Abstract
A series of metal ferrite (MeIIFe2O4) catalysts were prepared by a co-precipitation method with a variation of divalent metal component (MeII = Zn, Mg, Mn, Ni, Co, and Cu) for use in the oxidative dehydrogenation of n-butene to 1,3-butadiene. Successful formation of metal ferrite catalysts with a random spinel structure was confirmed by XRD, ICP-AES, and XPS analyses. The catalytic performance of metal ferrite catalysts in the oxidative dehydrogenation of n-butene strongly depended on the identity of divalent metal component. Acid properties of metal ferrite catalysts were measured by NH3-TPD experiments, with an aim of correlating the catalytic performance with the acid property of the catalysts. It was revealed that the yield for 1,3-butadiene increased with increasing surface acidity of the catalyst. Among the catalysts tested, ZnFe2O4 catalyst with the largest surface acidity showed the best catalytic performance in the oxidative dehydrogenation of n-butene.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Selective oxidation of olefins has been considered to be an important subject for the production of various chemical intermediates in the petrochemical industries [1–4]. In particular, oxidative dehydrogenation of n-butene has attracted much attention as a promising process for producing 1,3-butadiene, an important raw material for manufacturing a large number of chemicals such as ABS (acrylonitrile-butadiene-styrene), BR (butadiene rubber), and SBR (styrene-butadiene rubber) [4–6]. A number of catalysts have been investigated for the oxidative dehydrogenation of n-butene, including ferrite-type catalyst [7–10], Cu-Mo catalyst [11], vanadium-containing catalyst [12], and Bi-Mo-based catalyst [13, 14]. Among these catalysts, metal ferrite catalysts have been extensively studied as efficient catalysts for this reaction [15–17].
Metal ferrite catalysts have a general form of MeIIFe2O4 such as ZnFe2O4 (zinc ferrite), MgFe2O4 (magnesium ferrite), CoFe2O4 (cobalt ferrite), and CuFe2O4 (copper ferrite) [15–17]. It can be inferred that the identity of divalent metal component (MeII) inevitably affects the catalytic performance of metal ferrite catalysts in the oxidative dehydrogenation of n-butene. Nonetheless, no systematic investigations on the effect of divalent metal component on the catalytic performance of metal ferrite catalysts in the oxidative dehydrogenation of n-butene have been reported yet.
Many attempts have been made to find a crucial factor determining the catalytic performance in the oxidative dehydrogenation of n-butene [18, 19]. Although the fundamental reaction mechanism has not been clearly elucidated, many researchers agree that the reaction mechanism by way of π-allyl intermediate is the most feasible reaction pathway for the formation of 1,3-butadiene from n-butene [6, 18, 20, 21]. According to this mechanism, an initial abstraction of α-hydrogen from n-butene to form π-allyl intermediate is the rate-determining step in the oxidative dehydrogenation of n-butene. This means that acid property of the catalyst plays a very important role in abstracting α-hydrogen from n-butene (a base molecule) in this reaction [22, 23]. It has been demonstrated in our previous works [15, 24] that the catalytic performance of zinc ferrite catalyst in the oxidative dehydrogenation of n-butene was closely related to the surface acidity of the catalyst.
In this work, a series of metal ferrite (MeIIFe2O4) catalysts with different divalent metal (MeII = Zn, Mg, Mn, Ni, Co, and Cu) were prepared by a co-precipitation method, and were applied to the oxidative dehydrogenation of n-butene to 1,3-butadiene. The formation of metal ferrite catalysts was confirmed by XRD, ICP-AES, and XPS analyses. NH3-TPD experiments were conducted to measure the acid properties of metal ferrite catalysts. A correlation between catalytic performance and acid property of metal ferrite catalysts was then established.
2 Experimental
2.1 Preparation of Metal Ferrite (MeIIFe2O4) catalysts
Metal ferrite (MeIIFe2O4) catalysts with different divalent metal (MeII = Zn, Mg, Mn, Ni, Co, and Cu) were prepared by a co-precipitation method. A total of 1,000 mL of sodium hydroxide solution (0.6 N NaOH from Sigma-Aldrich) was heated to 70 °C. 1.42 g of zinc chloride (ZnCl2 from Sigma-Aldrich) and 5.61 g of iron chloride (FeCl3 · 6H2O from Sigma-Aldrich) were separately dissolved in 100 mL of distilled water at 70 °C. The metal precursor solutions were then added into the sodium hydroxide solution as quickly as possible under vigorous stirring. After the resulting solution was stirred vigorously at 70 °C for 1 h, it was aged overnight at room temperature. The precipitate was filtered and washed with distilled water to obtain a solid product. The solid product was dried at 175 °C for 16 h, and finally, it was calcined at 475 °C for 3 h to yield the zinc ferrite (ZnFe2O4) catalyst. 1.19 g of magnesium chloride (MgCl2 from Sigma-Aldrich), 2.15 g of manganese chloride (MnCl2 · 4H2O from Sigma-Aldrich), 2.54 g of nickel chloride (NiCl2 · 6H2O from Sigma-Aldrich), 2.54 g of cobalt chloride (CoCl2 · 6H2O from Sigma-Aldrich), and 1.78 g of copper chloride (CuCl2·2H2O from Sigma-Aldrich) were used as a divalent metal precursor for the preparation of corresponding metal ferrite catalyst.
2.2 Characterization
Formation of metal ferrite (MeIIFe2O4) catalysts was confirmed by XRD (MAC Science, M18XHF-SRA) measurements. Atomic ratios of constituent metal components in the prepared catalysts were determined by ICP-AES (Shimadz, ICP-1000IV) analyses. Surface areas of the catalysts were measured using a BET apparatus (Micromeritics, ASAP 2010). XPS analyses (Thermo VG, Sigma Probe) were conducted to measure the binding energies of Fe ions in the metal ferrite catalysts using C 1s peak (284.6 eV) as a reference.
Acid properties of metal ferrite catalysts were measured by NH3-TPD experiments. Each catalyst (0.3 g) was charged into a tubular quartz reactor of the conventional TPD apparatus. The catalyst was pretreated at 200 °C for 1 h under a flow of helium (20 mL/min) to remove any physisorbed organic molecules. About 20 mL of NH3 was then pulsed into the reactor every minute at room temperature under a flow of helium (5 mL/min), until the acid sites were saturated with NH3. The physisorbed NH3 was removed by evacuating the catalyst sample at 50 °C for 1 h. The furnace temperature was increased from room temperature to 600 °C at a heating rate of 5 °C/min under a flow of helium (10 mL/min). The desorbed NH3 was detected using a GC-MSD (Agilent, MSD-6890N GC).
2.3 Oxidative Dehydrogenation of n-Butene
Oxidative dehydrogenation of n-butene to 1,3-butadiene was carried out in a continuous flow fixed-bed reactor in the presence of air and steam. Each catalyst (0.33 g) was pretreated at 470 °C for 1 h with an air stream (16 mL/min). Water was sufficiently vaporized by passing through a pre-heating zone and continuously fed into the reactor together with n-butene and air. C4 raffinate-3 containing 57.9 wt.% n-butene (1-butene (7.5 wt.%) + trans-2-butene (33.9 wt.%) + cis-2-butene (16.5 wt.%)) was used as a n-butene source, and air was used as an oxygen source (nitrogen in air served as a carrier gas). Feed composition was fixed at n-butene:oxygen:steam = 1:0.75:15. The catalytic reaction was carried out at 420 °C. GHSV (gas hourly space velocity) was fixed at 475 h−1 on the basis of n-butene. Reaction products were periodically sampled and analyzed with gas chromatographs. Conversion of n-butene and selectivity for 1,3-butadiene were calculated on the basis of carbon balance as follows. Yield for 1,3-butadiene was calculated by multiplying conversion and selectivity.
3 Results and Discussion
3.1 Formation of Metal Ferrite (MeIIFe2O4) Catalysts
Successful formation of metal ferrite (MeIIFe2O4) catalysts was confirmed by XRD and ICP-AES measurements. Figure 1 shows the XRD patterns of metal ferrite catalysts with different divalent metal (MeII = Zn, Mg, Mn, Ni, Co, and Cu). Each phase was identified by its characteristic diffraction peaks using JCPDS. The XRD patterns are in good agreement with those reported in previous works [16, 19, 25], indicating the successful formation of metal ferrite catalysts. As listed in Table 1, Fe/MeII atomic ratios of metal ferrite catalysts determined by ICP-AES analyses were in the range of 1.90–2.15, in good agreement with the theoretical value of 2.0. This result also supports that metal ferrite catalysts were successfully prepared in this work. BET surface areas of metal ferrite catalysts are also summarized in Table 1.
To confirm the structure of metal ferrite catalysts, binding energies of Fe cations were measured by XPS analyses. Metal ferrite catalysts have a spinel crystalline structure (AB2O4) with tetrahedral and octahedral sites for metal atoms [26]. Three types of spinel structures (normal spinel, inverse spinel, and random spinel) can be formed depending on the distribution of metal atoms; (i) a normal spinel structure in which divalent cations (A) are in the tetrahedral sites and trivalent cations (B) are in the octahedral sites, (ii) an inverse spinel structure in which divalent cations (A) occupy octahedral sites while trivalent cations (B) are equally distributed in the tetrahedral and octahedral sites, and (iii) a random spinel structure in which divalent (A) and trivalent cations (B) are randomly located at both tetrahedral and octahedral sites [7, 27]. In the metal ferrite catalysts with a form of MeIIFe2O4, therefore, Fe cations can be located at two different sites (tetrahedral and octahedral sites) in the spinel crystalline structure [21, 27]. This means that the type of spinel structure of metal ferrite catalysts can be identified by determining the location of Fe cations in the spinel structure. To identify the spinel structure of metal ferrite catalysts, XPS analyses for measuring the binding energies of Fe cations of metal ferrite catalysts were conducted.
Figure 2 shows the XPS spectrum of Fe3+ 2p3/2 of ZnFe2O4 catalyst. Deconvolution of the spectrum reveals that there are two types of Fe cations in the ZnFe2O4 catalyst which are located at the tetrahedral and octahedral sites. These two types of Fe cations were also observed in all the MeIIFe2O4 catalysts. The binding energies of Fe3+ 2p3/2 of metal ferrite catalysts are summarized in Table 2. It is noticeable that all the MeIIFe2O4 catalysts retained a satellite peak (Fe 3+sat 2p3/2) within the range of 718.3–719.3 eV, indicating that all Fe cations of MeIIFe2O4 catalysts are trivalent cations (Fe3+) [28]. A peak within the range of 710.3–710.9 eV was attributed to the Fe3+ cations located at the octahedral site (Fe 3+O 2p3/2) in the spinel crystalline structure, while a peak within the range of 713.2–714.1 eV was due to the Fe3+ cations located at the tetrahedral site (Fe 3+T 2p3/2) in the spinel crystalline structure [25]. The amounts of Fe3+ cations located at the tetrahedral site of metal ferrite catalysts are also summarized in Table 2. The result clearly shows that ca. 1/3 (32.2–37.6 %) of Fe3+ cations is located at the tetrahedral site of metal ferrite catalysts, regardless of the different identity of divalent metal. This result indicates that all the metal ferrite catalysts prepared in this work have a random spinel structure.
3.2 Catalytic Performance of Metal Ferrite (MeIIFe2O4) Catalysts
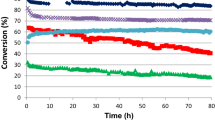
Figure 3 shows the catalytic performance of MeIIFe2O4 (MeII = Zn, Mg, Mn, Ni, Co, and Cu) catalysts in the oxidative dehydrogenation of n-butene at 420 °C after a 6 h-reaction. The catalytic performance was strongly dependent on the identity of divalent metal component. Conversion of n-butene, selectivity for 1,3-butadiene, and yield for 1,3-butadiene decreased in the order of ZnFe2O4 > MgFe2O4 > MnFe2O4 > NiFe2O4 > CoFe2O4 > CuFe2O4. Among the catalysts tested, ZnFe2O4 catalyst showed the highest yield for 1,3-butadiene (67.8%).
3.3 Correlation Between Catalytic Performance and Surface Acidity
An attempt has been made to correlate the catalytic performance (Fig. 3) with the BET surface area (Table 1) of metal ferrite catalysts. However, the catalytic performance was not directly correlated with the BET surface area of metal ferrite catalysts. In order to investigate the effect of acid properties on the catalytic performance of metal ferrite catalysts, NH3-TPD experiments were carried out. Figure 4 shows the NH3-TPD profiles of selected metal ferrite catalysts. All the catalysts showed a broad NH3-TPD peak. The metal ferrite catalysts exhibited a significant difference in total acidity (peak area) and a slight difference in acid strength (peak temperature) with a variation of divalent metal component (MeII). We attempted to correlate the catalytic performance with the acid properties (acidity and acid strength) of metal ferrite catalysts. Unfortunately, either total acidity or acid strength was not directly correlated with the catalytic performance of metal ferrite catalysts. However, a reliable correlation between catalytic performance and surface acidity of the catalysts was observed. The surface acidity was defined as the amount of adsorbed NH3 per unit surface area of a catalyst.
Figure 5 shows a correlation between yield for 1,3-butadiene and surface acidity of metal ferrite catalysts. The correlation clearly shows that the yield for 1,3-butadiene was closely related to the surface acidity of metal ferrite catalysts. This result was in good agreement with the previous work investigating the effect of acid property on the catalytic performance of zinc ferrite catalysts in the oxidative dehydrogenation of n-butene [15]. Among the catalysts tested, ZnFe2O4 catalyst with the largest surface acidity showed the best catalytic performance. As mentioned earlier, the acid property of metal ferrite catalysts plays an important role in adsorbing and activating n-butene, that is, in abstracting α-hydrogen from n-butene to form π-allyl intermediate [15, 24]. Therefore, it is reasonable to expect that a catalyst with large surface acidity (with large capacity to activate n-butene per unit surface area) is favorable for the facile adsorption and activation of n-butene (a base molecule) in the oxidative dehydrogenation of n-butene. In conclusion, the surface acidity of metal ferrite catalysts played a key role in determining the catalytic performance in the oxidative dehydrogenation of n-butene.
4 Conclusions
Metal ferrite (MeIIFe2O4) catalysts with different divalent metal (MeII = Zn, Mg, Mn, Ni, Co, and Cu) were prepared by a co-precipitation method, and were applied to the oxidative dehydrogenation of n-butene to 1,3-butadiene. Successful formation of metal ferrite catalysts was well confirmed by XRD and ICP-AES analyses. XPS analyses for measuring the binding energies of Fe cations of metal ferrite catalysts revealed that all the metal ferrite catalysts retained a random spinel structure. Among the catalysts tested, ZnFe2O4 catalyst showed the best catalytic performance in the oxidative dehydrogenation of n-butene. The catalytic performance of metal ferrite catalyst was closely related to the surface acidity of the catalyst. The yield for 1,3-butadiene increased with increasing surface acidity of the catalyst. The largest surface acidity of ZnFe2O4 catalyst was responsible for its high catalytic performance in the oxidative dehydrogenation of n-butene.
References
Oh SC, Lee HP, Kim HT, Yoo KO (1999) Korean J Chem Eng 16:543–547
Kim YH, Yang HS (2000) Korean J Chem Eng 17:357–364
Kung HH (1986) Ind Eng Chem Prod Res Dev 25:171–178
Hong F, Yang BL, Schwartz LH, Kung HH (1984) J Phys Chem 88:2525–2530
Liaw BJ, Cheng DS, Yang BL (1989) J Catal 118:312–326
Toledo JA, Valenzuela MA, Armendáriz H, Aguilar-Ríos G, Zapata B, Montaya A, Nava N, Salas P, Schifter I (1995) Catal Lett 30:279–288
Rennard RJ, Kehl WL (1971) J Catal 21:282–293
Massoth FE, Scarpiello DA (1971) J Catal 21:294–302
Rennard RJ, Innes RA, Swift HE (1973) J Catal 30:128–138
Toledo-Antonio JA, Bosch P, Valenzuela MA, Montoya A, Nava N (1997) J Mol Catal A Chem 125:53–62
Tiwari PN, Alkhazov TG, Adzamov KU, Khanmamedova AK (1989) J Catal 120:278–281
López Nieto JM, Concepción P, Dejoz A, Knözinger H, Melo F, Vázquez MI (2000) J Catal 189:147–157
Jung JC, Lee H, Kim H, Chung YM, Kim TJ, Lee SJ, Oh SH, Kim YS, Song IK (2007) J Mol Catal A Chem 271:261–265
Jung JC, Lee H, Kim H, Chung YM, Kim TJ, Lee SJ, Oh SH, Kim YS, Song IK (2008) Catal Commun 9:447–452
Lee H, Jung JC, Kim H, Chung YM, Kim TJ, Lee SJ, Oh SH, Kim YS, Song IK (2008) Catal Commun 9:1137–1142
Gibson MA, Hightower JW (1976) J Catal 41:431–439
Cares WR, Hightower JW (1971) J Catal 23:193–203
Kung HH, Kung MC (1985) Adv Catal 33:159–198
Toledo-Antonio JA, Nava N, Martínez M, Bokhimi X (2002) Appl Catal A Gen 234:137–144
Gibson MA, Hightower JW (1976) J Catal 41:420–430
Xu WQ, Yin YG, Li GY, Chen S (1992) Appl Catal A Gen 89:131–142
Cullis CF, Hucknall DJ (1982) In: Bond GC, Webb G (eds) A specialist periodical report: catalysis, vol 5. Royal Chemical Society, London
Grasselli RK, Burrington JD (1981) Adv Catal 30:133–141
Lee H, Jung JC, Kim H, Chung YM, Kim TJ, Lee SJ, Oh SH, Kim YS, Song IK (2007) Catal Lett doi:10.1007/s10562-007-9371-7
Li F, Liu X, Yang Q, Liu J, Evans DG, Duan X (2005) Mater Res Bull 40:1244–1255
Jebarathinam NJ, Eswaramoorthy M, Krishnasamy V (1996) Appl Catal A Gen 145:57–74
Bid S, Pradhan SK (2003) Mater Chem Phys 82:27–37
Mullet M, Guillemin Y, Ruby C (2008) J Solid State Chem 181:81–89
Acknowledgments
This work was supported by SK Energy Corporation (POST-BK21 Program) and Korea Energy Management Corporation (2005-01-0090-3-010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H., Jung, J.C., Kim, H. et al. Effect of Divalent Metal Component (MeII) on the Catalytic Performance of MeIIFe2O4 Catalysts in the Oxidative Dehydrogenation of n-Butene to 1,3-Butadiene. Catal Lett 124, 364–368 (2008). https://doi.org/10.1007/s10562-008-9476-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9476-7