Abstract
The effect of preparation method on MnO x –CeO2 mixed oxide catalysts for methane combustion at low temperature was investigated by means of BET, XRD, XPS, H2-TPR techniques and methane oxidation reaction. The catalysts were prepared by the conventional coprecipitation, plasma and modified coprecipitation methods, respectively. It was found that the catalyst prepared by modified coprecipitation was the most active, over which methane conversion reached 90% at a temperature as low as 390 °C. The XRD results showed the preparation methods had no effect on the solid solution structure of MnO x –CeO2 catalysts. More Mn4+ and richer lattice oxygen were found on the surface of the modified coprecipitation prepared catalyst with the help of XPS analysis, and its reduction and BET surface area were remarkably promoted. These factors could be responsible for its higher activity for methane combustion at low temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For the environment and energy consideration, complete oxidation of methane into harmless CO2 and H2O has been paid much attention in catalytic combustion field. Among the heterogeneous catalysts, the supported noble metal Pd-based catalysts show excellent activity at low temperature [1–3]. The temperature corresponding to 90% methane conversion (T 90) over Pd/SnO2 catalyst is 440 °C [2]. Pd/Sn x Zr1-x O2 catalyst has been also reported to possess high activity at low temperature for methane complete oxidation, over which T90 is 378 °C [3]. However, poor thermal stability and expensive cost of noble metals prevent the widespread application of these catalysts. Recently, the transition metal mixed oxides are attractive due to the relatively low price and as high as or slightly higher catalytic activity toward methane combustion at low temperature than supported noble metals [4,5].
Among the transition metal mixed oxides of interest for oxidation reactions, MnO x -bases mixed oxide catalysts exhibit great potential. It is generally believed that MnO x are compounds with a typical berthollide structure and contain labile lattice oxygen. Their catalytic properties are attributed to the ability for manganese to form oxides with variable oxidation states (MnO2, Mn2O3, Mn3O4, or MnO) and to their oxygen storage capacity in the crystalline lattice [6,7]. For methane oxidation reaction, the Mn4+ sites are stronger catalytic active sites than the Mn3+ sites [6,8]. The active site on supported MnO x is mainly identified as being Mn4+ of MnO2 in other oxidation processes [7,9]. In addition, CeO2 has been widely used as a promoter and an oxidation catalyst owing to its unique redox properties and high oxygen storage capacity [10,11]. Compared with pure MnO x and CeO2, MnO x –CeO2 mixed oxides showed higher catalytic activities because manganese and cerium oxides formed solid solution in which oxygen reservoir of CeO2 and the mobility of oxygen species were greatly enhanced [12,13].
The catalytic performance of MnO x –CeO2 mixed oxides is notably affected by the preparation methods and conditions. The MnO x –CeO2 catalyst modified with Ag exhibited much higher activity at low temperature for oxidation of formaldehyde because the content of lattice oxygen was apparently increased [14]. Previous studies have proved that the modified coprecipitation prepared MnO x –CeO2 catalyst was more active than those prepared by coprecipitation and sol–gel methods [13]. Considering the same catalytic mechanism (Mars-Van-Krevelen redox mechanism) as total oxidation of formaldehyde, modified coprecipitation method was used to prepare MnO x –CeO2 mixed oxide catalysts for methane combustion in this contribution. Meanwhile, as effective molecule activation and surface modification approaches, the plasma technique was also considered to prepare MnO x –CeO2 catalysts in this work. It was reported that the plasma prepared catalysts showed higher dispersion and enrichment on surface of active components, decrease of reduction temperature, etc. [15–17]. Since these favorable effects, the low-temperature activities of catalysts prepared by plasma method could be remarkably improved.
With the aforementioned background, the aim of the present work was to investigate the effect of preparation method on the properties of MnO x –CeO2 mixed oxide catalysts for methane combustion at low temperature. MnO x –CeO2 catalysts were prepared by coprecipitation, plasma and modified coprecipitation methods, respectively, and were characterized with BET, XRD, XPS, and TPR techniques.
Experimental procedure
Catalyst preparation
MnO x –CeO2 mixed oxides (Mn/(Mn+Ce) = 0.5, molar ratio) were prepared by three different methods. (1) Coprecipitation method: 50% Mn(NO3)2 solution and (NH4)2Ce(NO3)6 with a molar ratio of 5:5 was mixed in dissolved distilled water. Total of 2 M NaOH solution was added to the mixing solution at 50 °C drop by drop until the pH value reached 10.5 with stirring. The mixtures were further aged at 50 °C for 2 h in the mother liquid. After filtration and washing with distilled water, the obtained solid was dried at 110 °C overnight and calcined at 500 °C for 6 h in air. The catalyst was designed as CP. (2) Plasma method: the catalyst was prepared using the same process as the above. Furthermore, the catalyst precursor was treated 90 min by glow discharge plasma before calcination. The treatment approach was similar to that in Ref. [15]. The precursor was put into the discharging tube and decomposed in vacuum. The discharge parameters are as follows: frequency 13.56 MHz, discharge voltage 100 V, anodic current 100 mA. The prepared catalyst was denoted as PP. (3) Modified coprecipitation method: the coprecipitation procedure was the same as CP, but the metal oxide precursors contained KMnO4. The molar ratio of Mn(NO3)2, KMnO4 and (NH4)2Ce(NO3)6 was 1:4:5. The catalyst was nominated as MP. The precipitates were filtered and washed with distilled water many times during the preparation as Ref [13]. There should be no or considerable little potassium in the MP catalyst and the effect of traces of potassium on catalytic activity was not considered in this work. For a comparison purpose, pure MnO x was prepared by coprecipitation of Mn(NO3)2 and KMnO4 with the ratio of 1:4.
Catalyst characterization
The BET surface area of catalysts were determined by N2 adsorption experiments at −196 °C on a micromeritics NOVA 1000 e. Before each measurement, the sample was degassed in vacuum at 300 °C for 3 h.
X-ray powder diffraction (XRD) patterns were recorded on the DX-2000 diffractometer using Cu Kα (l = 1.54056 Å) radiation between 10°and 80°. The voltage and anode current were 40 kV and 30 mA, respectively. The average crystallite sizes of the cubic phase were evaluated from the half-width of the ceria (1 1 1) peak according to the Scherrer’s equation [18]. The lattice parameters were calculated according to the full profile fitting procedure.
X-ray photoelectron spectra (XPS) experiments were performed on the XSAM800 spectrometer with a Al anode for Ka (1486.6 eV) radiation. Charging effects were corrected by adjusting the binding energy of C1s peak from carbon contamination to 284.6 eV.
Temperature-programmed reduction (TPR) measurements were carried out at atmospheric pressure in a fixed-bed reactor. Fifty milligrams sample was loaded and pretreated with N2 at 300 °C for 1 h to remove the adsorbed carbonates and hydrates. After cooling down to 50 °C, the reduction agent of 4.22% H2/N2 with a flow rate of 30 mL/min was introduced. The temperature of the reactor was increased linearly from 50 to 650 °C at a rate of 10 °C/min by a temperature controller. The effluent stream was analyzed for hydrogen by a thermal conductivity detector.
Catalytic activity test
Methane combustion was performed in a fixed-bed reactor with a continuous flow at atmospheric pressure in the temperature range of 300–480 °C. In order to decrease the effect of exothermic reaction on bed-temperature, catalyst sample of 150 mg was diluted in 300 mg silica sand. Subsequently, the diluted catalyst was packed in a quartz tube that connected with a thermocouple centered in the catalyst bed. The reaction gases consisted of 20% CH4 and 40% O2 in Ar, and space velocity (SV) was 40,000 ml g−1 h−1. The analysis of the effluent from the reactor was performed by the 112A model gas chromatograph with a thermal conductivity detector (TCD).
Results and discussion
Catalyst activity in methane combustion
Figure 1 illustrates the results of activities of various catalysts for methane combustion. In this work, CO2 and H2O are the only reaction products of methane oxidation. As expected, the preparation methods significantly affect the catalytic activities of MnO x –CeO2 mixed oxides. For the CP, PP and MP catalysts, the temperatures responding to 50% CH4 conversion (T 50) were 408, 376 and 348 °C, respectively. The T 50 of the PP and MP catalysts decreased 32 and 60 °C in contrast to that of the CP catalyst, indicating that the MP and PP catalysts possessed much higher activity in the experimental temperature range, especially the MP catalyst. Moreover, the T 90 over the MP catalyst was as low as 390 °C. When the specific conversion (defined as methane conversion per surface area unit) was considered, the activity difference was still obvious below 390 °C. Moreover, the rates of methane combustion (mol/m2 s) on the three catalysts were compared, and similar results were also obtained. Thereby, it could be claimed that the MP catalyst was the most active at low temperature.
BET texture analysis
The surface area, pore volume and average pore diameter of MnO x –CeO2 mixed oxide catalysts are summarized in table 1. From table 1, it can be seen that the surface area and pore volume of the MP catalyst (106.40 m2/g and 0.214 cm3/g, respectively), are much larger than those of the CP catalyst. In addition, the surface area of the PP catalyst (80.11 m2/g) slightly increases in comparison with that of the CP catalyst (74.59 m2/g), while the average pore diameter decreases by 45%. All the average pore diameters of the catalysts are below 10 nm. The results of BET measurements suggest that the surface areas of MnO x –CeO2 mixed oxides are related to their preparation methods, and the MP catalyst shows a maximum surface area.
XRD characterization
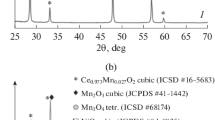
Figure 2 displays the XRD patterns of MnO x –CeO2 catalysts. There was no diffraction of manganese oxides in the figure. Only the broad peaks of cubic fluorite structure CeO2 (JCPDS #43–1002) were observed. The XRD qualitative analysis results show that MnO x –CeO2 mixed oxides crystallize in CeO2 type structure as reported in Ref [19]. Mn atoms probably replace some of Ce sites and enter the CeO2 lattice.
The refined lattice parameters and crystallite sizes of the MnO x –CeO2 mixed oxide catalysts are listed in table 2. As the ion radius of Mn (r(Mn3+) = 0.66 Å and r(Mn4+) = 0.60 Å) is smaller than the ion radius of Ce (r(Ce4+) = 0.92 Å), and Mn can enter CeO2 lattice, the lattice parameters of these mixed oxides are smaller than that of Pure CeO2 (5.411 Å). The XRD results identify that the preparation methods have no effect on the phase composition of MnO x –CeO2 mixed oxides. In addition, it can be also seen that the average crystallite sizes of the CP, PP and MP catalysts are 49, 29 and 42 Å, respectively. Obviously, the crystallite size of the PP catalyst decreased notably, indicating that the glow discharge plasma treatment significantly enhances the dispersion of active components.
According to XRD patterns showed in figure 3, MnO2 (JCPDS #44–0141) was the main phase of the pure MnO x with a much lower content Mn3O4(JCPDS #04–0732). Consequently, it might be accepted that manganese species was almost Mn4+ in the MP catalyst.
XPS analysis
The XPS spectra of Mn2p, O1s and Ce3d were measured for these three MnO x –CeO2 catalysts. From the XPS spectra of Mn2p, for the CP catalyst, the binding energies around 643.49s and 641.86 eV could be ascribed to the presence of Mn4+ and Mn3+ species, respectively. Similarly, the binding energies around 643.00 and 641.23 eV was also attribute to Mn4+ and Mn3+ species for the PP catalyst. In the MP catalyst, it is interesting to note that only one peak at about 642.55 eV corresponding to Mn4+ species was observed within the resolution of XPS. This result is in good consistent with the XRD measurements. According to the corresponding XPS spectra of O1s, two peaks (OI and OII) were displayed, which represent two different kinds of surface oxygen species. OI with BE from 529.15 to 529.31 eV is characteristic of the lattice oxygen (O2−) [19,20], while OII with BE of 530.22–531.65 eV belongs probably to the defect oxides or the surface oxygen ions with low coordination [13,14]. From the XPS spectra of Ce3d, the binding energies of Ce4+ were located at the characteristic values of 882.40–882.60 eV as reported in Ref. [21], suggesting that the oxidation state of cerium was CeO2 in all the catalysts. This is also in agreement with the XRD results.
The surface compositions calculated from the XPS spectra of the MnO x –CeO2 catalysts are summarized in table 3. The different preparation methods resulted in remarkable varieties of relative concentration of Mn4+ and OI. In the CP catalyst, the relative concentrations of Mn4+ was calculated to be about 30%, but the data in the PP and MP samples were almost 80% and 100%, respectively. The corresponding concentrations concentrations of OI were 13.63%, 36.58% and 53.77%, respectively. This reveals that plasma and modified coprecipitation methods can apparently increase concentrations of Mn4+ and lattice oxygen species on the surface in contrast to conventional coprecipitation method, especially modified coprecipitation method. Therefore, the MP sample possessed the most Mn4+ and richest lattice oxygen species. The surface condition of the MP catalyst obviously favors the oxidation of methane, and provides a good interpretation for the higher activity of the MP catalyst at low temperature.
H2-TPR measurements
The H2-TPR profiles of the pure MnO x and the MnO x –CeO2 mixed oxides are showed in figure 4. From the figure 4, H2-TPR profiles of the pure MnO x showed an intensive reduction peak with a maximum at about 370 °C, which could be attributed to the reduction of MnO2–MnO. Since the larger negative value of reduction potential, the reduction of MnO to Mn0 has not been observed even up to 950 °C [22]. Therefore, MnO was regarded as the final reduction state.
Compared with the reduction behaviors of pure MnO x , the reduction temperatures of the MnO x –CeO2 mixed oxides systematically shifted to lower regions. This indicated that the reductions of MnO x –CeO2 mixed oxides were promoted due to the formation of the solid solution. However, the reduction behaviors of the catalysts are significantly influenced by the preparation methods as showed in figure 4. The H2-TPR profile of the CP catalyst exhibited an intensive reduction peak with a maximum at 346 °C, followed by a weak reduction peak at about 430 °C. The two reduction peaks could be attributed to the reductions of Mn2O3 to MnO and surface CeO2, respectively. Comparatively, the reduction of the PP catalyst began in much lower region (at a maximum of about 250 °C). The low temperature reduction could be ascribed to the reduction MnO2/Mn2O3 to Mn3O4, and the high temperature reduction assigned to the combined reduction of Mn3O4 to MnO and surface oxygen removal of CeO2. For the MP catalyst, the H2-TPR profile exhibited overlapped strong reduction peaks from 170 to 450 °C. According to fitting multi-peaks (Gauss model) with origin 7.0 software, it was found that the overlapped reduction peaks consisted of three peaks with the maximums at about 276, 356, and 400 °C, respectively. In general, the reduction profile of MnO2 shows a typical two-step reduction process, and the reduction sequence is probably MnO2→ Mn3O4→ MnO [13,23]. However, Craciun et al. [6] presented a three-step reduction profile for unsupported MnO x . The first two peaks at about 327 and 417 °C correspond to two-step reduction of MnO2 (MnO2→ Mn2O3 → Mn3O4). The third peak at 510 °C represents the total reduction of Mn3O4 to MnO. Three reduction temperatures of the MP catalyst obviously shifted to lower regions compared with those of unsupported MnO x [6]. The solid solution between MnO x and CeO2 was formed in which mobility of oxygen species was greatly promoted. As a result, the reduction temperature of MnO2 in the MP catalyst was decreased significantly.
Based on the results of characterization and reaction, there were more MnO2 and lattice oxygen and larger surface area for the modified coprecipitation prepared catalyst, resulting in higher activity toward methane combustion at low temperature. As showed in XRD patterns, MnO2 and CeO2 in the MP catalyst formed the solid solution, leading to strongly synergistic interaction. According to three reactions represented by Ding et al. [24], the synergistic mechanism could be explained by considering the effective activation of molecule oxygen over the MnO2–CeO2 solid solution.
Conclusion
MnO x –CeO2 mixed oxide catalysts are highly active for methane combustion at low temperature. The catalytic activity primarily depended on the preparation methods. The catalyst prepared by modified coprecipitation exhibited much higher activity toward methane oxidation than those prepared by coprecipitation and plasma methods, and the methane conversion reached 90% at a temperature as low as 390 °C over the MP catalyst. XRD characterization identified that the preparation methods had no effect on the phase formation of MnO x –CeO2 catalysts. However, BET, XPS and H2-TPR measurements suggested that larger surface area, more Mn4+, richer lattice oxygen and easier reduction resulted in higher activity of the MP catalyst.
References
Gélin P., Primet M. (2002) Appl. Catal. B. 39:1
Sekizawa K., Widjaja H., Maeda S., et al. (2000) Catal. Today. 59:69
Lin W., Lin L., Zhu Y.X., et al. (2005) Appl. Catal. B 57:175
Zhou C.-J., Lin W., Zhu Y.-X., et al. (2003) Chinese J. Catal. 24:229
L.F. Liotta, G. Di Carlo, and G. Pantaleo, et al. (2005) Appl. Catal. B (doi:10.1016/j.apcatb.12.023)
Craciun R., Nentwick B., Hadjiivanov K., et al. (2003) Appl. Catal. A 243:67
Craciun R. (1998) Catal. Lett. 55:25
Machocki A., Ioannides T., Stasinska B., et al. (2004) J. Catal. 227:282
Hadjiivanov K., Lavalley J.-C. (2001) Catal. Commun. 2:129
Otsuka K., Wang Y., Nakamura M. (1999) Appl. Catal. A. 183:317
Trovarelli A., de Leitenburg C., Boaro M., et al. (1999) Catal. Today. 50:353
Silva A.M.T., Marques R.R.N., Quinta-Ferreira R.M. (2004) Appl. Catal. B. 47:269
Tang X., Li Y., Huang X. et al. (2006) Appl. Catal. B. 62:265
Tang X., Chen J., Li Y., et al. (2006) Chem. Eng. J. 118:119
Chen M.H., Chu W., Dai X.Y., et al. (2004) Catal. Today. 89:201
Zhang Y.-P., Ma P.-S., Zhu X., et al. (2004) Catal. Commun. 5:35
Wang J.-g., Liu C.-j., Zhang Y.-p., et al. (2004) Catal. Today. 89:183
Patterson A.L. (1939) Phys. Rev. 56:978
Machida M., Uto M., Kurogi D., et al. (2000) Chem. Mater. 12:3158
Chen H., Sayari A., Adnot A., et al. (2001) Appl. Catal. B. 32:195
Larachi F., Pierre J., Adnot A., et al. (2002) Appl. Surf. Sci. 195:236
Carno J., Ferrandon M., Bjornbom E., et al. (1997) Appl. Catal. A. 155:265
Alvarez-Galvan M.C., de la Pena O., rsquo., et al. (2003) Catal. Commun. 4:223
Ding Z.Y., Li L., Wade D., et al. (1998) Ind. Eng. Chem. Res. 37:1707
Acknowledgments
This work was supported by the National Natural Science Foundation of China (#205903603) and by the 973 project of the Ministry of Science and Technology of China (#2005CB221406).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, L., Chu, W., Qu, F. et al. Low-temperature catalytic combustion of methane over MnO x –CeO2 mixed oxide catalysts: Effect of preparation method. Catal Lett 113, 59–64 (2007). https://doi.org/10.1007/s10562-006-9012-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-006-9012-6