3, Cs3-W and Cs3-M salts. It should be noted, that similar suggestions concerning the polar interactions between water molecules and protons existing in Cs2.5 salt, have also been formulated by Okuhara et al. [19].
Table 1 demonstrates that at each temperature of catalytic test, the Cs2.5 samples exhibit the highest activity in ethanol conversions. This is not surprise because Cs2.5 salt is well known to be an excellent solid acid catalyst in various gas phase reactions [21]. The observed lower activity of Cs2 salt in ethanol conversion is also in agreement with the literature data. This low activity was attributed to very low specific surface area of Cs2 salt. The remarkable activity is also observed for all Cs3 samples, although no protons are expected from their formula. It should be noted that the activity for Cs3 salt was also observed by Essayem et al. [6] in the methanol conversion and by Narasimharao et al. [16] in the transesterification of tributyrin by methanol. However, as literature data show [2, 5] the presence of residual protons due to not completely stoichiometric precipitation of Cs3 salts can not be excluded and as McGarvey and Moffat [22] demonstrated the neutral Cs3 salt contained 0.1 H+ per Keggin unit. This may explain the observed catalytic activity of our Cs3 samples. The presence of protons in Cs3 salt may be also explained by dynamic dissociation of the water molecules owing to interaction with weakly charged external oxygen atoms of heteropoly anions according to the reaction [23]:
Moreover, recently published data shows that the substitution of protons by alkali cations can cause also dissociation of water molecules, enhances the number of “free” protons and other proton species [24].
Similar catalytic activities of samples prepared by standard and two-stage procedures (Table 1) indicate that the acidity of their surfaces is close to each other. This result can be explained in the following way. During re-dispersion of standard Cs2 salt in polar medium like water or methanol the aggregated state of Cs2 salt split to some extent to form primary particles. This implies that secondary structure is changed from solid solution to the system most probably consisting of Cs3PW12O40 core covered by heteropolyacid as a consequence of protons migration to the surface of primary particles. This process causes the accessibility of all the protons for cesium ions added. From this reason appropriate part of protons is neutralized and Cs2.5-W or Cs2.5-M as well as Cs3-W or Cs3-M salts are formed. One can speculate that the formation of protons by dissociation of polar solvents, and its capture on surface may also proceed. However, from the present results obtained in gas-phase catalytic reaction, a contribution of the latter process seems to be less important. All the Cs-samples after drying at 393 K form solid solutions again. As the consequence, the standard Cs2.5 salt and its Cs2.5-W and Cs2.5-M analogous, having the same secondary structure show similar catalytic activities in the dehydration of ethanol, i.e. catalytic reaction involving gaseous reactants. The same effect is also observed for Cs3-series samples.
In order to characterize properties of Cs-salts forming colloidal dispersion, the measurements of pH and conductivity in aqueous colloidal solutions of cesium salts were performed. It can be expected that migration of protons from bulk to surface layers of primary particles facilitated by water should cause the increase of acidity of colloidal solution. It means that as a result of equilibrium between the surface of primary particles and liquid medium surrounding the particles, the pH and the conductivity measured for the colloidal solutions would change. In fact the pH values increases from Cs2 up to Cs3 salt (Table 2). Literature data show that in homogeneous solution of HPW the Keggin anions are stable at strong acidic conditions (pH ~1) because of their transformation to lacunary forms at higher pH [25]. However, owing to very low solubility of Cs2.5 salt in water or methanol determined by Kozhevnikov et al. [26] to be 3.7 and 2.7%, respectively, this transformation seems to be neglected in our experiments. Moreover, the formation of lacunary forms resulted in some changes in the FT-IR spectrum, in particular the sharp band at 1,080 cm−1 splits to two components, at 1,085 and 1,040 cm−1 [27]. This effect is not observed in the present studies.
The change of pH values versus cesium content (x) in the colloidal solution of our Cs-salts is shown in Fig. 4. Curves TACT and CAS correspond to the concentrations of Cs-salts used in transesterification of triacetin (TACT) and castor oil (CAS) with methanol, respectively. It is observed that the pH of colloidal dispersions of cesium salts diminishes in the sequence Cs3 > Cs2.5 > Cs2. The most surprising phenomenon is relatively low pH of colloidal solution of Cs3 salt. However, as described before, the precipitation of Cs3 salt may be incomplete causing the presence of residual protons. Moreover, as described before, the pH may be also explained by dissociation of water molecules according to the interaction with heteropoly anions or Cs-cations [23, 24].
In contrast to the pH data, the conductivity of colloidal solutions changes in opposite direction, it increases in the order Cs3 < Cs2.5 < Cs2 (Table 2). Some explanation is needed relative to the fact that the conductivities of Cs-salts prepared by two-stage procedure are ca. 2-times higher than these for standard salts, whereas the values of pH are practically the same. Our previous studies [15] demonstrated that the size of colloidal particles of Cs2 salt changed to smaller after its re-dispersion in water. Because Cs2 salt was the base for Cs2.5-W and Cs3-W preparation the increase of external surface area as a result of smaller diameter of the colloidal particles was main reason why the conductivity of colloidal solutions of these samples are higher than those measured for dispersed standard Cs2.5 and Cs3 salts.
It is obvious that both parameters measured in colloidal solution depend on the number of protons existing in the solvent, which is determined by the equilibrium between the solvent and the surface layer of primary particles of cesium salts. Thus, the values of both parameters indicate that acidities of surface layers of primary particles grow from Cs3 through Cs2.5 to Cs2 sample irrespective of the procedure used for the preparation of Cs-salts, standard or two-stage methods.
The transesterification of triglycerides proceeds in an excess of methanol and is catalysed by protons. Thus, it can be expected that migration of protons enhanced by polar solvent, methanol, would also explain the order of catalytic activity for Cs-salts of different Cs content determined in transesterification of triglycerides with methanol. Here, methanolysis of two triglycerides, triacetin (TACT) and castor oil (CAS) was studied. Triacetin, a shortest triglyceride, has the same chemical functionality as other triglyceride molecules and its methanolysis to form methyl acetate and glycerol proceeds via the diacetin and monoactin (Scheme 1). Castor oil (derived from Riccinus communis plants) is mentioned frequently in the literature as a potential raw material for Biodiesel. The main constituent of castor oil is triglyceride of 12-hydroxy-9-octadecenoic acid (ricinoleic acid). Due to the presence of OH group at C-12 carbon, castor oil is well soluble in methanol and methyl esters formed in methanolysis. As a result, homogeneity of reaction mixture was attained under transesterification of castor oil with methanol and ethanol [15, 28]. Similarly, triacetin is readily soluble in methanol and methyl esters formed in the reactions. Hence, there was a single liquid phase system and no separate phases of methanol or triglycerides appeared. Therefore, mass-transfer effects resulting from the presence of two phases (oil–methanol) encountered during the transesterification of natural oils may be neglected. Moreover, by using a triacetin as the model compound we are able to gain some insight into the reactivity of Cs-salts for partial glycerides formation. In experiments, an excess of methanol giving methanol/triacetin molar ratio of 29:1 was used. Catalytic experiments were carried out in the presence of standard Cs2, Cs2.25, Cs2.5, Cs3 salts as well as the Cs2.5 and Cs3 salts obtained by two-stage procedures.
Figure 5 shows the conversion of triacetin versus reaction time in the presence of Cs-salts. It can be clearly seen that among studied cesium samples the Cs2 salt is the most active catalyst. The catalytic activity of Cs-salts decreases as the content of Cs in HPW grows, in the order Cs2 > Cs2.25 > Cs2.5 > Cs3. In methanolysis of castor oil, the yield of methyl-ester is considered to compare the activity of Cs-salts. As described in our previous paper [15] castor oil used in experiments contained small amount of triglycerides of other fatty acids and this makes difficult the calculation of its conversion. As Fig. 5 also shows, the highest yield to methyl-esters is obtained in the presence of Cs2 salt whereas the yield is definitively lower when Cs2.5 salt is the catalyst. This indicates the same order of activity in methanolysis of castor oil. Moreover, the activity of salts prepared by means of two-stage procedure is practically the same as those determined in the presence of standard Cs-salts.
Figure 6 shows the relationship between the yield to bioester and the values of pH determined in aqueous colloidal solutions of standard cesium salts. For the samples prepared by two-stage procedure similar relationships are also observed. It is clearly observable that the catalytic activity of Cs-salts in the transesterification of triglycerides rises with the decrease of pH and the increase of conductivity of colloidal solutions. As described before, these values may be considered to be in direct relation with the acidity of surface layers of primary particles. It seems to be obvious that in colloidal solution formed in polar medium as a result of vigorous stirring the aggregates split and the primary particles become dominating. Thus, due to the migration of protons to their surfaces, most of the protons become easily accessible for the reactants. Therefore, this migration process may explain why Cs2 salt is the most active catalyst in liquid phase reaction, which is accomplished in polar solvent.
As described before, the Cs-salts of HPW readily dispersed in highly polar medium such as water or alcohol forming a colloidal suspension. When Cs x H3− x PW12O40 salts of various Cs contents (ranging from x = 0.9 to x = 3) were studied in the hydrolysis of ethyl acetate performed in an excess of water the salts particles formed a colloidal solution [29]. Under these conditions their activity decreased monotonously with increasing Cs content or decreasing acidity and the observed change of catalytic activity was related by Izumi et al. [29] to the bulk (formal) acidity of the Cs-salts. Similar order of activity is observed in the present catalytic reactions performed in methanol medium when the particles of Cs-salts are in colloidal states. Hence, the order of catalytic activity of Cs-salts observed in gas-phase dehydration of ethanol does not correspond to that determined in transesterification of triglycerides carried out in polar medium. This phenomenon is in full agreement with the catalytic results presented by Okuhara, Misono and co-workers [10, 14].
Moreover, it has been shown by Highfield and Moffat [30] that methanol is irreversibly bonded by H3PW12O40 via strong interaction with protons from HPW forming protonated species [CH3OH2]+. The influence of this type of methanol–proton species on the catalytic activity of H3PW12O40 has been observed in the gas-phase catalytic reaction, synthesis of MTBE (methyl-tert-butyl ether) from methanol and isobutene [31]. It can not be excluded that similar protonated species may also be formed in the case of colloidal solution of Cs-salts in polar medium, like methanol or water.
Now, the question arises as to whether the catalytic activity of Cs-salts in polar solvent (methanol) is due to the surface protons only. Proton migration enhanced by polar medium leads to an enrichment of surface of primary particles by protons. In extreme case, this may lead to the system consisting of Cs3 core with epitaxial layer of heteropolyacid. To clarify the stability of this system in methanol medium catalytic performance of Cs-salts is compared to that of parent H3PW12O40, which is well soluble in methanol and it acts as a homogeneous catalyst, similarly to what was reported by Morin et al. [32]. In catalytic test the concentration of HPW was equal to that of Cs-salts, i.e. 0.00225 mol/dm3. A typical course of triacetin transesterification in the presence of soluble HPW is shown in Fig. 7.
In homogeneous catalysis the rate of triacetin conversion was distinctly higher than that over Cs-salts and the maximum conversion of triacetin attained ca. 82%. Under these conditions triacetin was reacted, via diacetin and monoacetin as the intermediates, to form methyl-ester and glycerol (Fig. 7). This reaction pattern is typical and almost identical to those reported in the literature for methanolysis catalysed by homogeneous bases (KOH, NaOH, LiOH) [33] as well as acids [34]. Under homogeneous reaction partial glycerides, di and monoglycerides, exhibit a maximum concentration shortly after the start of the reaction and in final solution practically all glycerides (triglycerides, di and mono) are converted to methyl esters and glycerol [34]. Similarly, in the transesterification of glyceryl tributyrate performed on solid alkaline Li/CaO catalyst [35] the concentrations of di and monoglycerides peaked at the beginning of the reaction. The same profile for partial glycerides has been observed by Lopez et al. [36] for triacetin, the shortest triglyceride. Suppes et al. [37] also observed the peak of partial glyceride concentration at the start of the reaction catalysed by zeolite. As Fig. 8 shows the products distribution diagram in the presence of Cs-salts has a different shape from that obtained in homogeneous reaction catalysed by HPW. Firstly, in the presence of Cs2 and Cs2.5 salts definitively lower maximum conversions of triacetin were obtained, they attained 47 and 41%, respectively. Secondly, in the presence of Cs-salts the content of diacetin reached a plateau after a short reaction time. After the plateau, the content of triacetin as well as that of diacetin only slowly decreases. Monoacetin is observed only when the conversion of triacetin is relatively high. In addition, the monoacetin level was very low, not exceeding ca. 8–10% in all cases throughout the whole reaction progress (Fig. 8). The effect of plateau reaching for diglyceride has been observed by Cantrell et al. [38] in methanolysis of glyceryl-tributyrate in the presence of alkaline Mg–Al hydrotalcites. It has been shown in these studies that the rate of tributyrate conversion and associated methyl-ester and diglyceride formation were both first order in triglyceride concentration during the initial stage of reaction (<25% tributyrate conversion). The reaction kinetics deviates from simple first order behaviour after this point when the diglyceride reached a plateau. Similar effect has also been observed in the presence of heterogeneous alkali supported on CaO and MgO [39]. The effect of plateau reaching by partial glycerides has been explained by these authors as due to mass transfer limitation resulting due to the creation of glycerol film onto the solid catalyst. Moreover, the authors concluded that partial glycerides could also coat the catalyst particles. The presence of this glycerol/glycerides film could have two important effects. It could present a mass transfer barrier to the methanol and triglycerides. Furthermore, it could facilitate the reverse reactions thus shifting the equilibrium to left, to the intermediate products i.e. partial glycerides. As the consequence, two effects were observed on the reaction profiles diagrams; the plateau for diglycerides and the relatively high concentration of partial glycerides in the final solution [39].
In Fig. 9 the concentrations of partial glycerides (diacetin, monoacetin) are plotted versus the conversion of triacetin. The data obtained in the presence of homogeneously acting HPW are compared with those for Cs-salts. It is observed that at a given conversion of triacetin, the content of partial glycerides and in particular that of monoacetin is higher in the presence of Cs-salts than over soluble HPW. This implies that the conversions of diacetin to monoacetin and monoacetin to glycerol slowed down more than the other reaction steps in the presence of both Cs2 and Cs2.5 salts. This effect was not observed in the presence of soluble HPW. Hence, an accumulation of partial glycerides/glycerol occurs on Cs-salts and the effect is more pronounced in the presence of Cs2.5 than over Cs2 catalyst. Following the literature observation, this accumulation effect may be a reason of low maximum yield of methyl esters obtained in the presence of Cs-salts. In order to examine the supposed accumulation of glycerol/glycerides by Cs-salts, the recovered catalysts were studied by FT-IR technique.
The Cs-catalysts after the reaction were filtered off and slowly dried in air at temperature ca. 353 K. The obtained “white gel type solids” were studied by the FT-IR technique and the spectra of recovered Cs2 and Cs2.5 salts are displayed in Fig. 10. For the comparison, the spectra of as-received Cs-salts, glycerol and triacetin are also reported. In the spectra of recovered catalysts, apart from the distinct bands originating from the Cs-salts (at 798, 890, 986 and 1,080 cm−1) very strong band located at ca. 1,040 cm−1 can be seen. In the spectra of most of the reagents, triacetin, monoacetin and in particular glycerol, a relatively strong band is observed at ca. 1,030–1,050 cm−1. On the other hand, in the spectra of glycerides, triacetin and monoacetin, strong band at ca. 1,740 cm−1 more intense than the band at ca. 1,040–1,050 cm−1 occurs. However, the bands within such region (ca. 1,740 cm−1) in the spectra of recovered Cs-salts are very weak, whereas the band at 1,040 cm−1 is very strong. This may indicate that glycerol is the reagent mostly accumulated by the spent catalyst whereas the glycerides are present to lesser extent.
To verify the tendency of Cs-salts for the accumulation of glycerol, the reaction was carried out in the presence of glycerol. Thus, known amounts of glycerol (corresponding to 50 and 100% of glycerol content which can be formed in the experiment) were added to the reaction mixture before the catalytic experiment. After heating glycerol-containing reaction mixture up to the desired temperature, the catalyst was introduced and the reaction was started. Experiments were performed using HPW, Cs2 and Cs2.5 salts and the obtained results are reported in Fig. 11. From the reaction products diagram it can be clearly seen that the presence of glycerol exerts only slightly the triacetin conversion in the presence of soluble HPW, i.e. under homogeneous conditions. Due to added glycerol the conversion of triacetin decreased by ca. 5–10% only and no strong changes with respect to the content of partial glycerides are observed. In contrast to homogeneous conditions, the influence of added glycerol is very strong in the case of Cs-salts. A strong suppression of triacetin conversion from the very beginning of reaction is observed (Fig. 11). At the same amount of added glycerol, much higher inhibition of reaction is observed on Cs2.5 salt than in the presence of Cs2 catalyst. Moreover, in the presence of added glycerol, the content of partial glycerides (diacetin) observed at a given conversion of triacetin is much higher than in the reaction without added glycerol. Our results support literature supposition that some of reagents, like partial glycerides and/or glycerol may to some extent accumulate on the Cs-salts before they are reacted to final products. This accumulation may result in partial blockage of catalytic centres similarly to what was reported by Pouilloux et al. [40] during esterification of oleic acids with glycerol catalysed by acidic resin, Amberlyst. Strong interaction of glycerol with SO3H groups of Amberlyst via the system of hydrogen bonds leaded to significant blockage of catalytically active protons. Thus, interaction of glycerol with the protons of present Cs-salts leading to a partial blockage of catalyst seems to be very probable.
Now, the question arises as to whether the catalytic activity of Cs-salts in polar-methanol medium, is in fact a heterogeneous in nature. This problem has already been discussed in the literature. For instance, in quite recently reported paper focused on the use of Cs2 salt in transesterification of rapeseed oil with ethanol Hamad et al. [17] demonstrated by number of techniques that Cs2 salt presents a relative good resistance for the leaching in ethanol. Furthermore, Narasimharao et al. [16] noted that the Cs x H3−x PW12O40 salts of cesium content higher than x = 1 were stable to methanol reflux and in consequence the Cs-salts of x > 1 were recoverable catalyst with no leaching of HPW during methanolysis of tributyrin. However, no experimental data supporting this conclusion were reported. On the other hand Okuhara et al. [19, 22, 41] claimed that the Cs2.5 salt is a “water-tolerant” solid acid catalyst for hydrolysis of esters in the presence of excess water. However, catalytic activity of Cs2.5 salt under such conditions gradually decreased with successive reuse of the salt. Similarly, pre-treatment of Cs2.5 salt by hot water (at 353 or 393 K for 24 h) resulted in lower activity compared to that of no water treated sample. This decrease in activity was attributed to the liberation of small heteropoly clusters to water [19, 42]. They present the H+-rich heteropoly clusters not ionically dissociated but retain essentially the secondary structure of Cs-salts. Also the slow leaching from Cs2.5H0.5PW12O40 catalyst during methanolysis of vegetable oil has been observed by Chai et al. [42]. After six runs of catalytic experiment ca. 5.6% of the starting amount of the catalyst was dissolved. This result is close to the recently presented data [26] showing the solubility of Cs2.5 salt in methanol ca. 2.7%.
Here, the recycling use in methanolysis of triacetin was studied for Cs2 salts. As described before Cs-salts of HPW form colloidal solution in polar medium such as water or methanol because they consist of ultrafine crystallites (ca. 10–20 nm in average) and separation of catalyst by hot filtration or centrifugation was impossible, similarly to what was observed by Hamad et al. [17] for Cs2 salt. Even on using filter paper Sartorius 100 nm no transparent filtrates were obtained. Therefore, after completion of reaction (typically 3 h) the reaction mixture was cooled to room temperature and then the catalyst was separated by centrifugation. Later, the catalyst was three times washed with methanol and THF (tetrahydrofurane) to remove organic material from the catalyst, centrifuged after washing with each solvent, dried in air and stored in a desiccator. In recycling experiments, the same amount of fresh reactants (triacetin and methanol) was used in each methanolysis tests under the same reaction conditions. The recycling tests were performed three times and the obtained activity results are shown in Fig. 12. It can be seen that the conversion of triacetin slightly but continuously decreased during the recycling use of Cs2 catalyst. The conversion decreased from 34.2% for fresh Cs2 salt to 32 and 30% during subsequent recycling of the salt. The decrease in conversion may be ascribed to slow leaching of Cs2 catalyst in reaction mixture, similarly to what was observed for Cs2.5 salt by Chai et al. [42] in methanol medium and Okuhara et al. [41] in water medium.
In conclusion, an essential difference in the catalytic performance of soluble HPW and Cs-salts observed in the presence work may be experimental evidence that in the presence of Cs-salts the reaction is accomplished due to the surface protons. This conclusion is supported by the result showing the preservation of Cs-salts catalytic activity during the recycling use in transesterification of triglycerides with methanol.
4 Conclusions
It was shown, in agreement with number of literature reports, that among Cs-salts, of Cs content within the range x = 2 up to x = 3, the Cs2.5H0.5PW12O40 salt is the most active catalyst in the gas phase dehydration of ethanol. In contrast, in liquid phase transesterification of triglycerides with methanol the Cs2HPW12O40 salt exhibited the highest catalytic activity. We observed that the order of activity for Cs-salts (x = 2, 3) might change, depending on the texture-morphology of the salts particles during the catalytic reaction under working state of catalyst. In dehydration of ethanol involving gaseous reagents, the salts are in aggregated state consisting of primary particles and “surface acidity” being the result of specific surface area is a crucial for their activity. In polar medium (methanol) colloidal dispersion is formed because the aggregates split to form primary particles (ca. 10–20 nm in diameter). It has been shown that the acidic Cs-salts, which form solid solution of HPW in Cs3PW12O40 salt after annealing at 393 K can change their secondary structure, after contacting with polar solvent, to the structure consisting of Cs3PW12O40 core with epitaxial layer of heteropolyacid. The migration of protons from the bulk to surfaces of primary particles enhanced by polar (water, methanol) medium causes an enrichment of surface layer by protons what influences the activity of Cs-salts in the transesterification of triglycerides by methanol. This resulted in much higher activity of Cs2HPW12O40 salt than that of other Cs-salts, of higher Cs-content per Keggin unit. The catalytic properties of Cs-salts in methanolysis of triglycerides essentially differed from that of soluble, homogeneously acting parent H3PW12O40. An accumulation of partial glycerides and in particular glycerol on the Cs-salts resulted in relatively low maximum conversion of triglycerides, maybe due to partial blockage of the catalytic centers. This effect and almost constant activity of Cs-salts under recycling use in the transesterification experiments are considered to be experimental evidence that methanolysis over Cs-salts was accomplished with the participation of surface protons.
References
Okuhara T (2002) Chem Rev 102:3641
Kozhevnikov IV (1998) Chem Rev 98:171
McGarvay GB, Moffat JB (1991) J Catal 130:483
Gao S, Rhodes C, Moffat JB (1998) Catal Lett 55:183
Okuhara T, Mizuno N, Misono M (1996) Adv Catal 41:113
Essayem N, Coudurier G, Fournier M, Vedrine JC (1995) Catal Lett 34:223
Matachowski L, Lalik E, Haber J, Gil B, Brożek-Mucha Z (2009) submitted to Inorg Chem
Bardin BB, Davis RJ (1998) Top Catal 6:77
Okuhara T, Nishimura T, Ohashi K, Misono M (1990) Chem Lett 1201
Nishimura T, Okuhara T, Misono M (1991) Appl Catal 73:L7
Okuhara T, Nishimura T, Watanabe H, Misono M (1992) J Mol Catal 74:247
Essayem N, Kieger S, Codurier G, Vedrine JC (1996) Stud Surf Sci Catal 101A:591
Corma A, Martinez A, Martinez C (1996) J Catal 164:422
Koyano G, Ueno K, Misono M (1999) Appl Catal A 181:267
Zięba A, Matachowski L, Lalik E, Drelinkiewicz A (2009) Catal Lett 127:183
Narasimharao K, Brown DR, Lee AF, Newman AD, Siril PF, Tavener SJ, Wilson K (2007) J Catal 248:226
Hamad B, Lopes de Souza RO, Sapaly G, Carneiro Rocha MG, Pries de Oliveira PG, Gonzales WA, Andrade Sales E, Essayem N (2008) Catal Commun 10:92
Nakato T, Toyoshi Y, Kimura M, Okuhara T (1999) Catal Today 52:23
Nakato T, Kimura M, Nakata S, Okuhara T (1998) Langmuir 14:319
Okuhara T, Watanabe H, Nishimura T, Inumaru K, Misono M (2000) Chem Mater 12:2230
Tatematsu S, Hibi T, Okuhara T, Misono M (1984) Chem Lett 6:865
McGarvey GB, Moffat JB (1991) J Catal 128:69
Ukshe EA, Leonova LS, Korosteleva AI (1989) Solid State Ionics 36:219
Holclajtner-Antunovic I et al (2009) Submitted to deview book. Solid State Ionics
Zhu Z, Tain R, Rhodes C (2003) Can J Chem 81:1044
Alsalme A, Kozhevnikova EF, Kozhevnikov IV (2008) Appl Catal A 349:170
Pope MT (1983) Heteropoly and isopoly oxometalates. Springer, Berlin
Plentz Meneghetti SM, Menghetti MR, Wolf CR, Silva EC, Lima GES, De Coimbra MA, Soletti JI, Carvalho SHV (2006) JAOCS 83:819
Izumi Y, Ono M, Kitagawa M, Yoshida M, Urabe K (1995) Microporous Mater 5:255
Highfield JG, Moffat JB (1995) J Catal 95:108
Małecka A, Poźniczek J, Micek-Ilnicka A, Bielański A (1999) J Mol Catal A Chem 138:67
Morin P, Hamad B, Sapaly G, Carneiro Rocha MG, Pries de Oliveira PG, Gonzalez WA, Andrade Sales E, Essayem N (2007) Appl Catal A 330:69
Arzamendi G, Arguinarena E, Campo I, Zabala S, Gandia LM (2008) Catal Today 133–135:305
Di Serio M, Tesser R, Pengmei L, Santacesaria E (2008) Energy Fuels 22:207
Watkins RS, Lee AF, Wilson K (2004) Green Chem 6:335
Lopez DE, Goodwin JG Jr, Bruce DA, Lotero E (2005) Appl Catal A 295:97
Suppes GJ, Dasari MA, Doskocil EJ, Mankidy PJ, Goff MJ (2004) Appl Catal A 257:213
Cantrell DG, Gillie LJ, Lee AF, Wilson K (2005) Appl Catal A 287:183
MacLeod CS, Harvey AP, Lee AF, Wilson K (2008) Chem Eng J 135:63
Pouilloux Y, Abro S, Vanhove C, Barrault J (1999) J Mol Catal AChem 149:243
Kimura M, Nakato T, Okuhara T (1997) Appl Catal A 165:227
Chai F, Cao F, Zhai F, Chen Y, Wang X, Su Z (2007) Adv Synth Catal 349:1057
Acknowledgments
The authors wish to acknowledge Dr D. Mucha for XRD measurements. A. Zięba acknowledges a Ph.D. grant of the Polish Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matachowski, L., Zięba, A., Zembala, M. et al. A Comparison of Catalytic Properties of Cs x H3−x PW12O40 Salts of Various Cesium Contents in Gas Phase and Liquid Phase Reactions. Catal Lett 133, 49–62 (2009). https://doi.org/10.1007/s10562-009-0149-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-009-0149-y