Abstract
A composite oxide ZnAl2O4 was prepared by microwave-assisted hydrothermal treatment, a precursor mixture of hydroxides obtained by precipitation of aluminium and zinc nitrates. Characterization by TEM, XRD and textural studies shows that ZnAl2O4 is nanosized and is a micro/mesoporous material with large a surface area (140 m2/g). The gas phase catalytic methylation of 4-hydroxypyridine in the presence of the ZnAl2O4 catalyst was performed in a continuous process at atmospheric pressure in the temperature range of 240–360 °C. A mixture of O- and N-alkylated products, namely 4-methoxypyridine and N-methyl-4-pyridone were obtained. The alkylation of 4-hydroxypyridine with methanol at 345 °C offered 87.6% selectivity towards N-methyl-4-pyridone with about 89% 4-hydroxypyridine conversion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aromatic heterocycles are the most important compounds for human beings: two-third of organic compounds belong to this class. Pyridine is their representative. A great number of pyridine derivatives are important biologically active compounds. 2- and 4-N-methylpyridones have been found among metabolites in human cells [1].
4-Hydroxypyridine 1 indicates antioxidant properties in inhibition of hyperglycaemia [2]. 4-Hydroxypyridine/4-pyridone, with regards to its mesogenic properties, is used for synthesis of liquid crystals with useful properties such as high birefringence and polarity including hydrogen bonding capability [3]. 4-Pyridone has also complexing capability and can be used for the preparation of materials with bulk supramolecular structure [4].
Hydroxypyridines are very interesting compounds due to the presence of two different reactive nucleophilic centres. During the alkylation of these compounds, O-substitution, as well as N-substitution, can occur. N-methylated derivative of 4-pyridone serves as useful intermediate chemicals for other derivatives advantageous for production of pharmaceuticals, pesticides, insecticides, fungicides, etc. It was found that N-methyl-4-pyridone 2 may find applications in manufacturing of photographic compounds with de-aggregating and dye-forming properties [5].
Recently, hydrothermally synthesised zinc aluminate spinel was described by us as a good and efficient catalyst in a continuous flow process of 2-hydroxypyridine alkylation with methanol [6]. The reaction was performed in the gas phase at atmospheric pressure. High yield of N-methyl-2-pyridone was obtained at a temperature range from 310 to 340 °C. Selectivity towards N-methyl-2-pyridone reached 95%. As a by-product 2-methoxypyridine was observed.
The purpose of the presented work was to prepare ZnAl2O4 using microwave-assisted hydrothermal method and to investigate its catalytic properties in the synthesis of N-methyl-4-pyridone (Scheme 1).
Microwave heating, as a uniform and effective heating method, is most often employed for hydrothermal synthesis of nanomaterials as an attractive alternative to conventional conductive heating with its inherent heterogeneous temperature distribution [7, 8]. Microwave irradiation can considerably increase the reaction kinetics, and promote the formation of uniform nanoparticles. Thanks to these, the heating time is much shorter than in conventional hydrothermal methods and obtained materials usually show advanced catalytic properties [9, 10].
2 Experimental
2.1 Catalyst Preparation
The ZnAl2O4 catalyst was prepared by the microwave-assisted hydrothermal method using a mixture of aluminium and zinc hydroxides as raw material. In order to synthesise the catalyst, first the precipitation at room temperature was performed by adding 25 wt% aqueous solution of ammonia to mixed aqueous solution of corresponding metallic nitrates, in 1:2 molar ratio, until pH value of about 9 was reached. During precipitation and after 30 min. ageing, the system was vigorously stirred. The hydrated precursors obtained in this way were separated by filtration and washed several times with distilled water until the disappearance of nitrate ions. The aqueous slurry (5%) of precursors was then subjected to hydrothermal treatment which was carried out at 200 °C for 15 min. in a microwave accelerated reaction system MW Reactor Model 02-02 (ERTEC, Poland). After cooling the autoclave, the obtained product was subsequently washed using a high-speed centrifuge and condensed through evaporation at elevated temperatures. The long wires of about 2 mm in diameter formed by extrusion of the obtained gel were air-dried overnight, calcined at 600 °C for 4 h, then crushed and sieved into the pieces of 0.6–1.2 mm. The resulting catalyst was characterized by physico-chemical methods and its catalytic properties in the alkylation of 4-hydroxypyridine with methanol were evaluated.
2.2 Characterization Methods
The purity and crystallinity of the as-prepared and heated samples were examined using powder X-ray diffraction (XRD, Cu Kα, DRON-3 Diffractometer), of which the data were collected in steps of 0.05° (2θ) min−1 from 10° to 80° (2θ). The ICDD database [11] was used for phase identification. With the software Celref of least-squares refinement of cell dimensions from powder data, the lattice parameters were calculated. The average grain size D was estimated according to the Scherrer’s equation, D = 0.9λ/βcos θ, where θ is the diffraction angle of the (311) peak of the cubic phase, β is the full width at half maximum (FWHM) of the (311) peak (in radians), which is calibrated from high purity silicon.
The morphologies and structures of the synthesized products were observed with a Tesla BS 500 transmission electron microscope (TEM) operated at 60 kV and Philips CM20 SuperTwin high-resolution transmission electron microscope (HRTEM) operated at 200 kV with 0.25 nm resolution. The specimens were prepared by placing a drop of ultrasonic sample dispersion in methanol on a carbon/formvar—coated copper grid and allowing the solvent to evaporate. The crystal structure was determined by analysis of selected area diffraction patterns (SAED) and was completed by analysis of high-resolution electron micrographs. The particle sizes were measured with a comparator and the average particle sizes and size distribution were determined based on the measurements of at least 50 particles from TEM or HRTEM micrographs.
The BET specific surface area (S BET) and pore volume (V p) distribution were measured by nitrogen adsorption at liquid nitrogen temperature, using an automatic volumetric instrument (FISONS Sorptomatic 1900), which was performed after outgassing the sample at 300 °C under vacuum, down to a residual pressure better than 10−3 Torr. The pore size (r p) distribution was calculated from the desorption branch of the isotherms, based on the Dollimore–Heal (D–H) method [12].
The total surface concentration of acid sites and acidity strength distribution was determined by a temperature programmed desorption of ammonia (NH3-TPD). The nature of the surface acid sites, i.e. the presence of Lewis or Brönsted centres and their concentration, were studied by FTIR spectroscopy measurements of the samples preadsorbed with pyridine as a probe molecule. Transmission IR spectra were recorded using Specord M80 spectrometer. The procedure applied for acidity measurements of ZnAl2O4 catalyst was the same as already described in our earlier studies [13, 14].
The analysis of basic properties of the catalyst, aiming at determining the strength and the basic sites distribution, was performed by temperature-programmed desorption (TPD) of CO2. The amount of CO2 chemisorbed and its desorption profile were measured using the mass spectrometer (Pfeiffer Vacuum, OmniStar QMS 200) by monitoring the signal of m/e = 44. The applied procedure (sample pretreatment, gas adsorption, etc.) was the same as already described in our earlier work [15].
Additionally, the competitive reaction (dehydration/dehydrogenation) of cyclohexanol was performed to determine and confirm acid-base nature of the catalyst. The experiment was performed in a down flow reactor in the gas phase at 300 °C at atmospheric pressure.
The formation of carbonaceous deposits on the catalyst surface was studied by infrared Fourier transform spectroscopy (FTIR). The FTIR spectra were recorded at room temperature in 4,000–400 cm−1 spectral range using Bruker IFS-88 spectrometer. The resolution was 2 cm−1. Specimens were prepared by mulling the powder sample with Nujol.
2.3 Catalytic Activity Studies
The alkylation of 4-hydroxypyridine with methanol were performed with 3 cm3 of microwave-prepared ZnAl2O4 catalyst. The catalytic activity in methylation reaction was studied in the gas phase at atmospheric pressure with no addition of a carrier gas. The catalyst’s particles (0.6–1.2 mm) were packed in an electrically heated standard down flow quartz reactor of an inner diameter 8.0 mm with a fixed bed. The space below the catalyst bed was filled with quartz wool. The temperature was controlled with a thermocouple placed in the centre of the catalytic bed. Our earlier studies with 2-hydroxypyridine methylation in the presence of the same catalyst [6] allowed us to choose the proper experimental conditions. The optimum 4-hydroxypyridine, methanol and water molar ratios were found to be 1:10:1, respectively, and flow rate of 3.0 cm3 of liquid per hour (load 1.0 h−1) was selected. The water was added to the reaction mixture to extend the activity of the catalyst [16, 17]. The mixture of substrates was supplied from the top of the reactor using a micro-feed syringe pump. The alkylation reactions were carried out continuously, starting at 250 °C and studied as the function of increasing temperature up to flexure of the 4-hydroxypyridine conversion curve. At chosen temperatures, after the activity reached a steady state, after approximately 1 h, the condensed reaction products, together with unreacted 4-hydroxypyridine, were collected and analysed. Analysis of the product mixture was carried out using a gas chromatograph (HP 6890) equipped with capillary column (30 m × 0.32 mm × 0.25 μm) filled with 5% phenyl methyl silicone and with FID detector. Helium was used as a carrier gas. The percentage of liquid products composition reported was based on methanol-free 4-hydroxypyridine. Mass spectroscopy was also used to identify reaction products by comparison with authentic samples. To study the influence of microwave-treatment on the catalytic activity of zinc aluminate additional experiment of 4-hydroxypyridine methylation in dependence of reaction temperature was performed in the presence of ZnAl2O4 prepared by conventional hydrothermal method. That catalyst was earlier used for 2-hydroxypyridine methylation in the vapour phase. It was prepared also using Al and Zn nitrates as starting materials and its detailed synthesis and properties were described earlier in [6]. Shortly afterwards, a hydrothermal prepared zinc aluminate catalyst (after heat-treatment at 600 °C), with mean crystallite size 3.6 nm, was characterised by S BET = 85 m2/g, V p = 0.15 cm3/g, total acidity evaluated by NH3-TPD measurements equal 0.48 mmol NH3/g and concentration of Lewis acid centres—85 μmol Py/g.
3 Results and Discussion
3.1 Catalyst Characterization
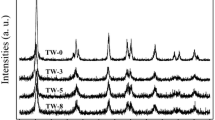
The typical XRD patterns of zinc aluminate catalyst prepared by microwave-assisted hydrothermal method, as-prepared and heated at 600 °C are shown in Fig. 1. As evident from the XRD analysis, the samples were single-phase material and all observed diffraction peaks in recorded XRD patterns could be indexed as ZnAl2O4 with cubic spinel structure (ICDD PDF No. 05-0669); no reflections from alumina or zinc oxide could be detected. Additionally, a chemical analysis using EDS was done indicating that no other impurities were present in the resulting product and confirmed the presence of only aluminium and zinc in the samples with the Al:Zn molar ratio equal 2:1. The considerable broadening of the diffraction peaks for the as-prepared sample indicates high crystallite dispersion. For the heated sample, the diffraction peak widths decreased while the intensity of the diffraction peaks increased due to an increase in the degree of crystallinity and in the crystallite size. The mean crystallite size, calculated from the half-width of the (311) line using Schererr`s formula, were 1.8 and 3.6 nm for the as-prepared and 600 °C heated sample, respectively. The cubic lattice parameter was calculated as a = 0.8070 nm, for the heated sample, what is close to the bulk spinel (a = 0.8085 nm); a reached the value of bulk zinc aluminate after heat treatment at much higher temperature (1,200 °C for 6 h).
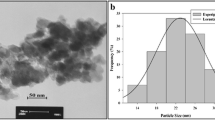
The morphology, size and shape of the zinc aluminate catalyst’s particles synthesized under microwave-assisted hydrothermal conditions were studied by electron microscopy. HRTEM images reveal highly dispersed nanoparticles with the uniform particle size distribution and that is an indication of higher quality of the present catalyst compared with the already reported for ZnAl2O4 catalyst prepared under traditional hydrothermal treatment and used in the methylation of 2-hydroxypiridine [6]. A typical TEM micrograph for the as-prepared sample is shown in Fig. 2. It should be stated that most of the nanoparticles exhibit lattice fringes of distances corresponding to the lattice planes of the spinel structure. Additionally, SAED patterns exhibit rings with d-spacings which could be attributed to the corresponding reflections of the cubic ZnAl2O4 structure while the broadening of the diffraction rings suggests small size or low crystallinity of the as-prepared particles. It is consistent with the results obtained from powder XRD and EDS analysis. The cubic lattice parameter of the spinel, calculated from the electron diffraction pattern (0.808 nm), is comparable to that of bulk ZnAl2O4. The particle size distribution, obtained from TEM images, shown in inset of Fig. 2, is narrow indicating the particle sizes between 1 and 3.5 nm. The mean particle size determined from direct TEM observations (obtained from the Gaussian fit of the histogram) as 2 nm agrees well with the crystallite size calculated from the Scherrer’s fitting of the broadened X-ray diffraction peaks. Electron microscopy studies of samples heated at 600 °C and higher temperatures (not shown) reveal some changes in particle shape and size: formation of aggregates of irregular particles is accompanied by visible increase in mean particle size which is connected with progressive sintering of nanoparticles.
Figure 3 shows representative adsorption–desorption isotherms of nitrogen obtained at the temperature of liquid nitrogen and pore size distribution for the as-prepared and heated ZnAl2O4 samples. According to the IUPAC classification [18], the shapes of the nitrogen adsorption–desorption isotherms are similar for type I and typical for type IV, respectively for the as-prepared and heated sample. Though, in the case of as-prepared sample adsorption that takes place mainly at very low relative pressures, which is a characteristic for microporous materials, some increase in the adsorbed volume of nitrogen can be also observed at the higher P/P 0 due to the capillary condensation, which is characteristic for mesoporous materials. Moreover, the beginnings of the closed hysteresis loop should be noticed which suggests the presence of mesopores in the sample, end the shape of the N2 adsorption–desorption isotherms for the as-prepared sample can be classified as a mixture between types I and II. The sample heated at 600 °C shows N2 adsorption–desorption isotherms really as type IV; the isotherm hysteresis loop is well developed and can be classified into type H2 in the IUPAC classification. This means that this sample is mesoporous with monomodal pore size distribution and a lower contribution of micropores. Generally, this type of hysteresis loops is characteristic for solids consisting of particles crossed by nearly cylindrical channels or made by aggregates (consolidated) or agglomerates (unconsolidated) of (quasi)spheroidal particles.
The pore size distributions, calculated from the corresponding desorption isotherms according to the D–H method, were shown in inset in Fig. 3. It indicates that pore size distributions are monomodal and narrow for both samples suggesting that ZnAl2O4 prepared under microwave-assisted hydrothermal conditions is formed from monodisperse particles. In the case of the as-prepared sample a lot of pores are in the microporous region and pore size distribution has very sharp curve centred at ∼1.5 nm while a little broad pore size distribution was observed for heated sample. It should be noticed that the mean pore size R is only slightly shifted in mesoporous region to ∼3.8 nm.
The as-prepared catalyst is characterized by large specific surface area S BET = 140 m2/g, which may be correlated with small spinel crystallite size. After heat treatment at 600 °C some decrease of S BET (to 105 m2/g) is observed. The total pore volume V p is similar for both samples (∼0.140 cm3/g at P/P 0 = 0.95) but the as-prepared catalyst has a significant contribution of micropore volume (V μ = 0.105 cm3/g.)
The catalyst surface concentration of acid sites was determined by the NH3-TPD measurements as 0.57 mmol NH3/g. It was assumed that one ammonia molecule reacts with one acid centre. Contribution of acid sites of equal strength indicates that investigated catalyst in the main part is characterized by medium acid sites which corresponds to the desorption temperature of ammonia from the range 300–400 °C. The nature of acid sites was studied by IR spectroscopy after the adsorption of the probe molecule on the catalyst surface. The spectra (not shown) recorded for the sample after the sorption of pyridine at room temperature contain two well resolved maxima at about 1,450 and 1,490 cm−1 related to the chemisorbed forms of pyridine. The band at 1,450 cm−1 is assigned to pyridine adsorbed on the Lewis acid sites while the peak at 1,490 cm−1 can be attributed to the adsorbed pyridine species on both Brönsted and Lewis acid centres. The lack of characteristic peak at ∼1,545 cm−1 related to the presence of Brönsted acid centres indicates that only Lewis acid sites are on the surface of investigated catalyst. The concentration of the Lewis acid sites was found to be 96 μmol Py/g. It should be noticed that the intensity of the bands assigned to the presence of Lewis acid centres decreases with an increase in desorption temperature.
The basic properties of mixed metal oxides are also important for determining the catalytic and adsorptive properties of catalytic materials. The distribution and strength of the basic centres on metal oxides can be examined by the temperature-programmed desorption of adsorbed CO2 (CO2 TPD). Typical thermogram, corresponding to CO2 TPD, is shown in Fig. 4. The received complex TPD profiles suggest that several binding sites are available on the surface of ZnAl2O4 catalyst. The profiles were deconvoluted in three overlapping CO2 desorption peaks, reaching maximum desorption rate in the ranges 125–131, 187–199 and 331–345 °C, denoted as α(low-temperature peak), β(middle-temperature peak) and γ(high-temperature peak), respectively. Table 1 shows the relative contribution of each individual desorption peak calculated by the integration of its profile and total amount of CO2 evolved during TPD. Di Cosimo et al. [19, 20] studying mixed metal oxides with spinel structure observed that TPD profiles could be deconvoluted in three peaks with different basic strength. The authors associated their results to those obtained from the IR spectra of adsorbed CO2 and related the α peak (low-strength basic sites) to surface hydroxyl groups and the β (medium-strength basic sites) and γ (high-strength basic sites) peaks to surface basic oxygen atoms.
The gas phase competitive reaction (dehydration/dehydrogenation) of cyclohexanol over ZnAl2O4 was additionally studied as a test for determining the acid–base character of the catalyst. It is commonly known that the highest activities in the dehydrogenation to cyclohexanone are related to basic catalysts, while for dehydration of cyclohexanol to cyclohexene take place in the presence of the acidic ones. The obtained results, nearly 90% selectivity for cyclohexene, indicated that the studied catalyst is mainly an acidic system. It confirmed the IR spectroscopy measurements of adsorbed pyridine.
The formation of carbonaceous deposits on the catalyst surface after the prolongated time of work at higher temperatures was studied using infrared technique. Figure 5 shows the FTIR spectra of the fresh catalyst and after work at 360 °C for 6 h. Significant differences are observed between both spectra, especially in the carbonyl C=O (1,700–1,600 cm−1) and aromatic C=C (1,650–1,500 cm−1) region. Table 2 summarizes the IR bands assignments which evidence the presence of carbonaceous deposits. The results indicate that the source of such deposits may be both the adsorbed and unreacted raw material (4-hydroxypiridine) as well as remains of products (4-methoxypiridine and N-methyl-4-pyridone). For both the samples, bands at 690, 565, 544 and 509 cm−1 originate from vibrations of ZnAl2O4 and residual amount of water manifests themselves by 3,418 cm−1 (stretching) and approximately 1,610 cm−1 (bending). If needed, the catalyst can be regenerated repeatedly without a loss of its activity by passing air through the catalyst bed heated at 500 °C.
3.2 Alkylation of 4-Hydroxypyridine with Methanol
In Table 3, the results of 4-hydroxypyridine 1 methylation over ZnAl2O4 prepared by microwave-assisted hydrothermal method are presented. The influence of the reaction temperature upon the conversion, yields of N-methyl-4-pyridone 2 and 4-methoxypyridine 3 were investigated.
The conversion of 4-hydroxypyridine increases with temperature up to 345 °C but at 360 °C the decreasing in conversion is observed. In the liquid reaction products, except unreacted 4-hydroxypyridine, N-methyl-4-pyridone and 4-methoxypyridine are found. Besides the unreacted 4-hydroxypyridine the reaction products contain other compounds (up to 7.6% at 360 °C); the latter were not identified and their amounts have been presented in the Σothers column. The formation of polymethylated products is not favoured over the ZnAl2O4 catalyst. At temperatures 240–250 °C 4-methoxypyridine is the major product (17.7%). As the temperature increases from 270 to 345 °C the yield of N-methyl-4-pyridone increases up to 77.8% (for conversion level 88.8% at 345 °C). For maximal conversion level the selectivity towards N-methyl-4-pyridone reaches 87.6%.
The results of 4-hydroxypyridine alkylation with methanol in the presence of conventional hydrothermally obtained zinc aluminate are presented in Table 4. That catalyst is actively less visible in the production of N-methyl-4-pyridone than ZnAl2O4 obtained by microwave-assisted hydrothermal method. The selectivity towards N-methylated 4-hydroxypyridine reaches 71.1% at temperature 320 °C. The comparison of selectivities over both catalysts is depicted in Fig. 6. In general, the zinc aluminate catalyst microwave-processed is more selective with respect to N-methylation leading to N-methyl-4-pyridone. The yield of O-methylation product, 4-methoxypyridine, is lower and its yield decreases with an increase in selectivity towards N-methyl-4-pyridone. It is very probable that both zinc aluminate catalysts 4-methoxypyridine undergo rearrangement reaction to N-methyl-4-pyridone with the increasing of temperature. Such rearrangement is very known in liquid phase. On heating 2- or 4-alkoxypyridines they rearrange to the corresponding N-alkylpyridones. The rearrangement is catalysed by alkylating reagents. The mechanism of this rearrangement is not clear [21]. Better results obtained over ZnAl2O4 catalyst prepared by microwave treatment may be caused by the fact that this catalyst is characterised by higher surface area and a higher acidity. Also others factors like better dispersion or narrow distribution of small possible size due to preparation under microwave heating may be responsible for the better catalyst activity. Over that catalyst the amount of by-products is also lower and does not exceed 7.6% at 360 °C.
The effect of time-on-stream on conversion of 4-hydroxypyridine and selectivity to N-methyl-4-pyridone was also studied. At selected temperatures, a state of dynamic equilibrium had been reached after about 30 min, and latter, as time passed, the composition of products stabilized. The reaction was performed at 285 °C (for ca. 50% conversion level) for 5 h and products were collected and analysed at every 0.5-h interval. The results are presented in Fig. 7. Almost constant 4-hydroxypyridine conversion ca. 50% was observed from 1 up to 4 h. At a longer time interval, the conversion decreased due to coke formation to ca. 47.5% but selectivity remained almost unchanged. If needed, a catalyst can be submitted to oxidative regeneration in air up to 480 °C. The activity of the regenerated catalyst in 4-hydroxypyridine alkylation by methanol is the same as in the case of a fresh one.
It is evident from our previous research, that in the case of 2-hydroxypyridine, the same reaction of alkylation over the ZnAl2O4 catalyst with methanol, is more efficient than in the case of 4-hydroxypyridine. This difference may be intrinsic property of the both substrates. In liquid phase 2-hydroxypyridine is more reactive towards alkylating reagents then 4-hydroxypyridine, although the difference is not outstanding. In competing experiments when the 1:1 mixture of potassium salts of 2- and 4-hydroxypyridine is methylated with methyl sulphate in ethanol, after the 50% conversion the yields of N-methyl-2- and N-methyl-4-pyridones are in 1.3:1.0 ratio. The second reason for higher reactivity of 2-hydroxypyridine can result from a much stronger bonding of 4-hydroxypyridine to the acidic (silica gel) as well to the basic (basic Al2O3) surfaces than 2-hydroxypyridine. The same is true for the pair N-methyl-4- and N-methyl-2-pyridone. When hydroxypyridines are bonded to the acidic surface of the catalyst their electrons are shifted out of their molecules toward the acidic centres and they lose their nucleophilic reactivity. As 4-hydroxypyridine is bonded more strongly to the surface of the catalyst it became less nucleophilic comparing with 2-hydroxypyridine. The crucial process in the described reaction is activation of methanol molecules over the surface of the ZnAl2O4 catalyst. Free molecules of methanol do not indicate the electrophilic reactivity. They are changed to the reactive electrophile when bonded to the acidic centres of the catalyst. Our mechanistic proposition the mode of the activation of methanol was depicted in the previous work [6].
4 Conclusions
The presented studies confirmed our supposition that ZnAl2O4 catalyst would be active and selective in N-methylation of hydroxypyridines. Alkylation of 4-hydroxypyridine with methanol in the presence of the zinc aluminate catalyst carried out at atmospheric pressure is an efficient method for obtaining N-methyl-4-pyridone with high yield and satisfactory selectivity, especially when the ZnAl2O4 catalyst is prepared by microwave-assisted hydrothermal method. It was found that proposed method of catalyst’s synthesis leads to material having attractive catalytic properties. Proposed method for obtaining N-methyl-4-pyridone is cheap, easy and environmentally friendly and can be interesting from technological point of view.
References
Electronic Medicines Compendium http://emc.medicines.org.uk/emc/assets/c/html/DisplayDoc.asp?DocumentID=6380
Stetinova V, Grossmann V (2000) Exp Toxicol Pathol 52:473
Dyer DJ, Lee VY, Twieg RJ (1997) Liquid Cryst 23:551
You F, Twieg RJ (1999) Tetrahedron Lett 40:8759
Gibson D, Clarke D, Winscom Ch (2000) US Pat 6,841,344
Grabowska H, Zawadzki M, Syper L (2006) Appl Catal A: Gen 314:226
Komarneni S, Katsuki H (2002) Pure Appl Chem 74:1537
Rao KJ, Mahesh K, Kumar S (2005) Bull Mater Sci 28:19
Malinger KA, Laubernds K, Son YC, Suib SL (2004) Chem Mater 16:4296
Ren ZW, Zhou DB, Tu SQ (2007) Chin J Catal 8:217
International Centre for Diffraction Date, ICDD, PDF-4 + 2005
Dollimore D, Heal GR (1964) J Appl Chem 14:109
Wrzyszcz J, Zawadzki M, Trawczyński J, Grabowska H, Miśta W (2001) Appl Catal A: Gen 210:263
Grabowska H, Miśta W, Trawczyński J, Wrzyszcz J, Zawadzki M (2001) Appl Catal A: Gen 220:207
Grabowska H, Zawadzki M, Syper L, Miśta W (2005) Appl Catal A: Gen 292:208
Sparks AK (1972) US Patent 3,670,030
Leach BE (1980) US Patent 4,227,024
IUPAC Recommendations (1994) Pure Appl Chem 66:1739
Di Cosimo JI, Diez VK, Xu M, Iglesia E, Apesteguia CR (1998) J Catal 178:499
Di Cosimo JI, Apesteguia CR, Gines MJL, Iglesia E (2000) J Catal 190:261
Beak P, Bonham J, Lee TJ Jr (1968) J Am Chem Soc 90:1569
Acknowledgments
The authors are very grateful to Mrs Ludwina Krajczyk for HRTEM studies and to Prof. Janusz Trawczyński for his help in acidity measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grabowska, H., Zawadzki, M. & Syper, L. Catalytic Method for N-Methyl-4-pyridone Synthesis in the Presence of ZnAl2O4 . Catal Lett 121, 103–110 (2008). https://doi.org/10.1007/s10562-007-9305-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-007-9305-4