Conventional (per)sulfated bulk zirconia, mesoporous sulfated zirconia and (per)sulfated zirconia supported on an ordered mesoporous silica, with or without aluminium incorporation, were examined as acid catalysts for the dehydrocyclisation of xylose into furfural. Furfural yields of up to 50% could be achieved at >90% conversion with the modified zirconia catalysts, which is better than that achievable with H2SO4 (using approximately the same equivalent amount of sulfur.)
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomass, or plant matter, is a renewable energy source that is one of the most important alternatives to crude oil [1–3]. It is made up of four major components: cellulose, hemicellulose, lignin and starch. Bio sugars obtained from these components by hydrolysis are the raw materials to make chemicals, consumer and industrial products, and energy from biomass. The two primary sugars are glucose, a 6-carbon sugar made from the cellulose portion of biomass, and xylose, a 5-carbon sugar made from the hemicellulose portion. Glucose may be processed into a wide variety of products including ethanol, sorbitol, mannitol, gluconic acid and 5-hydroxymethylfurfural. Xylose derivatives include ethanol, xylitol, furfural, furfuryl alcohol and furan. Furfural is of high commercial interest because it can be used for the production of a wide range of important non-petroleum-derived chemicals [4–6]. It is produced by the acid-catalysed dehydration of xylose. Presently, several industrial processes for furfural production use H2SO4 as the catalyst under homogeneous conditions. Due to the hazardous properties of liquid acids such as H2SO4 and the well known drawbacks of homogeneous catalytic processes, there has been a great effort to replace toxic liquid acid catalysts by more environmentally friendly solid acid catalysts [7–9].
The most widely studied solid acids based on inorganic oxides are zeolites and zeotypes, heteropolyacids, metal phosphates and sulfated metal oxides [7–10]. Some of these materials have been studied as catalysts for the transformation of carbohydrates. Moreau and co-workers found that microporous zeolites such as mordenites and faujasites catalyse the conversion of saccharides into furan derivatives with high selectivity (but only for conversions up to about 30%) [11]. Heterogeneous catalysts based on cubic zirconium pyrophosphates, γ-titanium phosphate and vanadyl phosphate exhibit good performances, in terms of selectivity and activity, for the dehydration of fructose to 5-hydroxymethylfurfural [12, 13]. Recently, we studied a series of composites comprising 12-tungstophosphoric acid, H3PW12O40 (PW), or cesium salts of PW, immobilized in micelle-templated mesoporous silicas, for the cyclodehydration of xylose in either toluene/water or dimethyl sulfoxide [14, 15]. The best systems exhibited furfural yields comparable with those obtained with H2SO4 under similar reaction conditions. Even better results, in terms of catalyst stability and recyclability, were obtained using microporous and mesoporous niobium silicates [16], and exfoliated titanate, niobate and titanoniobate nanosheets [17].
There is only one report concerning the use of sulfated metal oxides as catalysts for the conversion of xylose into furfural [18]. Furfural yields of about 60% at ca. 90% conversion were achieved by using conventional sulfated zirconia (SZ) or titania as solid acids and supercritical carbon dioxide as an extracting solvent, at 180 °C and 200 atm. The great interest in SZ as a solid acid catalyst arose due to its high catalytic activity and selectivity for the isomerisation of n-butane at low temperature [7, 10]. Various promoters have been found to increase the stability and/or activity of SZ, including Al, Mn, Fe, Ni and Pt [19–22]. Such promotional effects probably come from the synergism between stabilisation of the active tetragonal phase of zirconia, higher surface areas, higher sulfate contents, and modification of the surface acidity (e.g., an increase in the number of effective acid sites). Further improvements in catalytic performance are possible by modifying zirconia with persulfate rather than sulfate [23, 24].
Conventional SZ has a specific surface area usually in the range 80–100 m2 g−1 and a lack of ordered mesoporosity and textural homogeneity, making it suitable for traditional vapour-phase reactions involving small molecules but less amenable to liquid phase reactions. However, mesoporous versions can be made using either triblock copolymers or surfactants as structure-directing agents [25–31]. Wang and Mou synthesized a mesoporous SZ using Zr(O−nPr)4 as zirconium precursor, ammonium sulfate as sulfur source and hexadecyl trimethyl ammonium bromide as template [30]. With the addition of a proper amount of Al as promoter, the catalytic behaviour for n-butane isomerisation at low temperature was strongly promoted. Another method to improve the textural properties and acid site distribution of sulfated zirconias is to support SZ on an ordered mesoporous silica [25, 32–39]. The loading of persulfated alumina and zirconia into hexagonal mesoporous silica (HMS) gave a robust and reusable catalyst (even in the presence of HCl and H2O) for the dehydration of 2-propanol and alkylation reactions [37, 38].
In this paper, we report on the catalytic performance of several modified zirconia materials for the dehydrocyclisation of xylose into furfural, using a biphasic liquid system composed of water and toluene, at 160 °C. The materials examined were conventional (per)sulfated bulk zirconia, mesostructured sulfated bulk zirconia and (per)sulfated zirconia supported on a mesoporous silica. In addition to studying the effect of the sulfating agent on the catalytic performance for the first and third groups, the effect of Al incorporation was studied for all three types of modified zirconia solid acids.
Experimental
Catalyst preparation
All starting materials were purchased from commercial sources and used as received.
Synthesis of bulk zirconias
Aqueous ammonia was added dropwise to a solution of ZrOCl2 · 8H2O (1.5 g, 4.7 mmol) in water (17 mL) for the zirconia (denoted Z), and to a mixed solution of ZrOCl2 · 8H2O (3.0 g, 9.3 mmol) and Al(NO3)3 · 9H2O (0.069 g, 0.18 mmol) in water (15 mL) for the alumina modified zirconia (as synthesized AZ), until the pH was in the range of 9–10 [23]. After stirring overnight the solids were filtered and washed with water until a neutral filtrate and absence of chlorine ion was detected by AgNO3 tests. The solids were oven-dried at 110 °C for 24 h. For sulfation (S-treatment) and persulfation (PS-treatment), the solids Z and AZ were immersed in 0.5 M (NH4)2SO4 and (NH4)2S2O8, respectively, for 30 mins and then filtered, dried at 110 °C for 24 h and calcined at 650 °C in static air for 3 h resulting in SZ and PSZ, respectively, from Z, and SAZ and PSAZ, respectively, from AZ. The as-synthesized Z was calcined at 550 °C in static air for 3 h to get pure ZrO2.
Synthesis of mesoporous sulfated zirconias
The mesoporous sulfated zirconia (MSZ) was prepared by following the procedure originally described by Ciesla et al. [29] and later modified by Wang and Mou [30]. Hexadecyl trimethyl ammonium bromide (C16TMABr, 2.50 g, 6.86 mmol) was dissolved in deionized water (111 mL) and 37 wt.% HCl (18.8 mL). A 70 wt.% solution of Zr(O-nPr)4 in 1-propanol (5.7 mL, 12.7 mmol) was then added slowly with stirring, resulting in the immediate precipitation of a hydrolysis product. After the hydrolysis product had dissolved (ca. 30 min), a solution of (NH4)2SO4 (1.69 g, 12.8 mmol) in water (22 mL) was added and the mixture was stirred for 1 h at room temperature. The mixture was then transferred to a Teflon-lined autoclave and heated at 100 °C for 3 days. Calcination of the resultant solid product at 650 °C in static air for 5 h gave the sample designated as MSZ.
An alumina modified sample (MSAZ) was prepared by incipient wetness impregnation on uncalcined MSZ (1.0 g) with a solution of Al2(SO4)3 · 18H2O (0.20 g, 0.3 mmol) in deionized water (10 mL). After complete addition, the solid was dried at 120 °C for 1 h and then at 100 °C for 24 h. Calcination was carried out as described above for MSZ.
Synthesis of supported zirconia and supported alumina modified zirconia
A micelle-templated mesostructured silica was synthesized from a gel with the molar composition SiO2:0.29Na2O:0.50C16TMABr:150H2O, using sodium silicate solution (8% Na2O, 27% SiO2) as the silica source [40]. The gel was transferred to a 1 L PTFE bottle and heated under static conditions at 80 °C for 136 h. Then, the solid was filtered, washed with hot water, dried at 50 °C and calcined at 560 °C in static air for 6 h. Powder XRD (2θ/°, hkl in parentheses): 2.47 (100), 4.21 (110), 4.83 (200), 6.41 (210); a = 2d 100√ 3 = 41.34 Å.
ZrOCl2 · 8H2O (1.0 g, 3.1 mmol) (originating as-synthesized Z-MCM-41), or ZrOCl2 · 8H2O (0.96 g, 3.0 mmol) and Al(NO3)3 · 9H2O (0.04 g, 0.1 mmol) (originating as-synthesized AZ-MCM-41), were dissolved in water (10 mL) and added to the calcined mesoporous silica (2.0 g) by the incipient wetness technique [37]. After complete addition, the solids were dried at 120 °C for 1 h. The dried materials were hydrolysed by a flux of ammonia gas for 3 h and then washed with water to remove the chloride ions and dried for 2 h at 120 °C.
The sulfation and persulfation of the as-synthesized Z-MCM-41 and AZ-MCM-41 were carried out by adding 0.5 M (NH4)2SO4 or (NH4)2S2O8 (6 mL), respectively, to the solid material (1 g) by the incipient wetness technique. After complete addition, the solids were dried at either 120 °C for 1 h (for SZ-MCM-41 and PSZ-MCM-41) or 110 °C for 24 h (for SAZ-MCM-41 and PSAZ-MCM-41). The final materials were obtained by calcinations in static air at either 550 °C for 3 h (for SZ-MCM-41 and PSZ-MCM-41) or 650 °C for 3 h (for SAZ-MCM-41 and PSAZ-MCM-41).
Catalyst characterisation
ICP-AES measurements were carried out at the Central Laboratory for Analysis, University of Aveiro (by E. Soares). Microanalysis for S was carried out at the University of Aveiro (M.F. Lucas). Nitrogen adsorption measurements at −196 °C were carried out gravimetrically with a CI electronic MK2-M5 microbalance and an Edwards Barocel pressure sensor. Prior to measurement, the solids were out-gassed at 200 °C overnight to give a residual pressure of ca. 10−4 mbar. Powder X-ray diffraction (XRD) data were collected at room temperature on a Philips X’pert diffractometer with a curved graphite monochromator (Cu-Kα radiation), in a Bragg-Brentano para-focusing optics configuration. 29Si solid-state NMR spectra were recorded at 79.49 MHz on a 9.4 T Bruker Avance 400 spectrometer. 29Si MAS spectra were recorded with 40° pulses, a spinning rate of 5.0 kHz and 60 s recycle delays. 29Si CP MAS spectra were recorded with 5.5 μs 1H 90° pulses, a contact time of 8 ms, a spinning rate of 5.0 kHz and 4 s recycle delays. Chemical shifts are quoted in parts per million from tetramethylsilane. 27Al solid state NMR spectra were measured at either 104.26 MHz (for PSAZ) or 130.32 MHz (for MSAZ and PSAZ-MCM-41) with Bruker Avance 400 (9.4 T) and 500 (11.7 T) spectrometers, respectively. The spectra were acquired using a contact time of 0.6 μs, a recycle delay of 1 s, and spinning rates between 9 and 14 kHz. Chemical shifts are quoted in ppm from Al(H2O) 3+6 .
Catalytic reactions
Batch catalytic experiments were performed under nitrogen in a tubular glass micro-reactor equipped with a valve for gas purging. In a typical procedure, d-xylose (30 mg), powdered catalyst (20 mg), H2O (0.3 mL) and toluene (0.7 mL) were poured into the reactor. The reaction mixtures were stirred magnetically at 500 rpm and heated with a thermostatically controlled oil bath. It was found that the influence of the stirring rate on the initial reaction rate becomes negligible over 500 rpm. Zero time was taken to be the instant the micro-reactor was immersed in the oil bath. For the recycling tests, the solids were separated from the reaction solution after 4 h by centrifugation, washed with methanol, and heated to 350 °C (2° min−1) prior to re-use.
The samples were analysed by HPLC in isocratic mode. The products present in the aqueous phase were analyzed using a Knauer K-1001 HPLC pump and a PL Hi-Plex H 300 × 7.7 mm (i.d.) ion exchange column (Polymer Laboratories Ltd., UK), coupled to a Knauer 2300 differential refractive index detector (for xylose) and a Knauer 2600 UV detector (280 nm, for furfural). The mobile phase was 0.01 M H2SO4. Analysis conditions: flow rate 0.6 mL min−1, column temperature 65 °C. The furfural present in the organic phase was quantified using a Gilson 306 HPLC pump and a Spherisorb ODS S10 C18 column, coupled to a Gilson 118 UV/Vis detector (280 nm). The mobile phase consisted of 30% v/v methanol in an aqueous solution with 10% methanol (flow rate 0.7 mL min−1). Authentic samples of d-xylose and furfural were used as standards and calibration curves were used for quantification.
Results and discussion
Characterisation
Three main types of zirconia-containing materials were prepared by following published procedures or modifications thereof: (i) Conventional sulfated and persulfated bulk zirconia (SZ and PSZ, respectively); (ii) MSZ; (iii) sulfated and persulfated zirconia supported on an ordered mesoporous silica (SZ-MCM-41 and PSZ-MCM-41, respectively). For all types the effect of alumina doping is discussed. The incorporation of alumina (denoted A in the sample abbreviations) for types (i) and (iii) was carried out via the co-precipitation method, while for the mesostructured sulfated zirconia the impregnation method was used. For comparison, pure zirconia, ZrO2, was prepared in a similar way to that used for the type (i) samples.
The powder XRD patterns in the 2θ range of 20–65° for the type (i) and type (ii) zirconia catalysts showed that pure zirconia is formed in the monoclinic phase but after sulfation or persulfation the tetragonal phase is the major form present, with a small amount of the monoclinic phase (figure 1). For all samples, when Al is incorporated, the monoclinic phase tends to disappear due to stabilisation of the tetragonal phase, in agreement with literature results [20, 24, 30]. The powder XRD pattern of the calcined micelle-templated silica used to prepare the group (iii) materials was characteristic of mesoporous MCM-41 and exhibited four reflections in the 2θ range of 2–8°, indexed for a hexagonal cell (see section 2.1.3). The d value of the (100) reflection was 35.80 Å, giving a lattice constant of a = 41.34 Å. After supporting zirconia in the mesoporous silica, the intensities of these reflections decreased significantly (not shown). Although this could be due to a disruption of the hexagonal long-range order, it is equally likely that there is a contrast matching effect between the silica walls and the pore-filling material [37]. At angles above 10° 2θ there was no evidence for the formation of a crystalline zirconia phase. For all Al-promoted catalysts, the characteristic peaks of Al2O3 were not observed, suggesting that the alumina was homogeneously mixed with zirconia.
Table 1 presents the BET surface areas (SBET) and elemental analysis data for all catalysts. The SBET of the group (i) bulk zirconias are in the range of 85–91 m2 g−1, in agreement with reported values for similar materials [10, 20, 23, 24, 41]. The group (ii) zirconias exhibited type IV N2 adsorption-desorption isotherms according to the IUPAC classification (not shown), characteristic of mesoporous solids (pore width between 2 and 50 nm) [14]. SBET for the Al-promoted sample (MSAZ) was nearly double that measured for the MSZ sample. For both materials, the step corresponding to capillary condensation appeared in the relative pressure range of 0.4–0.6, showing a relatively uniform mesopore size distribution (PSD), which was narrower (as determined by the BJH method involving analysis of the adsorption branch) for MSAZ. The median pore widths were 4.2 nm for MSAZ and 5.0 nm for MSZ. The higher SBET and narrower PSD for MSAZ are consistent with previous findings which indicated that the addition of alumina to mesoporous sulfated zirconias can stabilize the mesostructure of the catalysts [30].
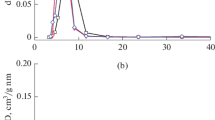
Pristine calcined MCM-41 also exhibited a type IV isotherm, with a capillary condensation step between p/p0 values of 0.21 and 0.35. The SBET of MCM-41 was 899 m2 g−1 and the total pore volume was 0.74 cm3 g−1. The modified MCM-41 materials possessed lower specific surface area (359–426 m2 g−1) and total pore volume (0.19–0.23 cm3 g−1). Despite the reductions in SBET, the values are far greater than those of bulk (unsupported) zirconia. The PSD remained rather narrow after the post synthesis modifications and the maximum decreased from 3.5 nm for pristine MCM-41 to 2.4–3.1 nm for the composite materials, in agreement with literature data (figure 2) [32]. Taken together with the XRD results, these findings indicate that the zirconia was well dispersed on the surface of the mesoporous silica support [33]. From the elemental analysis results we can calculate that the zirconia content was about 0.03 g/100 m2 for all four supported MCMs, which is likely to be well below the dispersion threshold of ZrO2 on MCM-41 [34–36]. For both the sulfated and persulfated materials, the maxima of the PSD plots for the Al-promoted samples are shifted by about 0.4 nm to higher values relative to the maxima for the corresponding Al-free samples. This may be related with the fact that the sulfur contents for the Al-promoted samples are lower than those for the Al-free samples (Table 1).
In contrast to the results obtained for the group (iii) materials comprising sulfated and persulfated zirconia supported on MCM-41, the incorporation of alumina in the group (i) bulk zirconias results in higher sulfur contents (for PSAZ) and slightly higher specific surface areas (Table 1), in agreement with published findings [20, 24]. The highest observed sulfur content of 0.45 mmol g−1 for PSAZ corresponds to a sulfur concentration of 3.0 atoms nm−2 (assuming that all sulfate groups are on the surface). This is slightly lower than the maximum concentration of 3.2 atoms nm−2 for monolayer coverage, calculated assuming that each group containing an atom of sulfur occupies 0.31 nm2 (based on the kinetic diameter of a sulfate group [42]). Incorporation of alumina in the group (ii) MSZ also resulted in a significantly higher final sulfur content (and higher specific surface area, as mentioned above), giving a sulfur concentration of 2.8 atoms nm−2, which is very similar to that calculated for PSAZ. The corresponding sulfur concentration for MSZ is 1.6 atoms nm−2.
The catalysts were also characterized by 27Al and 29Si MAS NMR spectroscopy. Representative 27Al MAS NMR spectra are shown in figure 3. The group (i) Al-promoted sulfated and persulfated zirconias gave spectra showing one sharp, strong peak at about 0 ppm, typical of octahedrally coordinated aluminium, and a much weaker, very broad signal centred around 30 ppm, which may be tentatively assigned to Al in 5-fold coordination [43]. A similar spectrum was obtained for the Al-promoted mesoporous sulfated zirconia (MSAZ), except that the broad 5-fold Al line peaking around 33 ppm was relatively more intense (figure 3). The spectra for the group (iii) materials SAZ-MCM-41 and PSAZ-MCM-41 showed only a sharp 6-fold Al line near 0 ppm, in agreement with literature results [33, 34]. No peak at about 50 ppm, corresponding to tetrahedrally coordinated Al, could be seen for either sample, indicating that Al was unlikely to be incorporated in the framework of the support. The 29Si MAS NMR spectrum of the pristine calcined MCM-41 support was described previously [14]. Two broad overlapping peaks at –101.4 and –109.4 ppm were assigned to Q3 and Q4 species of the silica framework, respectively, and a faint peak at –92 ppm was assigned to a small amount of Q2 environments [Qn = Si(OSi)n(OH)4-n].The 29Si CP MAS NMR spectrum showed a marked increase in the intensities of the Q2 and Q3 signals over that for the Q4 peak, confirming that the Q2 and Q3 silicons are attached to hydroxyl groups. After modification of this material to produce the group (iii) catalysts, the 29Si MAS NMR spectra were essentially unchanged, except that the intensities of the Q2 and Q3 signals were reduced (relative to that for the Q4 signal). This indicates that some surface silanol groups were consumed during the modification process, as has been reported previously [44].
Catalysis
The cyclodehydration of xylose involves a series of elementary steps in which the hydrogen ions transform hydroxyl groups of the pentose into H2O+ groups (the prerequisite for the liberation of water), resulting in the liberation of three water molecules per xylose molecule converted into furfural [4, 11]. A plausible mechanism for the xylose-to-furfural reaction consists of two 1,2-eliminations and one 1,4-elimination of water [4]. In general, loss of furfural is mainly due to condensation reactions that take place between furfural and intermediates of the xylose-to-furfural conversion, yielding products of higher molecular weight such as furfural pentose and difurfural xylose [1] (Scheme 1).
The cyclodehydration of xylose in the presence of the zirconia-based solid acids described above was investigated using a water-toluene solvent mixture, at 160 °C. The applied reaction conditions were optimized to minimize the non-catalytic, poorly selective, contribution over the catalytic one. Under these conditions, the non-catalytic reaction at 30 min is negligible, and after 4 h gave 12% conversion and <1% furfural yield. Throughout the discussion the results have not been corrected for the non-catalytic contribution. The biphasic solvent mixture was used to allow the simultaneous reaction of the polar reactant xylose (and intermediates) in the aqueous phase and the continuous extraction of furfural into the organic phase (where it preferentially dissolves). It has been previously reported for the dehydration of xylose and fructose into furfural and 5-hydroxymethylfurfural, respectively, that under aqueous-organic biphasic conditions higher yields of the desired products are obtained than if only water is used as solvent [40, 45].
The catalytic performance of pure (unmodified) zirconia is rather sluggish under the applied reaction conditions, yielding 9% furfural at 48% conversion, after 4 h. All of the modified zirconias exhibited superior catalytic activity and selectivity for furfural production, under similar reaction conditions (50–96% xylose conversion and 22–46% furfural yield, at 4 h). These results indicate that although both monoclinic and tetragonal phases are catalytically active for the conversion of xylose, the (per)sulfation treatments have a beneficial effect on the selectivity to furfural. According to the literature, the thermodynamically favoured monoclinic phase of ZrO2 is more basic than the metastable tetragonal one and the presence of sulfate groups on the zirconia surface enhances the fraction of the tetragonal phase, inducing surface acidity and diminishing the portion of basic sites [46, 47]. In the xylose conversion, the basic character of the surface of pure (monoclinic) ZrO2 is likely to favour undesirable aldol condensation reactions or cleavage of the carbon chain of xylose, whereas the acidity of modified (essentially tetragonal) zirconia favours the cyclodehydration pathway to furfural. When H2SO4 was used as the catalyst (with an intermediate amount of S equivalent to that present in the solid catalysts), the reaction was slower than that observed for the modified zirconia catalysts, yielding 25% furfural after 4 h reaction.
The kinetic profiles of xylose conversion in the presence of the modified zirconia catalysts are shown in figure 4. For the conventional sulfated and persulfated bulk zirconias in group (i), the initial activities (calculated at 30 min) vary between 7.4 and 8.3 mmol g −1cat h−1 (Table 2), and xylose conversion increases with time, reaching values in the range of 88–95% within 6 h reaction [figure 3(a)]. In contrast to results for n-butane isomerization [23, 24], zirconia modified by persulfate is not more active than zirconia modified by sulfate. The characterisation results (sulfur content, SBET and crystalline phases) do not show major differences for the group (i) materials, which may explain why the catalytic performances are similar. For the group (ii) MSZs, the initial catalytic activity of MSAZ is 1.7 times higher than that for MSZ, and compared with the group (i) catalysts xylose conversion is faster in the presence of MSAZ and slower in the presence of MSZ. The SBET values and sulfur contents for all of these modified bulk zirconia catalysts are congruent with this trend, decreasing in the order MSAZ > group (i) materials > MSZ (Table 1).
Xylose conversion versus time (a,c) and furfural selectivity versus conversion (b,d) in the presence of bulk [(a,b): SZ (*), PSZ (+), SAZ (□), PSAZ (Δ), MSZ (\({\diamondsuit}\)), MSAZ (–)] and supported [(c,d): SZ-MCM-41 (*), PSZ-MCM-41 (+), SAZ-MCM-41 (□), PSAZ-MCM-41 (Δ)] modified zirconia catalysts, at 160 °C.
The group (iii) materials comprising (per)sulfated zirconia supported on a mesoporous silica exhibited different catalytic performances compared with those observed for the bulk zirconia catalysts (figure 3(c), Table 2). The reaction rate (initial activity, mmol g −1cat h−1) increased in the order: SAZ-MCM-41 (5.3) < PSAZ-MCM-41 (7.5) < SZ-MCM-41 (8.9) < PSZ-MCM-41 (14.1). Hence, for these materials, using persulfate rather than sulfate as the sulfur source has a beneficial effect on catalytic activity. In contrast to the results for the group (ii) MSZs, the MCM composites promoted with Al are less active than the corresponding samples without Al. Another difference between these samples and the modified bulk zirconias in groups (i) and (ii) is that the Al-promoted MCM composites possess lower SBET and sulfur content than the corresponding samples without Al (Zr content is roughly the same for all four materials). For the group (i) and group (ii) materials, initial activity increases with SBET, whereas for the group (iii) materials the surface area does not seem to be very important, at least at our level of experimental variance.
A key observation for both the group (i) and group (ii) bulk zirconias, and the group (iii) supported materials, is that the initial catalytic activities (expressed per gram of catalyst) tend to increase with the sulfur content (figure 5). For groups (i) and (iii), TOF expressed per mole of sulfur is roughly constant at 18–22 and 10–13 mol −1 s h−1, respectively. Pârvulesco et al. proposed that the sulfur content of SZ catalysts is one of the main factors controlling the catalytic properties for the conversion of hydrocarbons (with higher contents leading to higher activities) [48]. The dehydration of carbohydrates can take place over Brönsted and Lewis acid sites [49]. The coexistence of Lewis and Brönsted acid sites and their relative amounts have been related to the sulfate content and to their topological distribution [46, 50]. The strength of Lewis acid sites (uncoordinated sites on the ZrO2 surface) is said to be enhanced by the electron-withdrawing (inductive) effect of the neighbouring sulfate groups [41, 51, 52]. Under the xylose reaction conditions, water adsorbed on the catalyst may convert strong Lewis acid sites into Brönsted sites [10, 53]. According to the literature, the acid strength of bulk zirconias differs from that of supported catalysts [25, 36], which may partly explain the differences in TOF (expressed per mole of S) for these materials.
Furfural selectivity for the three groups of materials tends to increase initially with xylose conversion, reaching values in the range of 37–58% at ca. 85% conversion [figure 4(b,d)]. The initial increase in selectivity with conversion has been reported previously for the xylose-to-furfural catalytic conversion in homogeneous and heterogeneous phase, and is most likely due to mechanistic reasons (because, as mentioned above, the reaction mechanism is complex, involving a series of elementary steps [17, 54]). The effect of Al doping on furfural selectivity is opposite to that observed for catalytic activity. At conversions >85%, Al doping has a beneficial effect on selectivity for the group (iii) supported catalysts, and a negative effect for the group (ii) MSZs. Whereas the MCM-41-supported SAZ and PSAZ catalysts gave a selectivity of about 51%, the corresponding supported SZ and PSZ catalysts were somewhat less selective (43–45%). On the other hand, the mesostructured bulk zirconia MSZ gave the highest selectivity (58%) and MSAZ the lowest (<36%). The conventional group (i) bulk zirconias gave intermediate values of selectivity (43–49%).
The catalyst stability was studied for all materials by recycling the solids twice (see section 2.3 for details). With the exception of SAZ-MCM-41 and PSAZ-MCM-41, xylose conversions after 4 h decreased between the first and third runs by factors ranging between 0.06 and 0.2. In the presence of SAZ-MCM-41, xylose conversion at 4 h actually increased with recycling runs, while with PSAZ-MCM-41 it remained constant (figure 6). Quite high stability was reported for HMS-supported PSAZ catalysts for reactions where HCl and water were co-products [38, 55]. No leaching of either Zr or Al was detected for the two MCM samples by ICP-AES. On the other hand, elemental analysis of the recovered solids showed that the sulfur content decreased by a factor of 5% for SAZ-MCM-41 and 28% for PSAZ-MCM-41. Apart from these two materials, the next most stable catalyst (in terms of conversions measured at 4 h) was MSAZ, for which xylose conversion decreased from 95% in the first run to 90% in the third run (figure 6). The sulfur content for this sample decreased 20%. For comparison, xylose conversion in the presence of MSZ decreased from 60% in the first run to 50% in the third run, and the sulfur content decreased 39%. It has been reported for common bulk SZ that a water washing treatment removes water-soluble, labile sulfate groups (which are recoverable by SO3 sulfation) and that the remaining sulfate groups are possibly bound in a form not permitting hydroxylation [46, 47].
In order to investigate the homo/heterogeneous nature of the reaction, an experiment was performed for the SZ-MCM-41 sample without adding xylose, at 160 °C for 4 h, after which the mixture was cooled to room temperature and the solid was separated from the water-toluene solvents by centrifugation. Subsequently, xylose was added to this solvent mixture and left to react for 4 h at 160 °C. The S content of SZ-MCM-41 decreased 43% and xylose conversion (32%) was approximately a third of that observed in the normal catalytic run with SZ-MCM-41 (94%, Table 2). Considering that, as mentioned above, 12% conversion is obtained when no catalyst is added, it seems that the leached species do not play a major role in the xylose conversion, under the applied reaction conditions.
Figure 6 also shows the furfural yields obtained after 4 h for the fresh and reused MSZ, MSAZ, SAZ-MCM-41 and PSAZ-MCM-41 catalysts. The furfural selectivity at 4 h for MSAZ and SAZ-MCM-41 was the same for all three catalytic runs (40 and 45%, respectively), and so the trends observed for the furfural yields mirror those observed with xylose conversion. The selectivities at 4 h for the other two catalysts MSZ and PSAZ-MCM-41 [and also the group (i) materials, SZ-MCM-41 and PSZ-MCM-41] decreased mainly from the first to the second run, with the result that the furfural yields decreased from 27% to a roughly constant 20% for MSZ, and from 41% to a roughly constant 34% for PSAZ-MCM-41. The good catalytic stabilities of the materials MSAZ, SAZ-MCM-41 and PSAZ-MCM-41, in terms of furfural yield at 4 h for the recycled catalysts, are probably due to a synergism between various factors, such as favourable textural properties (large surface area and pore volume) and resistance to sulfur leaching. Previous reports have indicated that the incorporation of Al into sulfated zirconia catalysts improves catalyst stability for n-butane isomerisation [24, 25].
Conclusions
Several bulk and ordered mesoporous silica-supported zirconia catalysts were tested as catalysts for the cyclodehydration of xylose into furfural, in a water-toluene solvent mixture at 160 °C. All of the modified zirconias exhibited superior catalytic activity and selectivity for furfural production than pure ZrO2, under similar reaction conditions. Furfural yields of up to 50% could be achieved at more than 90% conversion with the mesostructured bulk and silica-supported zirconia catalysts. The catalytic results are better than those obtained with H2SO4 as catalyst, under similar reaction conditions. Initial catalytic activity correlates fairly well with the sulfur content and was highest for PSZ-MCM-41 (14.1 mmol g −1cat h−1), which had a sulfur content of 1.2 mmol g−1. As observed for all the other composites, no leaching of Zr from PSZ-MCM-41 took place during the reaction, but sulfur leaching was the highest at about 50%, which compromises the reusability of the catalyst. In this sense, of all the materials prepared here, SAZ-MCM-41 seems to be the most attractive catalyst for aqueous phase conversion of xylose, since it was the most stable to sulfur leaching and exhibited increasing activity and no significant loss of selectivity to furfural in three recycling runs. For a better understanding of the structure-activity relationships a more detailed characterisation of the surface properties is needed, such as monitoring the surface acidity under similar conditions to those used for the reaction of xylose to elucidate the nature of the active sites and their influence on the reaction mechanism.
References
Clark J.H., Budarin V., Deswarte F.E.I., Hardy J.J.E., Kerton F.M., Hunt A.J., Luque R., Macquarrie D.J., Milkowski K., Rodriguez A., Samuel O., Tavener S.J., White R.J., Wilson A.J. (2006) Green Chem 6:853
Petrus L., Noordermeer M.A. (2006) Green Chem 6:861
Lichtenthaler F.W. (1998) Carbohydr. Res 313:69.
Zeitsch K.J. (2000) The Chemistry and Technology of Furfural and Its Many By-Products, first ed., in: Sugar Series, vol.13, Elsevier, The Netherlands
Gandini A., Belgacem M.N. (1997) Prog. Polym. Sci. 22:1203
Moreau C., Belgacem M.N., Gandini A. (2004) Top. Catal. 27:11
Corma A. (1995) Chem. Rev. 95:559–614
Okuhara T. (2002) Chem. Rev. 102:3641
Clark J.H. (2002) Acc. Chem. Res. 35:791
Yadav G.D., Nair J.J. (1999) Microporous Mesoporous Mater 33:1
Moreau C. (2002) Agro-Food-Industry Hi-Tech 13:17
Benvenuti F., Carlini C., Patrono P., Galletti A.M.R., Sbrana G., Massucci M.A., Galli P. (2000) Appl. Catal. A: Gen. 193:147
Carlini C., Patrono P., Galletti A.M.R., Sbrana G. (2004) Appl. Catal. A: Gen. 275:111
Dias A.S., Pillinger M., Valente A.A. (2006) Microporous Mesoporous Mater 94:214
Dias A.S., Lima S., Pillinger M., Valente A.A. (2006) Carbohydr.Res. 341:2946
Dias A.S., Lima S., Brandão P., Pillinger M., Rocha J., Valente A.A. (2006) Catal. Lett. 108:179
Dias A.S., Lima S., Carriazo D., Rives V., Pillinger M., Valente A.A. (2006) J. Catal. 244:230
Kim Y.-C., Lee H.S. (2001) J. Ind. Eng. Chem. 7:424
Gao Z., Xia Y., Hua W., Miao C. (1998) Top. Catal. 6:101
Kim S.Y., Lohitharn N., Goodwin Jr J.G., Olindo R., Pinna F., Canton P. (2006) Catal. Commun. 7:209
Miao C., Hua W., Chen J., Gao Z. (1996) Catal. Lett. 37:187
Tábora J.E., Davis R.J. (1996) J. Catal. 162:125
Y.D. Xia, W.M. Hua, Y. Tang and Z. Gao, Chem. Commun. (1999) 1899
Xia Y., Hua W., Gao Z. (1999) Appl. Catal. A: Chem. 185:293
Xiao F.-S. (2005) Top. Catal. 35:9
Hudson M.J., Knowles J.A. (1996) J. Mater. Chem. 6:89
Huang Y.-Y., McCarthy T.J., Sachtler W.M.H. (1996) Appl. Catal. A: Gen. 148:135
McIntosh D.J., Kydd R.A. (2000) Microporous Mesoporous Mater 37:281
Ciesla U., Fröba M., Stucky G., Schüth F. (1999) Chem.Mater. 11:227
Wang J.-H., Mou C.-Y. (2005) Appl. Catal. A: Gen. 286:128
Sun Y., Yuan L., Wang W., Chen C.-L., Xiao F.-S. (2003) Catal.Lett. 87:57
Chen C.-L., Li T., Cheng S., Lin H.-P., Bhongale C.J., Mou C.-Y. (2001) Microporous Mesoporous Mater 50:201
Chen C.-L., Cheng S., Lin H.-P., Wong S.-T., Mou C.-Y. (2001) Appl. Catal. A: Gen. 215:21
Chen C.-L., Li T., Cheng S., Xu N., Mou C.-Y. (2002) Catal. Lett. 78:223
Wang W., Wang J.-H., Chen C.-L., Xu N.-P., Mou C.-Y. (2004) Catal.Today 97:307
Sun Y. , Zhu L., Lu H., Wang R., Lin S., Jiang D., Xiao F.-S. (2002) Appl. Catal. A: Gen. 237:21
Yadav G.D., Murkute A.D. (2004) Adv. Synth. Catal. 346:389
Yadav G.D., Murkute A.D. (2004) Langmuir 20:11607
Yadav G.D., Pathre G.S. (2005) J. Phys. Chem. A 109:11080
Dias A.S., Pillinger M., Valente A.A. (2005) J. Catal. 229:414
Miao C.X., Gao Z. (1997) Mater. Chem. Phys. 50:15
Katada N., Endo J., Notsu K., Yasunobu N., Naito N., Niwa M. (2000) J. Phys. Chem. B 104:10321
Bore M.T., Marzke R.F., Ward T.L., Datye A.K. (2005) J. Mater. Chem. 15:5022
Sawant D.P., Vinu A., Jacob N.E., Lefebvre F., Halligudi S.B. (2005) J. Catal. 235:341
Román-Leshkov Y., Chheda J.N., Dumesic J.A. (2006) Science 312:1933
Li X., Nagaoka K., Lercher J.A. (2004) J. Catal. 227:130
Li X., Nagaoka K., Olindo R., Lercher J.A. (2006) J. Catal. 238:39
Pârvulesco V., Coman S., Pârvulesco V.L., Grange P., Poncelet G. (1998) J. Catal. 180:66
Armaroli T., Busca G., Carlini C., Giuttari M., Galletti A.M.R., Sbrana G. (2000) J. Mol. Catal. A: Chem. 151:233
Morterra C., Meligrana G., Cerrato G., Solinas V., Rombi E., Sini M.F. (2003) Langmuir 19:5344
Morterra C., Cerrato G., Signoretto M. (1996) Catal. Lett. 41:101
Morterra C., Cerrato G., Meligrana G., Signoretto M., Pinna F., Strukul G. (2001) Catal. Lett. 73:113
Corma A., García H. (2003) Chem. Rev. 103:4307
Dias A.S., Pillinger M., Valente A.A. (2005) Appl. Catal. A: Gen. 285:126., and references cited therein
Yadav G.D., Murkute A.D. (2004) J. Phys. Chem. A 108:9557
Acknowledgments
This work was partly funded by the FCT, POCI and FEDER (project POCI/QUI/56112/2004). The authors wish to express their gratitude to Prof. C.P. Neto for helpful discussions, Dr. D. Evitiouguine (CICECO) and Dr. F. Domingues (Department of Chemistry) for access to HPLC equipment, and M.F. Lucas for assistance in the HPLC analyses. We also wish to thank Prof. J. Rocha for generous support. A.S.D. and S.L. are grateful to the FCT for PhD and post-doctoral grants, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dias, A.S., Lima, S., Pillinger, M. et al. Modified versions of sulfated zirconia as catalysts for the conversion of xylose to furfural. Catal Lett 114, 151–160 (2007). https://doi.org/10.1007/s10562-007-9052-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-007-9052-6