Abstract
The CuO dispersed on ZnCr2O4 catalysts derived from Cu–Zn–Cr hydrotalcite like layered double hydroxide precursors with varying Zn/Cr ratios have been synthesized, characterized by BET—Surface area, X-ray diffraction (XRD), temperature programmed reduction (TPR), electron spin resonance (ESR), N2O titrations and the activities were evaluated for single step dimethyl ether (STD) synthesis from syngas. It is observed that the copper species were in highly dispersed state over Cu–ZnO–Cr2O3 at high Zn/Cr ratios while the copper cluster were present at low Zn/Cr ratios. The ESR analysis revealed signals due to isolated Cu2+ at high Zn/Cr ratios and clustered Cu2+ at low Zn/Cr ratio in fresh catalysts and only Cr3+ species in used catalysts. The TPR results indicated that the reduction peak shifted to high temperatures with an increase in chromium content due to large copper crystallites, which was supported by XRD analysis. The conversion of syngas to DME was well correlated with the copper metal surface areas, indicating that STD synthesis can be controlled by methanol synthesis rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Single step dimethyl ether synthesis from syngas is mainly composed of methanol synthesis, methanol dehydration and water gas shift reaction.
Single step dimethyl ether synthesis from syngas has technological advantages over the two step process of methanol synthesis and the following methanol dehydration. The advantages being (1) high one-through conversion in thermodynamic point of view [1] and (2) low operating cost in the economic point of view [2]. The STD synthesis has been conducted on the admixed catalysts of methanol synthesis and methanol dehydration. For the STD synthesis, the methanol synthesis catalysts were chosen from Cu/Zn oxides dispersed on Al2O3 and/or Cr2O3 systems, while methanol dehydration is carried out over solid acid catalysts [3–6]. The effects of solid acid catalysts on STD synthesis from syngas were studied with the admixed catalysts of Cu/ZnO/Al2O3 and ZSM-5 [7]. However, STD synthesis rates are not dependent on the acidity of the solid acid catalysts, but on the amount of methanol synthesis catalysts in the admixed catalysts, although methanol dehydration rate is closely proportional to the acidity of the solid acid catalysts. Therefore, it is suggested that the STD reaction rate on the admixed catalysts could be dependent on methanol synthesis rate, over the solid acid catalysts with moderate acid sites such as γ-Al2O3 are used. It is in general agreement that methanol synthesis rates are proportional to Cu metal surface areas, although the higher activity of Cu–Zn based catalysts for methanol synthesis has been studied for several decades in the aspects of electronic properties of Cu and ZnO [8], stabilizations of special morphologies [9, 10], spillover phenomena [11–14], and specific interaction at the Cu/ZnO interface [15]. It is reported that methanol synthesis rates are linearly proportional to Cu surface areas irrespective of the supports [16]. Another important aspect is the synergistic effect of Cu and ZnO are suggested for methanol synthesis. Nevertheless, it is shown that linear correlations of rate of methanol synthesis with Cu metal surface areas are obtained but the slopes of the curves dependent upon the support material [17–20]. The stability of Cu/ZnO catalysts has been discussed in view of the sintering of Cu via brass formation [21–24]. It is suggested that the sintering of Cu via brass formation could be prevented in Cu–ZnO–Cr2O3 catalysts obtained from Cu–Zn–Cr hydrotalcite-like precursor [25]. Thermally stable catalysts are required because STD synthesis is highly exothermic reaction as compared to methanol synthesis. Therefore, Cu–Zn–Cr catalytic systems prepared by the hydrotalcite-like precursor are used for STD synthesis in this study. It is generally known that precursors of copper containing hydrotalcites are very sensitive towards thermal treatments and the copper dispersion is dependent upon the preparation conditions such as pH, Zn/Cr ratios and dispersing agent. This paper illustrates the synthesis and characterization of Cu on ZnO–Cr2O3 matrix derived from the Cu–Zn–Cr layered double hydroxide (LDH) precursors. A series of Cu–ZnO–Cr2O3 (with Zn/Cr = 1.2–6.8) catalysts have been synthesized and characterized. The conversions of CO to DME synthesis are evaluated on the admixed catalysts composed of Cu–Zn–Cr and γ-Al2O3.
The physico-chemical properties of Cu–ZnO–Cr2O3 catalysts, which were elucidated by BET—surface area, X-ray diffraction (XRD), electron spin resonance (ESR), temperature programmed reduction (TPR) analyses, and N2O titrations are very well correlated with the conversion of syngas to DME.
2 Experimental
2.1 Catalyst Preparation
Cu–Zn–Cr hydrotalcite-like precursors were prepared by coprecipitation method with constant Cu and various Zn/Cr mole ratios in order to investigate its influence on copper dispersion, since the copper particles are dispersed and stabilized by ZnO and Cr2O3. In a typical method, required amounts of copper, zinc and chromium nitrate salts were dissolved in 600 mL of distilled water (solution A) and a solution B containing 2 M NaOH and 1 M Na2CO3 (1:1 = v/v) were added simultaneously to 2.0 L of distilled water under vigorous stirring. The mole ratios of Cu:Zn:Cr of the prepared catalysts denoted as CZC were 7:6:0.88 (CZC-6.8), 7:5.3:1.6 (CZC-3.3), 7:4.6:2.4 (CZC-1.9), 7:3.8:2.7 (CZC-1.4) and 7:3.6:2.9 (CZC-1.2) respectively. The CZC-X indicates the mole ratio of Zn/Cr = X. A constant pH ∼8.9 is maintained while adjusting the flows of solution A and solution B during the co-precipitation of the samples. The coprecipitation was carried out at room temperature and the precipitate was aged for 1 h, washed several times until pH of the gel reached the pH of the distilled water. The residual Na concentration is found to be <0.03% by atomic absorption spectroscopy. The resulting precipitate was filtered, oven dried at 100 °C for 24 h and calcined in static air at 400 °C/3 h at a ramping rate of 4 °C/min. For the comparison, the Zn–Cr (Zn:Cr = 3:1 mol ratio) and Cu–Cr (Cu:Cr = 1:1 mol ratio) samples were prepared under similar conditions adopted as mentioned above [26].
2.2 Characterization
The BET-surface areas of the calcined catalysts were obtained by means of dinitrogen physisorption at ca. 77 K using a Micromeritics ASAP 2000 instrument. Prior to the measurements, the catalysts were degassed at 120 °C for 0.5 h. The X-ray diffraction analysis of the oven dried and calcined samples were carried out in the 2θ range from 5 to 90° using a Rigaku Diffractometer employing Ni filtered Cu K∝ radiation at 40 kV and 126 mA. The temperature programmed reduction (TPR) and the N2O decomposition were carried out using a fixed bed reactor connected to a GC with thermal conductivity detector. For TPR analysis 5% dihydrogen in Ar was used. In case of N2O titrations, Porapak N column was used for the N2O and N2 separation. About 30–80 mg of catalyst loaded was reduced in 5% H2/Ar stream at 250 °C for 1 h and the N2O titrations were carried out at 90 °C. Several replicate experiments were carried out and the averaged value was chosen for Cu metal surface areas. In this investigation, a surface copper density of 1.46 × 1019 atoms/m2 was used for the copper metal area calculations [27]. The Electron Spin Resonance (ESR) analysis was performed at room temperature using JEOL, JES-FA200 ESR spectrophotometer by X-band equipment with an operating frequency ν = 9.029 GHz.
2.3 Activity Measurements
DME synthesis was carried out using a fixed bed micro reactor loaded with finely mixed catalysts composed of 0.5 g of activated methanol synthesis catalyst (Cu–Zn–Cr) and 0.5 g of methanol dehydration catalyst i.e. γ-Al2O3. Prior to the reaction the catalysts were reduced in a stream of 5% H2/Ar in a sequential reduction steps as follows: 1st step: treatment at 100 °C/0.5 h, 2nd step: reduction at 180 °C/0.5 h, 3rd step: reduction at 240 °C/2 h, 4th step: reduction at 280 °C/0.5 h. The activity of the catalysts for the STD synthesis was tested under the reaction conditions of the H2–CO ratio of 1.5, a GHSV of 6000 h−1, pressure of 600 psig and the reaction temperature ranges from 240 to 270 °C. The reaction mixture was passed through a purifier in order to eliminate traces of water, oxygen and iron carbonyl that would deactivate the catalyst. The effluent gas mixture was analyzed by an on-line gas chromatograph equipped with thermal conductivity detector using Porapak Q column. The experimental error in the evaluation of the catalysts was found to be ±2% unless otherwise mentioned.
3 Results
3.1 Activity Dependence on Copper Metal Surface Area
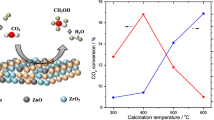
DME synthesis rates on the admixed catalysts of the prepared Cu/ZnO/Cr2O3 and γ-Al2O3 were plotted with respect to Cu surface areas as shown in Fig. 1. The STD reaction rates, rco increased proportionally with Cu metal surface area, suggesting that the DME synthesis rates are directly proportional to the copper metal surface area. In previous results, DME synthesis rates increased with an increase in amounts of Cu in Cu/ZnO based catalysts in the admixed catalysts [7]. This increase is found to be equivalent to the increase in specific Cu surface areas of the admixed catalysts. There is general agreement that rate of methanol synthesis is well correlated with Cu surface area, although one slope independent on Cu supports was suggested by some authors and different slopes dependent on Cu supports were done by the other authors [28]. Therefore, this experimental result supports that the STD reaction rate on the admixed catalysts could be dependent on methanol synthesis rate. It means that methanol synthesis rate on Cu–Zn–Cr2O3 catalyst part in the admixed catalysts can be much lower than methanol dehydration on γ-Al2O3 part in STD synthesis reaction.
3.2 BET Surface Area and XRD Analysis
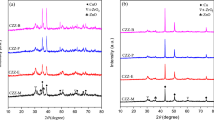
The BET surface areas of the calcined samples, the crystalline phases of the oven dried and calcined samples of Cu–Zn–Cr catalysts, and Cu metal surface areas by N2O titration are reported in Table 1. The BET surface areas of Cu–Zn–Cr2O3 catalysts are in the range of 41.4–63.6 m2/g and no dependency of the BET surface areas on the ratios of Zn to Cr is found. The XRD patterns of the oven-dried samples reveal the diffraction lines of Cu–Zn–Cr LDH structure as shown in Fig. 1 [29, 30]. The XRD patterns show the decrease of crystallinity with an increase in chromium loading. At high Zn/Cr ratios the diffraction lines were intense and sharp. The intensities of Cu–Zn–Cr HT peaks increased with an increase in Zn concentration and Frost and Ding report similar phenomenon at high zinc concentration over Cu–Zn–Cr HT samples [31]. For comparison purpose, the Zn–Cr (mole ratio = 3/1) and Cu–Cr (mole ratio = 1/1) are also prepared under identical preparation conditions and their diffraction patterns are shown in Fig. 2. Presence of CuO phase in oven dried samples is ascribed to the Jahn-Teller effect due to the deviant behavior of copper when the Cu/Zn ratio >1 is maintained in the ternary Cu–Zn–Cr catalysts [32].
XRD patterns of the calcined samples are shown in Fig. 3 and the observed phases are reported in Table 1. The HT structure of Cu–Zn–Cr collapsed upon thermal decomposition in air at 400 °C/3 h. Phases due to CuO [ICDD: 80-0076] and ZnO [ICDD: 89-1397] are predominant and the ZnCr2O4 phase [ICDD: 22-1107] is minor in intensity. It is also observed that some of the diffraction lines of ZnO and ZnCr2O4 are superimposed. The intensities of CuO and ZnO peaks increased with an increase in Zn/Cr ratio up to 1.9 and the ZnO characteristic peaks were predominant at Zn/Cr ratio higher than 1.9. This indicates the high interaction between CuO and ZnO particles at high Zn/Cr ratios. The very weak diffraction lines due to CuCr2O4 [ICDD: 72-1212] are observed over Cu/Cr2O3 and the Cu/ZnO/Cr2O3 (Zn/Cr mole ratio = 1.2) samples. None of the catalysts showed diffraction lines due to Cr2O3 phase even at low Zn/Cr = 1.2, this is possibly due to amorphous nature of Cr2O3 or might be due to an interaction of Cr2O3 with ZnO particles at high Zn/Cr and with CuO at low Zn/Cr ratios present in the catalyst [33]. From this it can be concluded that higher is the interaction between Cu and Zn, lesser is the possibility of Cu and/or Zn—Cr2O4 formation.
After DME synthesis reactions under the condition at temperatures of 240–270 °C, at 40.8 atm, and the GHSV of 6000 h−1, the used admixed catalysts of Cu/ZnO/Cr2O3 and γ-Al2O3 are subjected to XRD analysis and their patterns are reported in Fig. 4. Diffraction lines due to metallic copper [ICDD: 85-1326] and ZnO [ICDD: 89-1397] phase are observed. The catalysts with low Zn/Cr ratios displayed strong lines due to metallic copper as compared to those with high Zn/Cr ratios. The CuO peaks in calcined samples (Fig. 3) disappeared after the syngas reaction, which is due to the reduction of Cu2+–Cu0 phase [34]. It is also observed that CuCr2O4 peak intensities decreased in used catalysts as compared to fresh catalysts.
3.3 Electron Spin Resonance analyses (ESR)
The ESR spectroscopy is used to investigate the paramagnetic species of Cu2+, Cr3+ and Cr5+ present in the Cu–Zn–Cr catalysts. The room temperature ESR spectra of the fresh and used catalysts are shown in Figs. 5 and 6 respectively. From Fig. 5 it is evident that the peak shapes of the signals attributed to Cu2+ changed with increase in Zn/Cr ratio and the spectra are anisotropic with clearly defined g|| and g⊥ regions as expected for Cu2+ ions in a surrounding with axial symmetry. The well-resolved hyperfine structure due to isolated Cu2+ species appeared at g|| = 2.355, and A|| = 12.1 mT, and the unresolved pattern at g⊥ = 2.104 and A⊥ over CZC-6.8 and CZC-3.3 catalysts. These experimental results are in good agreement with the reported literature [35–37]. At low Zn/Cr ratios (Fig. 5c, d) the peaks are broadened and shifted to higher values and the broad signals are probably due to the Cr3+ species present in the catalyst [38]. The broad ESR signal of Cr3+ can be due to CuCr2O4 species [39], which is observed at low Zn/Cr ratios in XRD analysis. This result suggests the g|| region, which is ascribed to isolated Cu2+ species and is disappeared over the catalysts with low Zn/Cr ratios while the g|| region, ascribed to the Cr3+ species, appeared. It could be explained as to the participation of some part of Cu2+ ions in the formation of an oxide complex and/or a copper chromate particularly over CuO–ZnO–Cr2O3 with Zn/Cr = 1.2 and the XRD analysis showed CuCr2O4 phase supporting this explanation [40]. Lack of hyperfine structure (Fig. 5c–e) at low Zn/Cr ratios is due to the CuO cluster formation that is also demonstrated in (Fig. 3) XRD analysis. Signals due to Cr5+ species could not be seen in all the samples.
The ESR patterns of the used catalysts are shown in Fig. 6. Highly symmetrical broad signals, attributed due to Cr3+ centered at 328.0 mT with corresponding g = 1.97, are observed the peaks with line widths of 56.4, 61.9, 59.9, 52.9, 43.0 mT for Cu–Zn–Cr of Zn/Cr = 6.8, 3.3, 1.9, 1.33, 1.2 catalysts respectively [41]. These Cr3+ species are ascribed due to the presence of ZnCr2O4 in the catalyst and it is unlikely that these species belong to CuCr2O4 since XRD patterns of the used catalysts did not show any peaks due to CuCr2O4 phase. Cu2+ signals are not observed in used catalysts indicating that all Cu2+ species are in the reduced form.
3.4 Temperature Programmed Reduction (TPR)
The temperature programmed reduction profiles of the fresh catalysts are shown in Fig. 7. The CuO/Cr2O3 sample showed a reduction peak (Tmax ∼230 °C) with a shoulder and the ZnO/Cr2O3 sample showed a single reduction peak at 325 °C. These results indicate that copper oxide in the proximity of chromium particles undergoes reduction below 270 °C and the chromium oxide in the proximity of zinc oxide particles completely was reduced at 350 °C.
It is also observed that the copper oxides in Cu–ZnO–Cr2O3 samples are reduced in three stages and these peaks are presumably due to dispersed copper oxide, CuO interacted with ZnO or ZnCr2O4 phase and the clustered CuO. The reduction peak is slightly shifted toward high temperatures with an increase in Cr content. The small reduction peak at the temperature below 200 °C can be assigned to the finely dispersed CuO. The dispersed Cu2+ is reduced at temperatures lower than the clustered/bulk CuO species [42]. The intensity of the peak at the temperature below 200 °C is clearly observed to be high with CZC-6.8 and CZC-3.3 catalyst, while it is too little with CZC-1.4 and CZC-1.9 catalysts. In ESR analysis, the isolated Cu2+ species are observed with CZC-6.8 and CZC-3.3 catalysts, but disappeared with CZC-1.4 and CZC-1.9 catalysts. The peak at about 220 °C is due to sequential reduction of bulk Cu2+ into Cu0 and the peak at 249 °C is probably due to strongly interacted copper oxide either with ZnO or ZnCr2O4 phase. The shift in reduction peak towards high temperatures (Fig. 7) with an increase in chromium content (at low Zn/Cr ratios) indicates the formation of large CuO cluster as also evidenced in the XRD analysis (Fig. 3).
3.5 N2O Titrations
The copper metal surface areas of the fresh catalysts measured by N2O titrations are reported in Table 1. The Cu/ZnO/Cr2O3 samples with the high Zn/Cr ratio showed copper metal surface areas higher than the samples with low Zn/Cr ratio. From this it is reasonable to note that CZC-3.3 (SCu = 6.5 m2/g) and CZC-6.8 (SCu = 5.2 m2/g) catalysts produce finely dispersed copper on the catalyst surface. The Cu metal surface areas of CZC-1.2, CZC-1.4 and CZC-1.9 with low Zn/Cr ratio are 0.92, 1.44 and 2.6 m2/g, respectively. The high copper metal surface areas of CZC-3.3 and CZC-6.8 can also be supported from the ESR analysis (Fig. 5a, b). The strong axially symmetrical signals over CZC-3.3 and CZC-6.8 catalysts at g|| = 2.35 are generally attributed to highly dispersed or isolated Cu2+ species interacted with support material. As shown in Fig. 4, the broad reflections of CuO and ZnO phases over CZC-3.3, CZC-6.8 catalysts suggest that copper particles are in intimate contact with ZnO matrix [43]. On the contrary, XRD patterns of CZC-1.2, CZC-1.4 and CZC-1.9 catalysts showed (Fig. 3) sharp CuO peaks which can be assigned to the CuO cluster, which is also evidenced from the asymmetrical peaks with narrow line width and the disappearance of isolated Cu2+ species in the g|| = 2.35 region as shown in Fig. 5 (ESR analysis). From this observation, it can be concluded that ZnO plays an important role to disperse copper on the catalyst surface by increasing the isolated Cu+2 in the calcined samples.
3.6 Activity Measurements
The temperature dependence on CO hydrogenation activities are reported in Fig. 8. Increase in reaction temperature increased CO conversion was up to 270 °C over all the catalysts. The catalysts showed higher CO conversions at high Zn/Cr ratios and maximum is found over the catalyst with Zn/Cr = 3.3. The STD synthesis rate is proportional to the copper metal surface areas of the catalysts as observed from Fig. 1. The selectivity towards DME is ∼70 and 30% towards CO2 over all the catalysts. The activities of Zn–Cr and Cu–Cr catalysts are tested under the above reaction conditions at 270 °C. The CO conversions on Zn–Cr and Cu–Cr catalysts are found to be less than 1% and CO2 is the only product observed.
4 Discussion
From Table 1, it is clearly evident that CZC-3.3 is found to have higher copper metal surface area over the other catalysts. It appears that the optimum Zn/Cr ratio for high syngas conversion lies between 3.2 and 6.8 in this case which is also evidenced from the ESR analysis at low chromium contents; isolated/dispersed Cu2+ peaks are predominant than at high chromium loadings. It is also evident that at low Zn/Cr ratios isolated Cu2+ signals disappeared (Fig. 5d, e) with the evolution of broad ESR signals attributed to clustered Cu2+ species. Unfortunately, at present we have no direct experimental evidence showing how these Cu2+ species can contribute to the CO hydrogenation activity. However, the disappearance of isolated Cu2+ (dispersed CuO) in high chromium loaded catalysts demonstrated the low CO conversions in contrast to the low Cr loaded CZC-3.3 and CZC-6.8 catalysts. From this it can be concluded that isolated copper species play an important role during syngas conversions. XRD analysis of these catalysts revealed that sharp diffraction lines due to CuO at high chromium loadings (Fig. 3) as well as the CuCr2O4 phase although in minor intensity only in CZC-1.2 catalyst. On the contrary as aforesaid at high Zn/Cr ratios the CuO and ZnO peaks are not resolved and increase in ZnO lines observed; eventually the diffraction lines are broad, which indicates the close contact between copper and zinc particles. This suggests that interaction between copper and zinc particles are necessary in order to achieve high syngas conversions. Another factor that supports our results is based on the good agreement of copper metal surface areas (Table 1) against syngas conversions.
The XRD patterns of the used catalysts showed the decrease in peak intensities due to CuCr2O4 phase however, the ZnCr2O4 peaks remained intact. The used catalysts are obtained after reductive pretreatment with 5% H2/Ar at 280 °C and subsequent syngas exposure at 240–270 °C for prolonged time and subsequently passivated in N2 atmosphere. The syngas reaction at 270 °C and reduction temperature i.e. 280 °C is sufficient enough to completely reduce the CuCr2O4 species and complete reduction of ZnCr2O4 seems unlikely at temperature below 280 °C. Hence, the intensities of the diffraction lines due CuCr2O4 peaks decreased and those of the ZnCr2O4 peaks remained the same in the used samples. This observation is in good agreement with the TPR analysis where reduction of Zn–Cr sample occurred at high temperatures i.e. 350 °C.
The low syngas conversions at high chromium loadings is attributed to the formation of CuCr2O4 and the role of ZnCr2O4 appears to enhance the copper dispersion as observed from high copper metal surface areas over the samples with high Zn/Cr ratios i.e. CZC-3.3 and CZC-6.8 catalysts. Even if there was no observable difference in the XRD and ESR data obtained for Cu2+, similar change is expected to take place on the surface of the catalyst, where isolated/dispersed Cu2+ species are significantly higher over high Zn/Cr catalysts which is further supplemented from the N2O titrations, in other words at higher Zn loading the Cu2+ is highly dispersed or stabilized on the catalyst surface and high syngas conversions are obtained [44].
5 Conclusions
The Cu–Zn–Cr LDH precursors are synthesized (keeping copper content constant and varying the Zn/Cr ratios in order to see the influence of both Zn and Cr on the dispersion of copper), characterized and evaluated for syngas conversions. Based on the results obtained the following conclusions are drawn.
At high Zn/Cr ratios the XRD analysis of the fresh calcined catalysts disclosed the unresolved diffraction lines of CuO and ZnO attributed to intimate contact between copper and zinc particles. ESR analysis indicated the isolated Cu2+ signals at high Zn/Cr ratios in the g|| = 2.35 region, which disappeared at low Zn/Cr samples presumably due to the formation of CuCr2O4 phase. The TPR profiles showed that reduction peak due to Cu2+ shifted to high temperatures with increase in chromium loading suggesting the formation of large copper crystallites, which is also evidenced from the XRD analysis. The diffraction lines due to metallic copper, ZnO phases in major and ZnCr2O4 in minor intensities are observed in the used catalysts. The ESR analysis gave supporting evidence due to the presence of only Cr3+ species since the Cu0 is ESR inactive. It appears that isolated copper species play an important role on syngas conversion and ZnCr2O4 seems to disperse the copper on the catalyst surface. Finely dispersed copper particles are obtained at Zn/Cr mole ratio = 3.3 as evident from the high copper metal surface area over CZC-3.3 catalyst at a ca. 6.5 m2/g obtained by N2O titrations. The optimum Zn/Cr mole ratio lies between 3.3 and 6.8 and high syngas conversion is obtained over catalyst with Cu:Zn:Cr = 7:6:1.88 (mol ratios) catalyst. The high syngas conversion over Cu–Zn–Cr with Zn/Cr = 3.3 catalyst is attributed to the high copper metal surface area as compared to the other catalysts. Finally it can be concluded that higher yields of DME is obtained in single step over a composite catalyst comprised of finely mixed Cu–Zn–Cr (Zn/Cr = 3.3) with γ-Al2O3.
References
Fujimoto K, Asami K, Shikada T, Tominaga H (1984) Chem Lett 2051
Hansen JG, Voss B, Joensen F, Siguroardottir ID (1995) SAE Technical Paper Series 950063
Klier K, Chatikavanij V, Herman RG, Simmons GW (1982) J Catal 74:343
Fleisch TH, Mieville RL (1984) J Catal 90:165
Busetto C, Del Piero G, Manara G, Trifiro F, Vaccari A (1984) J Catal 85:260
Campos-Martin JM, Guerrero-Ruiz A, Fierro JLG (1995) J Catal 156:208
Kim JH, Park M, Joo OS, Jung KD (2004) Appl Catal 264:37
Frost JC (1988) Nature 334:557
Klier K (1982) Adv Catal 31:243
Bartley GJJ, Burch R (1988) Appl Catal 43:141
Joo OS, Jung KD, Han SH, Uhm SJ (1995) J Catal 157:259
Joo OS, Jung KD, Han SH, Uhm SJ, Lee DK, Ihm SK (1996) Appl Catal 135:273
Fischer IA, Bell AT (1998) J Catal 178:153
Spencer MS (1998) Catal Lett 50:101
Miller GJ, Rochester CH, Bailey S, Waugh KC (1992) J Chem Soc Faraday Trans 88:2085
Chinchen GC, Waugh KC, Whan DA (1986) Appl Catal 25:101
Bart JCJ, Sneeden RPA (1987) Catal Today 2:1
Burch R, Golunski SE, Spencer MS (1990) J Chem Soc Faraday Trans 86:2683
Denise B, Sneeden RPA, Beguin B, Cherifi O (1987) Appl Catal 30:353
Fisher IA, Woo HC, Bell AT (1997) Catal Lett 44:11
Kiennemann A, Irdris H, Hindermann J, Lavalley J, Vallet A, Chaumette P, Courty P (1990) Appl Catal 59:165
Spencer MS (1987) Surf Sci 192:336
van Herwijnen T, de Jong WA (1974) J Catal 34:209
Jung KD, Joo OS, Han SH, Uhm SJ, Chung IJ (1995) Catal Lett 35:303
Jung KD, Joo O-S (2002) Catal Lett 84:21
Kloprogge JT, Hickey L, Frost RL (2005) Mater Chem Phys 89:99
Evans JW, Wainwright MS, Bridgewater AJ, Young DJ (1983) Appl Catal 7:75
Robinson WRAM, Mol JC (1990) Appl Catal 60:73
Porta P, Morpurgo S (1995) Appl Clay Sci 10:31
Morpurgo S, Jacano ML, Porta P (1995) J Solid State Chem 119:246
Frost RL, Ding Z (2003) Thermochim Acta 405:207
Trifiro F, Vaccari A, Piero GD (1988) In: Unger KK, Raoquerol J, Sing KSW, Kral H (eds) Characterization of porous solids. Elsevier, Amsterdam, p 571
Terlecki-Baricevic A, Jovanovic D, Grbic B, Marinova T, Kirilov-Stefanov P (1994) Appl Catal A: Gen 108:115
Wang Z, Liu Q, Yu J, Wu T, Wang G (2003) Appl Catal A: Gen 239:87
Sakata S, Nakai T, Yahiro H, Shiotani M (1997) Appl Catal A: Gen 165:467
Tanabe T, Iijima T, Koiwai A, Mijuno J, Yokota K, Isogai A (1995) Appl Catal B: Environ 6:145
Bahranowski K, Dula R, Gasior M, Labanowski M, Michalik A, Vartikian LA, Serwicka EM (2001) Appl Clay Sci 18:93
Poole CP Jr, Kehl WL, MacIver DS (1962) J Catal 1:407
Wojciechowska M, Haber J, Lomnicki S, Stoch J (1999) J Mol Catal A: Chem 141:155
Lee CY, Jung TH, Ha BH (1996) Appl Catal B: Environ 9:77
Ralek M, Gunsser W, Knappwost A (1968) J Catal 11:317
Campos-Martin JM, Fierro JLG, Guerrero-Ruiz A, Herman RG, Klier K (1996) J Catal 163:418
Robinson WRAM, Mol JC (1990) Appl Catal 60:61
Huang X, Ma L, Wainwright MS (2004) Appl Catal A: Gen 257:235
Acknowledgments
This work is financially supported by a program of Energy and Resources Technology Development. One of the authors AV acknowledges the KOFST, (Korea) for financial support and Director-IICT, Hyderabad and CSIR-New Delhi, India.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Venugopal, A., Palgunadi, J., Jung, K.D. et al. Cu–Zn–Cr2O3 Catalysts for Dimethyl Ether Synthesis: Structure and Activity Relationship. Catal Lett 123, 142–149 (2008). https://doi.org/10.1007/s10562-008-9408-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9408-6