Abstract
Pyridine(Py)-modified Keggin-type mono-vanadium-substituted heteropoly acids (Py n PMo11V, n = 1–4) were prepared by a precipitation method as organic/inorganic hybrid catalysts for direct hydroxylation of benzene to phenol in a pressured batch reactor and their structures were characterized by FT-IR. Among various catalysts, Py4PMo11V exhibited the highest catalytic activity (yield of phenol 9.0%) with the high selectivity for phenol, without observing the formation of catechol, hydroquinone and benzoquinone in the reaction with 80 vol% aqueous acetic acid, molecular oxygen and ascorbic acid used as the solvent, oxidant and reducing reagent, respectively. The influences of the reaction temperature, the pressure of oxygen, the amount of ascorbic acid, the amount of catalyst, and the reaction time on the yield of phenol were investigated to obtain the optimal reaction conditions for phenol formation. Pyridine can greatly promote the catalytic activity of the Py-free catalyst (H4PMo11VO40), mostly because the organic π electrons in the hydrid catalyst may extend their conjugation to the inorganic framework of heteropoly acid and thus dramatically modify the redox properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Numerous new structural types of Polyoxometalates (POMs) with fascinating topological beauty and important electronic, optical and catalytic properties have been developed during the past two decades [1, 2]. They attract a lot of studies on the heterogeneous and homogeneous catalysis [3–6]. The additional interesting aspects of POMs in catalysis are their inherent stability towards oxygen donors such as molecular oxygen and hydrogen peroxide. Keggin-type heteropoly acids have many advantages which make themselves economically and environmentally attractive in both academic and industrial significance. They are a kind of the acidic and redox catalysts for various reactions since their strong acidity and redox property can be controlled by replacing the protons with metal cations and/or by changing the heteroatom or the framework transition-metal atoms [7–9]. On the other hand, organic/inorganic hybrid materials are extremely interesting as catalytic materials [10–12]. Hybrid materials composed by POMs and organic species not only have the advantages of organic species, such as the ease in processing and the structural fine tuning with inorganic clusters, but also the close interaction of organic delocalized p electrons with the inorganic d electrons may bring exciting synergistic effects [13]. In redox reactions, this interaction may dramatically modify the redox properties of the cluster [14, 15].
Phenol is an important intermediate for the manufacture of petrochemicals, agrochemicals and plastics [16]. It is mainly produced by cumene process. However, this process gives low atom utilization, low phenol yield, high energy consumption, and the production of equal amount of acetone as the byproduct [17]. Hence, the direct hydroxylation of benzene for the production of phenol has been attracting much attentions for tens of years, especially using molecular oxygen as the oxidant [18–20]. Although various catalysts have been used [21–24], organic/inorganic hybrid catalysts were seldom tried in this reaction as yet [3, 25].
We have attempted the liquid-phase oxidation of benzene to phenol catalyzed by vanadium-substituted heteropoly acids using H2O2 as the oxidant [26] and by phase transfer catalysts (cyclodextrins complexes with vanadium-substituted heteropoly acids) using molecular oxygen as the oxidant [27], respectively. In this work, we consider that the secondary structure of the heteropoly acid manifests itself to exhibit an extremely high proton mobility and a “pseudo-liquid phase” [28–30] that can be facile used in the design of catalysts. In such cases, not only water but also a variety of polar organic molecules can pass in and out the interpolyanion space in the structure freely, and react there. Therefore, this paper employed the organic/inorganic hybrid materials i.e., the pyridine-modified vanadium-substituted heteropoly acids, as the catalysts for the liquid-phase hydroxylation of benzene with molecular oxygen as the oxidant. An obvious promotion effect of pyridine in the hybrid catalysts on the phenol yield was observed for this reaction.
2 Experimental
2.1 Preparation of Catalysts
All solvents and reagents were purchased from commercial sources and used without further purification.
Keggin-type vanadium-substituted heteropolymolybdic acid (H4PMo11VO40 · xH2O) was prepared at the P/Mo/V ratio of 1:11:1 using MoO3, V2O5, and aqueous 85% H3PO4 as reactants [31]. The detail procedure for the preparation was as follows: MoO3 (18.58 g, corresponding to 11.73 mmol Mo11) (Shanghai Chem. Reagent Co., AR) and V2O5 (1.07 g, corresponding to 11.73 mmol V) (Shanghai Chem. Reagent Co., AR) were suspended in 150 mL de-ionic water in a 500-mL three-necked flask equipped with a condenser under magnetic stirring in an oil bath at the reflux temperature. Aqueous 85% H3PO4 (Shanghai Chem. Reagent Co., AR) was added drop-wise to the boiling and stirred suspension of the reaction mixture. After the addition of the phosphoric acid, a clear orange-red solution was obtained. The solution was cooled to the room temperature and further dried via evaporation to get a solid product, into which a suitable amount of de-ionic water was added to obtain a solution, and then the solution was left at the room temperature overnight to re-crystallize for purification. The resulting fine orange-red powders were characterized and used in the hydroxylation of benzene.
Pyridine-modified molybdovanadophosphoric acid, denoted by Py n PMo11V, was prepared by a precipitation method. For example, Py1PMo11V was prepared by adding a specified amount of H4PMo11VO40 into 25 mL of an aqueous solution containing pyridine (Shanghai Chem. Reagent Co., AR) with the Pyridine/H4PMo11VO40 molar ratio of 1:1. Then the solution containing precipitates was evaporated to dryness at 343 K and the solid product obtained was further dried at the same temperature overnight in vacuum oven.
2.2 Liquid-phase Hydroxylation of Benzene
The liquid-phase hydroxylation of benzene was carried out in a custom-designed temperature controllable titanic reactor (100 mL) with a mechanical stirrer.
The typical reaction conditions were as follows: 0.10 g catalyst and 0.60 g ascorbic acid (Shanghai Chem. Reagent Co., AR), 25 mL of 80 vol% aqueous acetic acid and 2.0 mL of benzene (Shanghai Chem. Reagent Co., AR) were added into the reactor in turn carefully. When the reactor was subjected to the desired temperatures, oxygen was injected into the reactor up to the preset pressure. The hydroxylation was conducted for 10 h under stirring. After the reaction, 1.0 mL of 1,4-dioxane (Shanghai Chem. Reagent Co., AR), which was confirmed as unchanged during the aftertreatment, was added into the reaction mixture as an internal standard for product analysis.
Gas chromatographic (GC) measurements were performed on a SP-6890A equipped with a FID detector and a capillary column (SE-54; 30 m × 0.32 mm × 0.25 μm). This reaction system appeared to have a high selectivity since phenol was the only product detected by GC, and no catechol, hydroquinone and benzoquinone were observed.
2.3 Measurement of the IR Spectra of the Catalysts
The IR spectra of the catalysts were measured using a KBr disk mounted in an infrared spectrophotometer (Nexus 870). Samples were mixed and grounded with KBr for IR measurement.
3 Results and Discussion
3.1 Catalyst Characterization
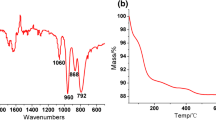
The IR spectra of pyridine, Py n PMo11V (n = 1–4) and H4PMo11VO40 are illustrated in Fig. 1. It can be seen that H4PMo11V and Py n PMo11V (n = 1–4) give all the IR vibration peaks assigned to a Keggin-type heteropoly acid, and the locations of featured peaks (PsO, 1,059 cm−1; MosOsMo, 967 cm−1; ModO, 860 and 777 cm−1) are in well agreement with those in the previous report [31]. The prepared H4PMo11VO40 was orange-red in color, very soluble in water, and took on a blue color upon treatment with a mild reducing reagent; these results can also provide qualitative support for the conclusion that the prepared vanadium-substituted heteropoly acid possesses the Keggin-type HPA structure. From 1,600 to 1,200 cm−1, the IR spectrum of pyridine clearly exists two peaks at 1,435 and 1,381 cm−1 (Curve A in Fig. 1). After the reaction of pyridine with H4PMo11VO40, the corresponding two peaks respectively shifts to 1,527 and 1,483 cm−1 (Curve B, C, D and E in Fig. 1). Those are known to relate to pyridine adsorbed on Brϕnsted acid sites and Lewis acid sites, which were generated with the formation of pyridinium ions in the bulk, as well as the formation of the primary oxygen-deficient Keggin structure [32]. The Lewis acid sites around the oxygen-deficient Keggin surface structure seem to be created by the removed lattice oxygen during the reaction of heteropoly acid with pyridine during the evaporation pretreatment. Because there is a strong electronic interaction between the metal oxygen cluster and the organic segment [15, 33], it is obvious that the strength of metal-O bond becomes weaker due to the increase of the electronic density of Keggin units by the incorporated pyridine.
3.2 Evaluation of Performances of Various Catalysts
The catalytic activities of H4PMo11VO40 and Py n PMo11V (n = 1–4) in the direct hydroxylation of benzene to phenol with molecular oxygen at 383 K are shown in Table 1. It can be seen that no phenol was detected without ascorbic acid used as the reducing reagent. When ascorbic acid was added into the reaction mixture without a catalyst, only 1.8% yield of phenol was achieved. Under the employed conditions, Py4PMo11V exhibited the highest catalytic activity among those catalysts. No other product rather than phenol was detected by GC analysis in all entries. By introducing pyridine into the heteropoly acid, the catalytic activities were substantially improved (3.1% for H4PMo11V vs. 3.5% for PyPMo11V, 6.6% for Py2PMo11V, 7.8% for Py3PMo11V, 9.0% for Py4PMo11V, respectively), which may be due to the strong electronic interaction between Keggin units and pyridine [15, 33]. It is proposed that the organic π electrons may extend their conjugation to the inorganic framework, which would weaken the strength of metal-O bond, and thus the catalytic activity would be promoted. Additionally, as is well known, not only water but also a variety of polar organic molecules can pass in and out the interpolyanion space in the structure freely and react there. When pyridine was adsorbed on the surface of Keggin units, it would help benzene enter the bluk of Keggin units easily and contact the catalytic center. Synergistic effect by the electronic interaction and the “pseudo-liquid phase” property may account for the improvement of the catalytic activity. The yield of phenol increases with the increase of the ratio of pyridine into PMo11V units. As shown in Table 1, when the ratio increased from 1 to 4, the corresponding yield of phenol increased from 3.5% to 9.0%.
3.3 Effect of the Reaction Temperature on the Yield of Phenol
The effect of the reaction temperature on the yield of phenol over Py4PMo11V is shown in Fig. 2. When the reaction temperature increased from 353 K to 373 K, the yield increased slowly from 4.3% to 5.8% and then sharply rose up to 9.0% when the temperature reached to 383 K. However, when the reaction temperature was further up to 393 K, the yield of phenol dropped quickly to 5.7%. This is mostly caused by the excessive oxidation of the product (phenol) at high temperatures, leading to the lower value of the phenol yield. Therefore, 383 K is considered as a suitable reaction temperature.
3.4 Effect of the Oxygen Pressure on the Yield of Phenol
The influence of the oxygen pressure on the yield of phenol was investigated using Py4PMo11V as the catalyst, and the results are illustrated in Fig. 3. The phenol yield was found to increase with the increase of the oxygen pressure up to 2.0 MPa where it reached to ca. 9.0%. Further increase of the oxygen pressure had a reverse influence on the phenol yield, which decreased to 6.8%. As is well known, the solubility of oxygen in water in general increases with the increase of the oxygen pressure, but too much oxygen may cause the excessive oxidation of the product (phenol). Therefore, 2.0 MPa is considered as a suitable reaction pressure.
3.5 Effect of the Amount of Ascorbic Acid on the Yield of Phenol
The results for the effect of the amount of ascorbic acid on the yield of phenol investigated at 383 K over Py4PMo11V are shown in Fig. 4. When the amount of ascorbic acid increased from 0.10 g to 0.40 g, the yield of phenol increased slowly from 2.8% to 4.2%, and then greatly increased up to 9.0% when the amount of ascorbic acid was up to 0.60 g, but a further increase in the amount of ascorbic acid inversely caused a decrease in the yield of phenol. The role of the reducing reagent was suggested to activate the oxygen molecule through the reduction of the V species, however, an extra ascorbic acid may decrease sharply the activated oxygen species which are necessary for phenol formation so that almost no activated oxygen species can be utilized for the benzene oxidation as well [34]. The yield of phenol had a maximum value at the amount of ascorbic acid of around 0.60 g. And thus the extra ascorbic acid is not in favor of the increase in phenol yield. Therefore, 0.60 g ascorbic acid is considered as a suitable amount in this reaction.
3.6 Effect of the Amount of Py4PMo11V on the Yield of Phenol
As shown in Fig. 5, when the amount of Py4PMo11V increased from 0.050 g to 0.10 g, the yield of phenol increased sharply from 4.6% to 9.0%. On the other hand, a further increase in the amount of Py4PMo11V caused a sharply decrease in the yield of phenol (from 9.0% for 0.10 g to 4.5% for 0.80 g). This may be due to the deep oxidation of benzene and/or the product (phenol) by the extra catalyst. So, 0.10 g Py4PMo11V is chosen as a suitable amount in this reaction.
3.7 Effect of the Reaction Time on the Yield of Phenol
The influence of reaction time on the yield of phenol over Py4PMo11V is shown in Fig. 6. It is obvious that the yield of phenol increased greatly from 3.8% to 9.0% when the reaction time increased from 4 h to 10 h, and after that, the yield decreased sharply with the further increase of reaction time. The sharply decrease of yield may be caused by the further oxidation of the product (phenol), so 10 h is chosen as a suitable reaction time in this work.
4 Conclusion
Organic/inorganic hybrid catalysts Py n PMo11V were easily prepared with H4PMo11VO40 and pyridine by the precipitation method and their Keggin-type structures remained well. Py4PMo11V was demonstrated to be a good catalyst in the direct hydroxylation of benzene to phenol with molecular oxygen and ascorbic acid as the oxidant and the reducing reagent, respectively. Pyridine may play an important role in the reaction for the improvement of the catalytic activity because of the strong electronic effect between pyridine and H4PMo11VO40 together with the “pseudo-liquid phase” property. The highest yield of phenol, 9.0%, was achieved in a batch reactor under optional reaction conditions: 0.10 g catalyst, 0.60 g ascorbic acid, 2.0 mL benzene, 25 mL of an aqueous solution containing 80 vol% acetic acid, 383 K, 2.0 MPa, and 10 h.
References
Zheng ST, Yuan DQ, Zhang J, Yang GY (2007) Inorg Chem 46:4569
Zhao JW, Li B, Zheng ST, Yang GY (2007) Cryst Growth Des 7:2658
Liu YY, Murata K, Inaba M (2005) Catal Commun 6:679
Bardin BB, Davis RJ (1999) Appl Catal A Gen 185:283
Zhang FM, Yuan CS, Wang J, Zhu HY, Wang CY (2006) J Mol Catal A Chem 247:130
Zhang FM, Wang J, Yuan CS, Ren XQ (2005) Catal Lett 102:171
Yang YY, Xu L, Gao GG, Li FY, Qiu YF, Qu XS, Liu H (2007) Eur J Inorg Chem 2500
Zhang FQ, Zhang XM, Wu HS, Jiao HJ (2007) J Phys Chem A 111:159
San Felices L, Vitoria P, Gutierrez-Zorrilla JM, Lezama L, Reinoso S (2006) Inorg Chem 45:7748
Saha PK, Dutta B, Jana S, Bera R, Saha S, Okamoto K, Koner S (2007) Polyhedron 26:563
Bigi F, Corradini A, Quarantelli C, Sartori G (2007) J Catal 250:222
Neumann R, Khenkin AM (2006) Chem Commun 2529
San Felices L, Vitoria P, Gutierrez-Zorrilla JM, Reinoso S, Etxebarria J, Lezama L (2004) Chem Eur J 10:5138
Xu BB, Peng ZH, Wei YG, Powell DR (2003) Chem Commun 2562
Lu M, Wei YG, Xu BB, Cheung CFC, Peng ZH, Powell DR (2002) Angew Chem Int Ed 41:1566
Lemke K, Ehrich H, Lohse U, Berndt H, Jahnisch K (2003) Appl Catal A Gen 243:41
Niwa S, Eswaramoorthy M, Nair J, Raj A, Itoh N, Shoji H, Namba T, Mizukami F (2002) Science 295:105
Gu YY, Zhao XH, Zhang GR, Ding HM, Shan YK (2007) Appl Catal A Gen 328:150
Mishra GS, Kumar A (2002) Catal Lett 81:113
Laufer W, Niederer JPM, Hoelderich WF (2002) Adv Synth Catal 344:1084
Li Y, Xia HA, Fan FT, Feng ZC, van Santen RA, Hensen EJM, Li C (2008) Chem Commun 774
Zhong YK, Li GY, Zhu LF, Yan Y, Wu G, Hu CW (2007) J Mol Catal A Chem 272:169
Lee CH, Lin TS, Mou CY (2007) J Phys Chem C 111:3873
Kubacka A, Wang ZL, Sulikowski B, Corberan VC (2007) J Catal 250:184
Liu YY, Murata K, Inaba M (2006) J Mol Catal A Chem 256:247
Zhang FM, Guo MP, Ge HQ, Wang J (2007) Chin J Chem Eng 15:4
Ge HQ, Leng Y, Zhou CJ, Wang J (2008) Catal Lett. doi:10.1007/s10562-008-9464-y
Takahashi K, Okuhara T, Misono M (1985) Chem Lett 841
Misono M, Okuhara T, Ichiki T, Arai T, Kanda Y (1987) J Am Chem Soc 109:5535
Misono M (1987) Catal Rev Sci Eng 29:269
Wang J, Lin Z, Han SY, Eum M, Lee CW (2003) J Ind Eng Chem 9:281
Li W, Oshihara K, Ueda W (1999) Appl Catal A Gen 182:357
Song IK, Kaba MS, Barteau MA (1996) J Phys Chem 100:17528
Ishida MA, Masumoto Y, Hamada R, Nishiyama S, Tsuruya S, Masai M (1999) J Chem Soc Perkin Trans 2:847
Acknowledgments
The authors thank the Natural Science Foundation of China (Nos. 20306011 and 20476046) and the “Qinglan” Project of Jiangsu Province for Young Researchers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ge, H., Leng, Y., Zhang, F. et al. Direct Hydroxylation of Benzene to Phenol with Molecular Oxygen over Pyridine-modified Vanadium-substituted Heteropoly Acids. Catal Lett 124, 250–255 (2008). https://doi.org/10.1007/s10562-008-9506-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9506-5