Abstract
Transition metal doping into the TiO2 lattice can expand the response of these metal oxide nano particles to the visible region. In view of this, Mo6+ ion is doped into the TiO2 lattice in order to understand the mechanism of its photo response. The prepared photocatalysts were characterized by X-ray diffraction, UV–Visible absorption spectroscopy, UV–Visible diffuse reflectance spectroscopy, SEM, EDX, FTIR and BET specific surface area techniques. The characterization results have confirmed the incorporation of metal ions into TiO2 lattice. XRD analysis shows no change in crystal structure except a slight variation in crystallite size and elongation along the c-axis with increase in the concentration of the dopant. Diffuse reflectance measurements showed a shift in the band edge position to longer wavelengths and an extension of the absorption to the visible region. The photo degradation efficiencies of these catalysts were studied with Tebuconazole pesticide as model pollutant. Under UV light, undoped catalyst showed higher activity than doped catalyst. But in the case of visible light irradiation Mo doped TiO2 with intermediate dopant concentration of 0.06 atom % had the highest photocatalytic reactivity. This may be due to the narrowing of band gap so that it could effectively absorb the light of longer wavelength. The degradation path way was followed by UV–Visible spectroscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanostructured semiconductors in heterogeneous photo catalysis have drawn considerable attention over the past few decades. This is due to their large surface area, high Redox potential of the phtogenerated charge carriers and selective reduction/oxidation of different classes of organic compounds. Among the semiconductors being studied, TiO2 is one of the most efficient photocatalyst under UV light irradiation [1, 2]. The basic principle of semiconductor photocatalysis involves the generation electron-hole pairs which serve as redox sources. These pairs are able to initiate oxidation and reduction reactions on the TiO2 photocatalyst [3]. The overall photo reactivity of TiO2 is determined by various factors such as generation of charge carriers, trapping of charge carriers and rate of formation of OH radicals. In photocatalysis process electron-hole pairs are generated in femtosecond time regime which must be trapped in order to avoid recombination which takes place around 100 nanoseconds. Duration of interfacial charge transfer and charge carrier recombination is around 100 nanoseconds. The competition between these processes governs the photocatalysis. The hydroxyl ion (OH−) traps the valence band holes, leading to the formation of hydroxyl radicals which are strong oxidants. The adsorbed O2 molecules acts as traps for conduction band electrons, leading to the formation of super oxide species (O −2 ) which are unstable, reactive and may involve in several reactions [4].
Although TiO2 is an efficient photocatalyst under UV light, many attempts have been made to study its photo response under visible light. But it is found that TiO2 utilizes only 3–5% of the solar spectrum. Doping methods have been extensively utilized for modifying the electronic structures of TiO2 nanoparticles to improve the photo response under solar light irradiation [5–7].
When a transition metal is doped into the TiO2 lattice, these dopants can either segregate on TiO2 nanostructure surface or they can be incorporated into the lattice. The dopants can be substitutional, interstitial or both when incorporated. Dopants with different locations have different impact on the TiO2 properties. It is mostly the substitutional dopant ions that contribute to the change of electronic structure and light absorption efficiency of the host. The substitutional dopant ions can induce an electronic coupling effect with the host atom and bring possible electron states within the band gap of the semiconductors [6]. The dopant related localized states on either above the valence band or below the conduction band are favorable to the band gap change, which in turn affects the photon absorption.
The location and the local bonding configuration of the dopants in TiO2 are difficult to predict. The coordination environment of the dopants and its position is affected not only by the nature of the dopant (ionic radii and concentration) but also on the synthesis method. The quantity of dopants in the lattice will be limited by its solubility in TiO2 lattice.
Doping of TiO2 is done by various methods such as Ion implantation, sol–gel reaction, solid-state reaction etc., In the present research the Mo doped TiO2 is prepared by using solid state reaction and its photocatalytic activity is studied with a pollutant tebuconazole pesticide under both UV/Solar light illumination.
Tebuconazole (TBZ) is an organo chlorine fungicide has gained renewed interest, particularly the threat of Asian soybean rust. TBZ is mainly used against diseases of turf grasses, vegetables, citrus, field crops and ornamental plants. TBZ can be applied as foliar sprays and in seed treatments. They may be applied as protectant or in curative treatments. If it is applied as a curative treatment, TBZ applications must be made early in the fungal infection process. Once the fungus begins to produce spores on an infected plant, the TBZ is not effective. Although TBZ doesn’t have the degree of systemic movement like many herbicides, they are xylem-mobile. They are readily taken up by the leaves and move within the leaf. Carbon-14 labeling studies have shown that three droplets of a labeled sample of TBZ fungicide applied to a soybean trifoliate leaf covered the entire leaf within 1 day. The TBZ is very specific in their mode of action; it inhibits the biosynthesis of sterol, a critical component for the integrity of fungal cell membrane. The brief contact with TBZ may cause slight skin irritation and redness, but inhalation of TBZ dust can cause irritation of the nose, lungs and throat. Photochemical degradation under natural sunlight is reported to be very slow in soil samples with 86% of the parent compound recovered after 34 days of irradiation. Similar inhibition effect was observed in aqueous medium [8].
The present paper focuses on the comparative study of photocatalytic efficiency of bare TiO2 and Mo doped TiO2 under UV/Solar light irradiation. These photo catalysts were characterized by various analytical techniques.
2 Materials and Methods
2.1 Reagents
Titanium (IV) Chloride is obtained from Merck Chemicals Ltd, (NH4)6Mo7O244H2O from Sisco-Chem Industries, Bombay. NH4OH, HCl,(NH4)2S2O8 and NaOH were from Sd Fine chemicals and used as supplied. Double distilled water was used for all the experiments.
2.2 Pesticide
Tebuconazole is an organo chlorine pesticide which is obtained from an industry (pure technical grade) and used as received. The molecular weight of pesticide is 307.8, molecular formula is C16H22N3ClO, IUPAC name is ((RS)-1-p-chlorophenyl-4, 4-dimethyl-3-(1H-1,2,4-triazol-1-ylmethyl)pentan-3-ol) and its λmax is 194 nm (Scheme 1).
2.3 Preparation of TiO2 and Mo Doped TiO2
The optically pure, fine grained anatase TiO2 is prepared by using sol–gel method as described below.
100 mL of high purity TiCl4 was carefully diluted by adding drop-wise to 250 mL of ice cold, well stirred double distilled water and the resulting solution was diluted to 500 mL. 25 mL of above diluted TiCl4 made acidic by adding around 1 mL of conc. H2SO4 in a beaker and diluted to 1 liter with double distilled water. Liquor ammonia was added till the pH of the diluted solution attains 7–8 to obtain hydroxide of Titanium gel. The gel obtained is allowed to settle down and the gel was washed several times to remove Chloride and ammonium ions. The precipitate obtained is oven dried for 12 h at 100 °C. The oven dried TiO2 was ground in a mortar. The fine powder obtained was subject to heat treatment at 600 °C to obtain the anatase form of TiO2 [9].
A known concentration of ammonium molybdate solution is added to TiO2 sample to get dopant concentration of 0.02, 0.04, 0.06 and 0.1 atom percent. These mixtures were manually grinded in a agate mortar. All these samples were oven dried at 100 °C for 12 h. During the process of heating the samples were repeatedly grinded in a mortar and finally calcined at 600 °C for 4–5 h. The Stoichiometry of the prepared samples is Ti1−x Mo x O2, where ‘x’is the atom percent of Mo.
2.4 Analytical Instruments
2.4.1 X-ray Diffraction
The XRD patterns of the powders were recorded using Phillips powder diffractometer Pw/1050/70/76 with Cu Kα radiation under the scan rate of 2°/min.
2.4.2 Absorption/Diffuse Reflectance Spectroscopy
The absorption and reflectance spectra were recorded by using UV–Visible Shimadzu double beam spectrophotometer 3101PC UV-VIS-NIR instrument. The spectra were recorded at room temperature in the range of 190–800 nm.
2.4.3 Scanning Electron Microscopy and Energy Dispersive X-ray Analysis
SEM was performed using a model JSM840 microscope operating at 25 kV on specimens upon which a thin layer of gold or carbon had been evaporated. An electron microprobe is used in EDX mode to obtain quantitative information on the amount and distribution of the metal species in the samples.
2.4.4 FTIR Spectral Analysis
FTIR spectra were recorded using Nicollet IMPACT 400 D FTIR spectrometer, over the range of frequencies from 4,000–400 cm−1 using KBr as the reference sample.
2.4.5 Specific Surface Area Measurements
The specific surface area measurements were determined by Digisorb 2006 surface area, pore volume analyzer-Nova Quanta Chrome Corporation instrument using multipoint BET adsorption.
2.5 Photo Reactor and Light Source
Both UV and Solar illumination were used as energy sources in this study. The UV light source is a medium pressure mercury vapor lamp and the photon flux is found to be 7.75 mW/cm2 as determined by ferrioxalte actinometry, the wave length of which peaks around 340–370 nm. The photo reactor consists of a glass vessel with the exposure area of 153.86 Sq.cm. The photo degradation experiments were carried out by direct exposure of light into the reaction mixture. All the experiments were performed at room temperature in presence of atmospheric oxygen with a constant lamp power of 125 W. The distance between the photo reactor and the lamp housing is 29 cm. Experiments using solar light were carried out from 11 am to 3 pm during the summer season in the months of April–June at Bangalore, India. The latitude and longitudes are 12.58 N and 77.38 E respectively. The average sunlight intensity was found to be around 1,200 Wm−2. The intensity of the solar light was concentrated by using a convex lens and the reaction mixture was exposed to this concentrated solar light.
The degradation path way was followed by examining the concentration of pesticide using their maximum absorbance in a UV–Visible spectrometer.
2.6 Experimental Procedure
The experiments were conducted with 250 mL of 2 ppm pesticide with 150 mg of TiO2/Mo doped TiO2. The reaction mixture was exposed to UV/Solar light for 150 min with constant stirring. The samples were withdrawn at regular intervals for analysis and centrifuged to separate the photo catalyst particles. The degradation path way was followed by UV–Visible Spectroscopy.
3 Results and Discussions
3.1 Structural Characterization of the Photo Catalysts
Catalytic properties of photo catalysts depend on its composition and structure on atomic scale. The performance of a catalyst depends on number and nature of active sites on the catalyst. Therefore the prepared catalysts were characterized by adopting following physico-chemical methods.
3.1.1 X-Ray Diffraction
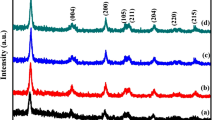
Figure 1 shows the crystal structure of undoped TiO2 and Molybdenum-doped TiO2 as determined by X-ray diffraction. The diffraction patterns of doped powders were similar with those of pure anatase TiO2. The average anatase crystallite size were determined by Scherer formula (D = kλ/βCOSθ, where D = Crystallite size, k = share factor, λ = wavelength, θ = diffraction angle, β = Full width at half maximum) and it is found to be 26.3 nm for TiO2 sample. The data obtained from the Scherer equation shows a slight increase in crystallite size with increase in Mo content. From the figure it is clear that X-ray diffraction peaks of crystal plane (101) shifts to higher diffraction angle with increase in the Mo content and hence a slight increase in crystallite size is observed (Fig. 1b). Further a change in lattice parameter corresponding to the elongation of the c-axis is also observed. The dopant can enter the TiO2 lattice either interstitially or it can act as substitutional impurity. Substitution is governed by the factors like atomic size, crystal structure, electro negativity and relative valency. If these properties can match closely, substitution is possible for all compositions. In the present case the Mo is in +6 oxidation state and Ti is in +4 oxidation state. The ionic radius of Mo6+ is 0.062 nm and that of Ti4+ is 0.0605 nm. Due to the similarity in the ionic radii of Mo6+ and Mo4+ (0.068), they can replace Ti4+ in the lattice. The charge compensation is achieved mainly by the ionized vacancies especially doubly ionized oxygen vacancies. The doping Mo ion in TiO2 lattice appears to follow Vegards’s law which states that “Change in the unit cell dimension should be linear with change in the composition” This confirms the possibility of Mo substitution in TiO2 lattice [10]. It can be observed from the Table 1 that unit cell parameter along c-axis and the crystallite size keep increasing with dopant concentration up to the dispersion capacity. This linearity confirms the fact that the Mo is incorporated as substitutional dopant (Fig. 1c). The change in lattice parameters may also indicate the oxygen vacancies which are usually created along with the incorporation of impurity [11]. In the case of metal oxides there is a critical value of dispersion capacity, at values lower than which the oxide might become highly dispersed on the support without the formation of crystalline phase. Since no characteristic peaks corresponding to Mo species is present, it can be concluded that the Mo loading is below the dispersion capacity. From the XRD data, unit cell parameters, unit cell volume, crystallite size and density of all the samples is presented in Table 1.
3.1.2 UV–Visible Absorption Spectra
The UV–Visible absorption spectra of different photocatalyst nanoparticles are shown in Fig. 2, The absorption spectrum of Mo doped TiO2 shows a red shift with increase in the dopant concentration in the TiO2 lattice. This may be due to the increase in the crystallite size. According to the energy band structure of TiO2, the optical absorption in the UV range is mainly attributed to the electronic transitions from the valence band of O2p to conduction band of Ti3d (O2p → Ti3d). The optical absorption in the wavelength range 400–550 nm results from sub-band transitions closely related to the ionized oxygen vacancies [12] and also due to the transitions from the mid band gaps formed by the Mo doping. According to the diffuse reflectance spectra the sub band levels created by ionized oxygen vacancies possibly exist below the conduction band. This may lead to different ways of electronic transitions (i) The electrons can be excited to conduction band from the top of the valence band (ii) The excitation of electrons can also take place to different levels of sub-bands related to oxygen vacancies.
Figure 2 shows the absorption band in the visible region which is mainly due to doping Mo into TiO2 lattice. The extent and magnitude of absorbance is increases with the Mo content in the TiO2 lattice. This may be also due to the increase in the surface oxygen vacanies amount. If the Mo6+ ion content is too high, TiO2 would be covered with the excess Mo on the surface (deposition of the metal on the photocatalyst). This can reduce the effective area of TiO2 surface for absorbing light. This will result in the decrease of absorbance for TiO2 nanoparticles. Therefore, it can be predicted that photo induced charges of TiO2 nanoparticles can be improved by doping an optimum amount of Mo6+ ions. This leads to the formation of mid band gaps in between the valence and conduction band.
The optical absorption spectra show two distinct maxima around 300–400 nm and 200–220 nm for both TiO2 and Mo doped TiO2 powders. These maxima corresponds to A1 (3.59 eV) and A2 (5.76 eV) bands reported for bulk crystals representing the electronic transition from Γ15 → Γ12 and Γ25 → Γ12 respectively [13–15]. The splitting of A2-A1 corresponds to 2.17 eV, this could be due to the presence of Mo6+ in TiO2 lattice. The electronic configuration of Mo6+is [Kr] 4d0 5s0. Since Mo6+ does not have any valence electron, it gives extra stability to participate in electronic transitions of the doped catalyst. The introduction of energy levels by the dopants into the band gap of TiO2 imparts color to the material. These results indicate that the Mo doped TiO2 can be a good catalyst under Visible light.
3.1.3 UV–Visible Diffused Reflectance Spectra
The reflectance data obtained was relative percentage reflectance to a non absorbing material (BaSo4) which can optically diffuse light. P. Kubelka and F. Munk developed a theory for the diffuse reflectance of powdered samples. The Kubelka–Munk equation for an “Infinitely thick” layer is shown in Eq. 1 [16].
where ‘R ∞’ is the absolute reflectance of the layer, ‘s’ is the scattering coefficient and ‘k’ is the molar absorption coefficient.
The band gap energies of photo catalysts were calculated by using Kubelka–Munk plots of (1 − R ∞)2/2R ∞ versus wavelength (Fig. 3).
The band gap energies of various photo catalysts are listed in Table 2. Incorporation of various atom % of Mo dopants into TiO2 lattice shifts the position of its fundamental absorption edge towards the longer wavelength. In the case of 0.06% Mo doped TiO2, the steeply diminishing region of Kubelka–Munk plot is around 480 nm, corresponds to the band gap of 2.58 eV. This absorption edge could be caused by the substitutional Mo doping. The electronic transition in undoped TiO2 is from O-2p valence band to Ti-3d conduction band. When Mo is incorporated into TiO2, it can dissolve inside the crystallites or settle in the grain boundaries. They can also agglomerate if the maximum solubility is exceeded. It can precipitate either at the center of crystallites or at the grain boundaries. As the mechanisms involved are complex and multiple, it is difficult to predict the exact role of dopant levels acting as active centers which are incorporated into the TiO2 crystal lattice. Main possible reactions are shown below.
If Molybdenum replaces titanium, a chemical reaction using Kröger and Vink [17] notation can be written in this way
The temperature has been supposed high enough to ionize the Molybdenum atoms so that each gives up one electron. If this is not the case, the number of ionized molybdenum atoms is settled by the ionization energy at the given temperature according to Fermi-dirac distribution [18]. Instead of creating conduction band electrons, charge compensation can also be achieved by lattice defects such as interstitial oxygen’s or titanium vacancies [19].
If Mo2O6 is dissolved substitutionally in TiO2 and if the predominating defects are oxygen vacancies, the reaction may be written as
where MoTi is a Molybdenum ion on a Titanium lattice site, \(V_{Ti}^{\prime \prime \prime \prime} \) is titanium vacancy (four times charged) and \(O_{i}^{\prime \prime} \) is oxygen vacancy in interstitial position(two times charged), MoiTi is molybdenum ion on titanium lattice in interstitial position.
The electronic transitions in Mo doped TiO2 may be from O-2p valence band to Mo-5d-V ••o level created just below the conduction band edge by 0.58 eV. Since incorporation of certain metal ions into TiO2 lattice is usually accompanied by vacancy in oxygen site as shown in the Eqs. 3–5. These vacancies can be singly ionized, doubly ionized or neutral. The newly created energy level below the conduction band edge at 0.58 eV with in the band gap is responsible for the activation of the catalyst under visible light illumination.
The magnitude of the reflectance increases with increase in the dopant concentration. The color of the obtained catalyst depends on the amount of the dopant into the TiO2 lattice [20, 21].
3.1.4 SEM
The micro structural characterization of TiO2 and Mo doped TiO2 nanoparticles were carried out via scanning electron microscopy. Figure 4 shows the SEM image of the nanoparticles. The SEM image confirms that Mo doped TiO2 presents a porous and sponge like network of high roughness and complexity and this results in a high surface area. Further it can also be noted that particle size decreased after Mo incorporation, which results an increase in surface area for the modified systems.
3.1.5 EDX
The elemental composition of the TiO2 and Mo doped TiO2 samples were determined using EDX analysis and the results are listed in Table 3 From the EDX data, it is clear that theoretical atom % of the samples were very close to the experimental values. Thus by this solid state reaction it is possible to get desired loading of the metals into TiO2 lattice.
3.1.6 FTIR Absorption Analysis
The FTIR spectra of the TiO2 and Mo doped TiO2 samples are illustrated in Fig. 5. The spectrum of TiO2 displays strong absorption bands at 488 and 548 cm−1 which are due to the vibration of the Ti–O bond in the TiO2 lattice [22, 23]. The peaks around 807–850 cm−1 may be assigned to stretching vibration of O–O. The peak observed at 660 cm−1 may be assigned to the vibration of the Ti–O–O bond [23]. The new peaks obtained at 1,317 and 2,351 cm−1 for the Mo doped TiO2 powders, can be assigned to the Mo–O vibrations. The shifting of the peaks to higher wavelengths and formation of new peaks confirms the doping of Mo into TiO2 lattice.
The IR spectrum of OH groups in the parent TiO2 shows band at 3,402 cm−1. This peak was assigned to isolated surface hydroxyl groups [24, 25]. The band intensity of the free hydroxyl groups increases with Mo content and the wave number shifts to higher frequencies from 3,402 to 3,436 cm−1. Simultaneously a peak at 2,934 cm−1 appears in Mo doped TiO2. This band is indicative of OH groups at Mo atoms on non-framework positions and represents acidic hydroxyls of varied strengths.
3.1.7 Specific Surface Area Measurements
The Nitrogen adsorption–desorption curve shows the characteristic type II isotherms (IUPAC classification) with small hystersis loop. The surface area values are higher for Mo doped samples. Specific surface area, total pore volume, and average diameter of modified TiO2 are summarized in Table 4. It can be observed that the surface area of the TiO2 photocatalyst increases with the introduction of Mo into the TiO2 lattice and the pore volume and average diameter almost remains constant for doped samples. Therefore it can be inferred that when Mo is incorporated, the metal is homogeneously distributed in the system.
3.2 Comparative Study of Photocatalytic Activities of TiO2/Mo Doped TiO2 Nanoparticles Under UV/Solar Light Illumination
3.2.1 UV Light Illumination
Photocatalytic experiments were conducted under UV light for a period of 2.5 h using TiO2 and Mo doped TiO2 Photocatalysts. The extent of degradation of the pesticide is studied by withdrawing samples for analysis at any desired time interval during irradiation. The apparent kinetics of disappearance of TBZ was followed by UV–Visible spectroscopic technique. The kinetics of disappearance of TBZ was estimated by the standard calibration curve at its λmax. The plot of C/C0 versus Time, where C0 is the initial concentration of the pesticide, C is the concentration of pesticide at given instant of time ‘t’ is shown in the Fig. 6.
C/C0 versus Time under UV light. a—illumination of only pesticide solution, b—pesticide with only TiO2, c—pesticide solution with TiO2 in presence of (NH4)2S2O8, d—pesticide solution with 0.04% Mo doped TiO2 in presence of (NH4)2S2O8, e—pesticide solution along with 0.06% Mo doped TiO2 and (NH4)2S2O8, f—pesticide solution along with 0.1% Mo doped TiO2 and (NH4)2S2O8
From the figure it can be observed that 95% degradation of TBZ is achieved with TiO2 in presence of (NH4)2S2O8 (Curve c). But in presence of TiO2, only 20% of degradation takes place (Curve b). Further the photo degradation of TBZ is only 5% under UV-light alone even after 6 h of irradiation. Therefore in the presence of both catalyst and Oxidizing agent the degradation is more efficient. These Oxidizing agents act as electron acceptors. They act as conduction band electron traps, which reduces the recombination rate thereby increasing the photo degradation efficiency. These acceptors are known to generate hydroxyl radicals which are thought to be the primary oxidizing species. They are known to react with aromatic hydrocarbons leading to the formation of hydroxylated derivatives and subsequent reaction of these intermediates results in complete mineralization of the pesticide.
Incorporation of Mo ion into TiO2 lattice decreases the photocatalytic efficiency under UV light. This decrease could be explained on the basis of rate of generation of conduction band electrons and valence band holes and their life time. Usually electron hole recombination drastically lowers the rate of degradation. The estimated duration for electron trapping is 10 nanoseconds and holes trapping is 100 picoseconds. In the Mo ion doped TiO2, the mid band gaps created due to the doping may act as recombination centers under UV light [26].
Figure 7 shows the plot of ln C/C0 versus Time. The kinetic parameters like rate and order of the reaction are calculated from this plot. Higher negative slope indicates the faster rate of degradation for the experiment with TiO2 and (NH4)2S2O8. Fairly good linear plots can be observed, indicating that all the reactions followed the first-order kinetics. The linear regression factor R 2 calculated for the plot ln C/C0 versus Time shows the value >0.9 suggesting the linearity of the plots except for the catalyst with higher dopant concentration.
Process efficiency, rate of the reaction, rate constant, order and the percentage of degradation of TBZ with various photo catalysts under UV illumination are shown in Table 5.
3.2.2 Solar Light Illumination
Figure 8 shows the plot of C/C0 versus Time for various catalysts in absence of oxidizing agent under solar light irradiation. The highest photocatalytic activity was observed for the Mo doped catalyst (0.06 atom %). This may be due to the shift in the band gap to the visible region by the creation mid band gaps by the dopant. This enhances the absorption of more photons from solar light illumination. However the degradation observed was 70% for 2.5 h for the most efficient catalyst. The rate of degradation decreases thereafter. Significant increases in the degradation rate were observed by the addition of electron acceptors like (NH4)2S2O8. Visible light induced degradation in presence of (NH4)2S2O8 is presented in Fig. 9. It can be observed that only 60% of degradation of TBZ occurred in presence of TiO2 with an oxidizing agent for 2.5 h of irradiation. But 93% degradation occurred with 0.06% Mo doped TiO2 in presence of an oxidizing agent. The percentage of degradation, rate constant and other parameters are listed in Table 5. It is very clear that the degradation efficiency increased with increasing the Mo content from 0.02 to 0.06 atom %. This intermediate concentration of dopant narrows the band gap and shifts the absorption band to 480 nm making the catalyst effective for absorbing solar light. Further the degradation efficiency decreased with increase in Mo content beyond 0.06 atom %. The enhanced photo response of Mo doped TiO2 under solar light irradiation can be accounted in the following way (i) fast interfacial electron transfer rate due to creation of new energy levels at 0.54 eV from which electron can be easily promoted to conduction band (ii) slower recombination rate of electron-holes (iii) Mo6+-V ••o state is acting as electron donor below the conduction band edge.
The plot of ln C/C0 versus (Fig. 10) shows the highest negative slope for the 0.06 atom % of Mo doped TiO2 photocatalyst under solar light illumination, which shows the faster rate of degradation. All most all the reactions shows the linearity (linear regression factor, R 2 > 0.9), which indicates the first order kinetics.
4 Conclusion
The doping of Mo into TiO2 lattice is successfully achieved by solid state reaction. The samples were characterized by various analytical techniques and from the analysis the following conclusions can be drawn.
-
The XRD analysis showed the increase in crystallite size and elongation along the c-axis with increase in the dopant concentration. The change in crystallite size and lattice parameter confirms the doping of Mo into TiO2 lattice. In the case of doped metal oxides there is a critical value of dispersion capacity. At values lower than which the oxide might become highly dispersed on the support without the formation of a separate crystalline phase. Since no characteristic peaks corresponding to Mo species is present, it can be concluded that the Mo loading is below the dispersion capacity.
-
UV–Visible absorption spectra and the diffused reflectance spectroscopic analysis showed a shift in the band gap transition to longer wavelengths and an extension of the absorption in to the visible region. The mid band gap energy level created below 0.54 eV of conduction band of TiO2 facilitates the faster interfacial electron transfer and reduces the electron-hole recombination rate for 0.06% Mo doped TiO2 under solar light. This accounts for the enhanced photo response of doped TiO2 under visible region.
-
The peaks obtained at 1,317 and 2,351 cm−1 in FTIR confirms the presence of Mo in TiO2 matrix. These peaks may be assigned for the Mo–O vibrations.
-
The comparative study of photocatalytic activity of TiO2 and Mo doped TiO2 shows the faster degradation of tebuconazole pesticide with TiO2 in presence of UV light. Further an appropriate amount of Mo (0.06 atom %) dopant enhanced the photo catalytic activity of Mo doped TiO2 under visible light irradiation. Most of the photocatalytic reactions followed the first order kinetics.
References
Pelizzetti E, Serpone N (eds) (1986) Homogeneous and heterogeneous photocatalysis. Reidel, Dordrecht
Schiavello M (1988) Photocatalysis and environment. Trends and applications. Kluwer Academic Publishers, Dordrecht
Fujishima A, Rao TN, Tryk DA (2000) J Photochem Photobiol C: Photochem Rev 1:1–21
Augugliaro V, Loddo V, Marchi G, Palmisano L, lopezmunoz MJ (1997) J Catal 166:272
Guha S, Ghosh K, Keeth JG, Ogale SB, Shinde SR, Simpson JR, Drew HD, Venkatesan T (2003) Appl Phys Lett 83:3296
Weng HM, Yang XP, Dong JM, Mizuseki H, Kawasaki M, Kawazoe Y (2004) Phys Rev B 69:125219
Matsumoto Y, Murakami M, Shono T, Hasegawa T, Fukumura T, Kawasaki M, Ahmet P, Chikyow T, Koshihara S, Koinuma H (2001) Science 291:854
Katagi T (2004) Rev Environ Contam Toxicol 182:1–195
Gomathi Devi L, Krishnaiah GM (1999) J Photochem Photobiol A: Chem 121:141–145
Azaroff LV (1977) Introduction to solids, TMH edn. McGraw-Hill Inc., New York
Prasad K, Bally Philippe AR, Schmid E, Lévy F, Benoit J, Barthou C, Benalloul P (1997) J Appl Phys 36:5696–5702
Lee JD (1996) Concise inorganic chemistry, 5th edn. Chapmann and Hall Publication, London
Kutty TRN, Gomathi Devi L (1985) Mater Res Bull 20:793
Fuller MP, Griffiths PR (1978) Anal Chem 5013:1906–1910
Cardona M, Harbeke G (1964) Phys Rev 137A:1467
Fuller MP, Griffiths PR (1978) Anal Chem 50(13):1906–1910
Kröger FA, Vink HJ (1956) Solid state physics. Academic Press, New York
Bernasik A, Radecka M, Rekas M, Sloma M (1993) Appl Surf Sci 65:240
Mizushima K, Tanaka M, Asai A, Ilda S, Goodenough JB (1979) J Phys Chem Solids 40:1129
Suda Y, Kawasaki H, Ueda T, Ohshima T (2004) Thin Solid Films 453–454:162
Wawrzyniak B, Morawski AW, Tryba B (2006) Int J Photoener 2006:1–8
Yoko XT, Kamiya K, Tanaka K (1990) J Mater Sci 25:3922
Zhang J, Boyd I, Sullivan BJO, Hurley PK, Kelly PV, Senateur JP (2002) J Non-Cryst Solid 303:134
Yamakata A, Aki Ishibashi T, Onishi H (2002) Bull Chem Soc Jpn 75:1019
Jackson P, Parfitt GD (1971) Trans Faraday Soc 67:2469
Maruska HP, Ghosh AK (1979) Solar Energy Mater 1:237
Acknowledgements
Financial support by UGC-Major Research Project (2007–2010), UGC-DRS, and UGC-FIST is greatly acknowledged. One of the author B. Narasimha Murthy wishes to acknowledge CMR Institute of Technology, Bangalore for its support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomathi Devi, L., Narasimha Murthy, B. Characterization of Mo Doped TiO2 and its Enhanced Photo Catalytic Activity Under Visible Light. Catal Lett 125, 320–330 (2008). https://doi.org/10.1007/s10562-008-9568-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9568-4