Abstract
Cyclodextrins (CyDs) complexes with vanadium-substituted heteropoly acids (PMoV n -β-CyDs, n = 1, 2) were prepared by simple mixing and their structures were characterized by FT-IR. Among various catalysts, PMoV1-β-CyDs, an efficient phase transfer catalyst, exhibited the highest yield (13.1%) of phenol without observing the formation of catechol, hydroquinone and benzoquinone in direct hydroxylation of benzene to phenol in 80 vol% aqueous acetic acid with molecular oxygen and ascorbic acid used as the oxidant and the reducing reagent, respectively. The influences of the reaction temperature, the pressure of oxygen, the amount of ascorbic acid, the amount of catalyst, and the reaction time on the yield of phenol were investigated to obtain the optimal reaction conditions for phenol formation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phenol is an important intermediate for the manufacture of petrochemicals, agrochemicals and plastics [1–3]. It is mainly produced by cumene process. However, this process consists of three steps, and has many disadvantages. For example, the yield of phenol remains only 5%, accompanied by great energy consumption especially in the distillation process for separation among the products and the reactants [4]. Hence, finding a one-step process for the production of phenol by direct hydroxylation of benzene attracts much attention in recent years [5–11].
The direct synthesis of phenol from benzene and nitrous oxide, a very toxic and global-warming related gas molecule, has been described [12], but this method is only cost-effective when nitrous oxide is available cheaply as a by-product [13]. Therefore, there is a need for the search of new processes to produce phenol without by-products, with a high selectivity and at the reaction conditions as mild as possible. Bahidsky et al. reported the gas phase hydroxylation of benzene to phenol catalyzed by the Cu-modified phosphate catalysts in the presence of oxygen and ammonia at 723 K and a very poor yield of phenol was obtained [14]. Lemke et al. investigated the direct hydroxylation of benzene to phenol by hydrogen peroxide on vanadium oxide catalysts supported on MCM-41, MCM-48, silica of Aerosil type and AMM [3]. Low to moderate yield of phenol and selectivity were achieved. As has been recently shown, active peroxide-type species can be obtained by an in situ reductive activation of molecular oxygen when a catalytic oxidation of hydrocarbons is conducted in the presence of co-reductants [2, 15–18]. Tsuruya and co-workers [19–21] reported the use of molecular oxygen as the oxidant for the one-step oxidation of benzene to phenol using Cu-ZSM-5 as a catalyst. Only 4.9% of the yield with ca. 30% selectivity was achieved at their optional reaction conditions. In 2007, Kubacka et al. developed the Cu ion-exchanged ZSM-5 system for the one-step formation of phenol by direct oxidation of benzene, in presence of molecular oxygen as well as an oxygen/hydrogen mixture [8]. They found that the yield of phenol increased with temperature up to 673 K and approached a high value of ca. 10%. Glacial acetic acid was employed as the solvent for the first time in the direct hydroxylation of benzene to phenol at room temperature using vanadium-substituted heteropolyacids and hydrogen peroxide as the catalysts and the oxidant, respectively [22]. A yield of 26% was achieved with a selectivity of 91%.
Catalytic function of polyoxometalates has attracted much attention because their acidic and redox properties can be controlled at atomic and molecular levels. The strong acidity and oxidizing property of polyoxometalates attract a lot of studies on the heterogeneous and homogeneous catalysis [23–31]. The additional interesting aspects of polyoxometalates in catalysis are their inherent stability towards oxygen donors such as molecular oxygen and hydrogen peroxide. Therefore, polyoxometalates are useful catalysts for liquid-phase oxidation of hydrocarbons.
We have attempted the liquid-phase oxidation of benzene to phenol catalyzed by vanadium-substituted heteropoly acids using H2O2 as the oxidant [32]. In extending the study of liquid-phase benzene oxidation, this paper reports the catalytic behaviors of the cyclodextrins (CyDs) complexes with vanadium-substituted heteropoly acids (PMoV n -β-CyDs, n = 1, 2) for the liquid-phase hydroxylation of benzene under the various reaction conditions, with molecular oxygen and ascorbic acid used as the oxidant and the reducing reagent, respectively.
2 Experimental
2.1 Preparation of Catalysts
All solvents and reagents were purchased from commercial sources and used without further purification.
Keggin-type molybdovanadophosphoric acids (H3+n PMo12−n V n · xH2O) were prepared at the P/Mo/V ratio of 1:(12 − n):n (n = 1, 2) using MoO3, V2O5, and aqueous 85% H3PO4 as reactants [33]. For example, H4PMo11V was prepared as follows: MoO3 (18.580 g, corresponding to 11.73 mmol Mo11) (Shanghai Chem. Reagent Co., AR) and V2O5 (1.067 g, corresponding to 11.73 mmol V) (Shanghai Chem. Reagent Co., AR) were suspended in 150 mL de-ionic water in a 500-mL three-necked flask equipped with a condenser under magnetic stirring in an oil bath at the reflux temperature. Aqueous 85% H3PO4 (Shanghai Chem. Reagent Co., AR) was added drop-wise to the boiling and stirred suspension of the metal oxides. After the addition of the phosphoric acid, a clear orange-red solution was obtained. The solution was further dried via evaporation to get a solid product, into which a suitable amount of de-ionic water was added to obtain a solution, and then the solution was left at room temperature overnight to re-crystallize for purification. The resulting fine orange-red powders were characterized and used as the control catalyst in the hydroxylation of benzene.
The cyclodextrins (CyDs) complexes with vanadium-substituted heteropoly acids(PMoV n -β-CyDs, n = 1, 2) were prepared by adding the specified amount of HPMoV n into 25 mL of a hot aqueous β-cyclodextrins (Shanghai Chem. Reagent Co., AR) solution to form the β-CyDs/PMoV n molar ratio of 1:1. The precipitates obtained (PMoV n -β-CyDs, n = 1, 2) were dried at 343 K overnight in vacuum oven and used as the catalyst in the hydroxylation of benzene.
2.2 Liquid-Phase Hydroxylation of Benzene
The liquid-phase hydroxylation of benzene was carried out in a custom-designed temperature controllable titanic reactor (100 mL) with a mechanical stirrer.
The typical reaction conditions were as follows: 0.100 g catalyst and 0.400 g ascorbic acid (Shanghai Chem. Reagent Co., AR), 25.0 mL of 80 vol% aqueous acetic acid and 2.0 mL of benzene (Shanghai Chem. Reagent Co., AR) were added into the reactor in turn carefully. When the reactor was subjected to the desired temperatures, oxygen was injected into the reactor up to the preset pressure. The hydroxylation was conducted for 10 h under stirring. After the reaction, 1.0 mL of 1,4-dioxane (Shanghai Chem. Reagent Co., AR), which was confirmed as unchanged during the treatment, was added into the reaction mixture as an internal standard for product analysis. The final product mixture after reaction and prior to the GC analysis was observed to be in the homogenous state.
Gas chromatographic (GC) measurements were performed on a SP-6890A equipped with a FID detector and a capillary column (SE-54; 30 m × 0.32 mm × 0.25 μm). This reaction system appeared to have a high selectivity since phenol was the only product detected by GC. Among all catalysts, phenol was produced as the only reaction product without observing the formation of catechol, hydroquinone and benzoquinone.
2.3 Measurement of the IR Spectra of the Catalysts
The IR spectra of the catalysts were measured using a KBr disk mounted in an infrared spectrophotometer (Nexus 870). Samples were mixed and grounded with KBr for IR measurement.
3 Results and Discussion
3.1 Catalyst Characterization
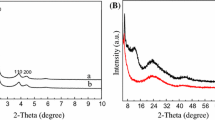
The IR spectra of PMoV n , PMoV n -β-CyDs (n = 1, 2) and β-CyDs are illustrated in Fig. 1. From Fig. 1, it can be seen that PMoV n and PMoV n -β-CyDs (n = 1, 2) in this work showed all the IR vibration peaks assigned to a Keggin-type heteropolyacid, and the locations of featured peaks (PsO, 1,059 cm−1; MosOsMo, 959 cm−1; ModO, 870, 785 cm−1) are in well agreement with those in the previous report [33]. The prepared PMoV n (n = 1, 2) catalysts were orange-red in color, very soluble in water, and took on a blue color upon treatment with a mild reducing reagent, such as ascorbic acid. These results also provided qualitative support for the conclusion that the prepared vanadium-substituted heteropoly acids have Keggin-type HPA structures.
The IR spectra of PMoV n -β-CyDs (n = 1, 2) and β-CyDs from 1,200 to 1,500 cm−1 are very similar to each other and the OH group peaks of PMoV n -β-CyDs (n = 1, 2) are obviously weaker than that of β-CyDs, which showed that PMoV n (n = 1, 2) have been interacted with β-CyDs possibly via the hydrogen bond and retain their Keggin-type structures well.
3.2 Evaluation of Performances of Various Catalysts
The catalytic activity of PMoV n and PMoV n -β-CyDs (n = 1, 2) in the direct hydroxylation of benzene to phenol with molecular oxygen at 383 K is shown in Table 1. It can be seen that no phenol was detected without ascorbic acid used as the reducing reagent. When ascorbic acid was added into the reaction mixture without a catalyst, only 1.7% yield of phenol was achieved. Under the working conditions, PMoV1 and PMoV2 displayed the same catalytic activity. PMoV1-β-CyDs exhibited the highest catalytic activity among those catalysts. No other products were detected by GC analysis in all entries. β-CyDs can obviously improve the catalytic activity (3.0% for PMoV1 vs. 5.2% for PMoV1-β-CyDs, 3.0% for PMoV2 vs. 3.3% for PMoV2-β-CyDs, respectively), which maybe owe to the unique cavity of β-CyDs. The cavity of β-CyDs is apolar, and thus any reactions, which proceed rapidly in apolar media, should be accelerated simply by a “microsolvent effect” [34]. As is well known, the solubility of apolar guest compounds in water is in general increased when they forms inclusion complexes with β-CyDs. Thus, β-CyDs is a potent phase-transfer catalyst [35].
As suggested in Fig. 2, the hydrophobic substrate (S) benzene in the organic phase enters the cavity of β-CyDs and contacts the catalytic center PMoV1. After the reaction, the product (P) phenol is released into the aqueous phase at the liquid/liquid interface and the transfer cycle can go on [36]. What’s more, the product can be rapidly released from the catalytic center and avoid to be oxidized further, which may account for the result that only phenol was detected as the product over all of the β-CyDs complexes in the direct hydroxylation of benzene.
3.3 Effect of the Reaction Temperature on the Yield of Phenol
The effect of the reaction temperature on the yield of phenol over PMoV1-β-CyDs is shown in Fig. 3. The yield increased slowly from 3.1% to 5.2% when the reaction temperature was enhanced from 333 to 383 K, and then sharply raised up to 9.9% when the temperature reached to 393 K. However, when the reaction temperature was further up to 423 K, the yield of phenol sharply dropped to 2.3%. This is mostly caused by the excessive oxidation of the product (phenol), and these deep oxidized products could not be well determined by GC analysis. Therefore, 393 K was considered as a suitable reaction temperature.
3.4 Effect of the Oxygen Pressure on the Yield of Phenol
The yield of phenol as functions of the oxygen pressure is illustrated in Fig. 4. The phenol yield was found to increase with the increase of the oxygen pressure up to 1.5 MPa where it reached to ca. 13.1%. Further increase of oxygen pressure had a reverse influence on the phenol yield, which quickly decreased to 1.9%. As is well known, the solubility of oxygen in water in general increases with increase of the pressure of oxygen, but too much oxygen may cause the excessive oxidation of the product (phenol). Therefore, 1.5 MPa is considered as a suitable reaction pressure.
3.5 Effect of the Amount of Ascorbic Acid on the Yield of Phenol
The results for the effect of the amount of ascorbic acid on the yield of phenol investigated at 393 K over PMoV1-β-CyDs are shown in Fig. 5. Almost no product was produced when no ascorbic acid was present as the reducing reagent. The yield of phenol increased with increasing amount of ascorbic acid up to 0.400 g, but a further increase in the amount of ascorbic acid inversely caused a decrease in the yield of phenol. The role of the reducing reagent was suggested to activate the oxygen molecule through the reduction of the V species, however, an extra ascorbic acid may decrease the activated oxygen species as well as [37]. The yield of phenol had a maximum value at the amount of ascorbic acid of around 0.400 g. The activated oxygen species are necessity for phenol formation, without utilizing for the benzene oxidation and thus the extra ascorbic acid is not in favor of the increase in phenol yield. Therefore, 0.400 g ascorbic acid was considered as a suitable amount in this reaction.
3.6 Effect of the Amount of PMoV1-β-CyDs on the Yield of Phenol
As shown in Fig. 6, when the amount of PMoV1-β-CyDs increased from 0.010 to 0.100 g, the yield of phenol increased sharply from 2.3% to 13.1%. When the amount of PMoV1-β-CyDs increased from 0.100 to 0.200 g, the yield of phenol decreased sharply from 13.1% to 3.7%. On the other hand, a further increase in the amount of PMoV1-β-CyDs caused a slight decrease in the yield of phenol (from 3.7% for 0.200 g to 1.1% for 0.800 g). As is well known, benzene and phenol can be completely oxidized by containing vanadium catalysts [38–40]. The reason causing the decrease of the yield of phenol with the increase of the catalyst may be benzene and (or) the product (phenol) were deep oxidized by the extra catalyst. So, 0.100 g PMoV1-β-CyDs was chosen as a suitable amount in this reaction.
3.7 Effect of the Reaction Time on the Yield of Phenol
The influence of reaction time on the yield of phenol over PMoV1-β-CyDs is shown in Fig. 7. It is obvious that the yield of phenol increased greatly with the increase of reaction time up to 10 h, and after that, the yield decreased sharply with the increase of reaction time. The sharply decrease of yield may be caused by the further oxidation of the product (phenol), so 10 h was chosen as a suitable reaction time in this work.
4 Conclusions
PMoV n can easily be interacted with the β-CyDs and their Keggin-type structures remained well. PMoV1-β-CyDs is demonstrated to be a good phase-transfer catalyst in the direct hydroxylation of benzene to phenol with molecular oxygen as the oxidant. The highest yield of phenol, 13.1%, is achieved in a batch reactor under optional reaction conditions: 0.100 g catalyst, 0.400 g ascorbic acid, 2.0 mL benzene, 25.0 mL of an aqueous solution containing 80 vol% acetic acid, 393 K, 1.5 MPa, and 10 h.
References
Stockmann M, Konietzni F, Notheis JU, Voss J, Keune W, Maier WF (2001) Appl Catal A Gen 208:343
Ehrich H, Berndt H, Pohl MM, Jahnisch K, Baerns M (2002) Appl Catal A Gen 230:271
Lemke K, Ehrich H, Lohse U, Berndt H, Jahnisch K (2003) Appl Catal A Gen 243:41
Niwa S, Eswaramoorthy M, Nair J, Raj A, Itoh N, Shoji H, Namba T, Mizukami F (2002) Science 295:105
Bianchi D, Bortolo R, Tassinari R, Ricci M, Vignola R (2000) Angew Chem Int Ed Engl 39:4321
Zhong YK, Li GY, Zhu LF, Yan Y, Wu G, Hu CW (2007) J Mol Catal A Chem 272:169
Tada M, Bal R, Sasaki T, Uemura Y, Inada Y, Tanaka S, Nomura M, Iwasawa Y (2007) J Phys Chem C 111:10095
Kubacka A, Wang ZL, Sulikowski B, Corberan VC (2007) J Catal 250:184
Gu YY, Zhao XH, Zhang GR, Ding HM, Shan YK (2007) Appl Catal A Gen 328:150
Tang Y, Zhang J (2006) Transit Met Chem 31:299
Yamaguchi S, Sumimoto S, Ichihashi Y, Nishiyama S, Tsuruya S (2005) Ind Eng Chem Res 44:1
Liptakova B, Hronec M, Cvengrosova Z (2000) Catal Today 61:143
Uriarte AK, Rodkin MA, Gross MJ, Kharitonov AS, Panov GI (1997) In: 3rd World congress on oxidation catalysis, vol 110. Elsevier Science Publ B V, Amsterdam, p 857
Bahidsky M, Hronec M (2004) Catal Today 91–92:13
Duprat AF, Capdevielle P, Maumy M (1991) J Chem Soc Chem Commun 464
Ohtani T, Nishiyama S, Tsuruya S, Masai M (1995) J Catal 155:158
Jintoku T, Takaki K, Fujiwara Y, Fuchita Y, Hiraki K (1990) Bull Chem Soc Jpn 63:438
Lin MR, Hogan TE, Sen A (1996) J Am Chem Soc 118:4574
Hamada R, Shibata Y, Nishiyama S, Tsuruya S (2003) PCCP Phys Chem Chem Phys 5:956
Yamanaka H, Hamada R, Nibuta H, Nishiyama S, Tsuruya S (2002) J Mol Catal A Chem 178:89
Shibata Y, Hamada R, Ueda T, Ichihashi Y, Nishiyama S, Tsuruya S (2005) Ind Eng Chem Res 44:8765
Zhang J, Tang Y, Li GY, Hu C (2005) Appl Catal A Gen 278:251
Wang JM, Yan L, Qian G, Lv GM, Li GX, Suo JS, Wang XL (2007) React Kinet Catal Lett 91:111
Wang JY, Hu CW, Jian M, Zhang J, Li GY (2006) J Catal 240:23
Gu YB, Wei RP, Ren XQ, Wang J (2007) Catal Lett 113:41
Zhang FM, Yuan CS, Wang J, Zhu HY, Wang CY (2006) J Mol Catal A Chem 247:130
Zhang FM, Wang J, Yuan CS, Ren XQ (2005) Catal Lett 102:171
Wang J, Lin Z, Han SY, Eum M, Lee CW (2003) J Ind Eng Chem 9:281
Weinstock IA (1998) Chem Rev 98:113
Klemperer WG, Wall CG (1998) Chem Rev 98:297
Mizuno N, Misono M (1998) Chem Rev 98:199
Zhang FM, Guo MP, Ge HQ, Wang J (2007) Chin J Chem Eng 15:4
Bardin BB, Davis RJ (1999) Appl Catal A Gen 185:283
Dodziuk H (ed) (2006) Cyclodextrins and their complexes: chemistry, analytical methods, applications. WILEY-VCH
Trifonov AZ, Nikiforov TT (1984) J Mol Catal 24:15
Monflier E, Tilloy S, Blouet E, Barbaux Y, Mortreux A (1996) J Mol Catal A Chem 109:27
Ishida MA, Masumoto Y, Hamada R, Nishiyama S, Tsuruya S, Masai M (1999) J Chem Soc Perkin Trans 2:847
Bellobono IR, Ascari F, Lagrasta C, Pinacci PL, Tozzi PM, Di Carlo MS, Simoncelli C (2003) Fresenius Environ Bull 12:1536
Bielanski A, Najbar M (1997) Appl Catal A Gen 157:223
Vassileva M, Moroz E, Dancheva S, Ushakov V, Andreev A (1994) Appl Catal A Gen 112:141
Acknowledgments
The authors thank the Natural Science Foundation of China (Nos. 20306011 and 20476046) and the “Qinglan” Project of Jiangsu Province for Young Researchers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ge, H., Leng, Y., Zhou, C. et al. Direct Hydroxylation of Benzene to Phenol with Molecular Oxygen over Phase Transfer Catalysts: Cyclodextrins Complexes with Vanadium-Substituted Heteropoly Acids. Catal Lett 124, 324–329 (2008). https://doi.org/10.1007/s10562-008-9464-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9464-y