Abstract
Low survival rate of grafted mesenchymal stem cells (MSC) in injured tissue is one of the major limitations of stem cell therapy. One of the most important factors that limits the MSCs survival rate and retention is ischemic stress, which can lead to damage to all components of the cell. In particular, it can damage mitochondria, that play an important role in apoptosis with releasing apoptotic factors. Therefore, we investigated the protective effects of Acetyl-l-carnitine (ALCAR) against serum and glucose deprivation (SGD) in adipose-derived mesenchymal stem cells (AD-MSCs). We measured cell viability, proliferation, and apoptosis in cells experiencing SGD stress for 8 h with exposure to varying concentrations of ALCAR. Results showed that ALCAR protects cells against SGD stress by reducing apoptosis. Its protective effects are associated with reductions in cleaved caspase-3 and attenuation of apoptosis. Result showed that ALCAR exhibits protective effects against SGD-induced damage to AD-MSCs by enhancing the expression of survival signals and by decreasing the expression of death signals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stem cell (MSC) therapy has provided a potentially effective treatment of wide range of disorders such as traumatic injuries, ischemic strokes, degenerative and autoimmune diseases (Brodhun et al. 2004; Kassem and Abdallah 2008). MSCs can be separated from diverse sources, like bone marrow, adipose tissue and umbilical cord and will be extended easily in vitro (Deng et al. 2006; Kern et al. 2006; Kingham et al. 2007). MSCs have multipotent differentiation capacity and transplanted MSCs can reduce inflammation, apoptosis and fibrosis. Also, they have immunomodulatory properties and may alleviate immune disorders by regulating immune responses (Kingham et al. 2007). Such characteristics of MSCs make them more attractive for regenerative medicine and tissue engineering. (Ghayour et al. 2015; Gimble et al. 2007). Nevertheless, their therapeutic potential is constrained by the poor survival rate and retention of transplanted MSCs (Potier et al. 2007). Most transplanted MSCs have been shown to be lost due to the harmful microenvironment of damaged tissues, such as oxidative stress, energy loss, lack of growth factor, inflammatory response and eventual apoptosis (Potier et al. 2007; Toma et al. 2002). Apoptosis is a programmed cell death cycle characterized by morphological alterations such as shrinkage, membrane blebbing, nuclear fragmentation and chromosomal nuclear DNA fragmentation (Hollville and Martin 2016). Therefore, methods to enhance survival rate and retention capacity of MSCs are desirable to improve the efficacy of their clinical application. Stress conditions including serum deprivation and oxidative stress induced apoptosis mainly through a mitochondrial apoptotic pathway (Green and Reed 1998; Martinou and Youle 2011). Also, Mitochondria are well known as the source of reactive oxygen species (ROS) production (Fleury et al. 2002). Therefore, it seems that mitochondrial protection may be a good approach for enhancing MSCs survival and retention. Mitochondrial protective agents can be a treatment to influence MSCs behavior. In this regard, Acetyl-l-carnitine (ALCAR) is an acetylated carnitine derivative that interacts with the mitochondrial membrane and plays a significant role in the mitochondrial oxidation of fatty acids and cellular energy production (Rebouche 2004). Some studies suggest the anti-apoptotic and antioxidant properties of ALCAR (Ferreira and McKenna 2017). It would appear that ALCAR decreased cytochrome-C cytosolic levels and eventual caspase-3 activation. However, little information is available about the effects of ALCAR on MSCs function and viability. Therefore, we investigated the protective effects of ALCAR on rat adipose-derived mesenchymal stem cells (AD-MSCs) survival and proliferation under stressful conditions. AD-MSCs were selected because of their ease of isolation, high harvesting yield, easy culture and fast growth rate (Kingham et al. 2007).
Materials and methods
Ethics statement
All experiments were carried out in accordance with the Helsinki Declaration and the guidelines of the Ethics Committee of the Ferdowsi University of Mashhad (Iran).
Isolation, culture and identification of AD-MSCs
As previously described, AD-MSCs were extracted from the abdominal adipose tissue of adult male Wistar rats (Kingham et al. 2007). Briefly, the adipose tissue was digested enzymatically with 0.15% (w/v) type I collagenase (Invitrogen, UK) at 37 °C for 1 h. Afterwards, by adding phosphate buffered saline (PBS) combined with 1% (v/v) fetal bovine serum (FBS), enzyme activity was neutralized. Then, the stromal-vascular fraction (SVF) was gathered by centrifugation at 800 g for 5 min. SVF was subsequently re-suspended in low glucose DMEM supplemented with 10% (v/v) FBS (Gibco, USA), 100 unit/mL of penicillin and 100 μg/ml of streptomycin then, cells were cultured in 75 cm2 flasks (SPL) at a density of 1 × 104/cm2. Then, cultured in a humidified atmosphere with 5% CO2 at 37 °C. For our experiments passage 3 cells were used. To validate multipotency capacity, according to the manufacturer’s protocol, AD-MSCs were cultivated in adipogenesis and osteogenesis differentiating media (Invitrogen, USA) at passage 3. The media was changed every 2 days. AD-MSCs had been exposed to Oil Red O staining for adipogenesis after 3 weeks of culture. Also, for evaluating the mineralisation of the matrix, osteogenesis has been stained with Alizarin Red. The phenotype of cultured AD-MSCs at passage 3 was analyzed by flow cytometry and surface expression of the CD11b, CD29, CD31, CD45 and CD90 markers was determined using a cytofluorometer (BD Biosciences, USA). Cells stained with FITC-conjugated IgG1κ, IgA and IgM were used as isotype controls. Flow cytometry was performed by BD FACSCalibur flow cytometer (BD Biosciences) and analysis was carried out using FlowJo v 10 (TreeStar) software (Ghayour et al. 2019).
In vitro model of AD-MSCs apoptosis and ALCAR treatment
Serum and glucose deprivation (SGD) was used to simulate ischemia and subsequent apoptosis of cells (Bialik et al. 1999; Liu et al. 2003; Zhu et al. 2006). Briefly, AD-MSCs were cultured in 96 well plates at a density of 1 × 104 cells/well in DMEM supplemented with 10% FBS for 24 h. Then, AD-MSCs were washed with serum-free DMEM and incubated in serum-free and no glucose DMEM at 37 °C with 5% CO2 for a period of 8 h. After this time, the viability assay and apoptosis assay were performed. For the ALCAR protection experiments, AD-MSCs were incubated in fresh DMEM supplemented with 10% FBS and ALCAR (0.1, 1 and 10 mM) 12 h before the SGD. Also, for evaluating the effects of ALCAR on AD-MSCs viability and proliferation under normal culture condition, AD-MSCs were seeded in 96-wells plate and incubated in complete DMEM and ALCAR at concentration of 0.1, 1 and 10 mM for 24, 48, 72 and 96 h. After treatment, cells viability was evaluated.
MTT assay
The MTT assay was performed to evaluate AD-MSCs viability and proliferation by measurement of metabolic activity, under normal and stress culture conditions. After being subjected to the experimental treatments, the cells were incubated with 20 μl MTT reagent (5 mg/ml; St. Louis, MO, USA) in the dark at 37 °C for 4 h. After aspiration of the medium, the resulting formazan crystals were dissolved in 200 μl of dimethyl sulfoxide (DMSO) for 1 h. The absorbance of solubilized formazan crystals was measured at 570 nm by an ELISA reader (model 550; Bio-Rad Laboratories, Hercules, CA, USA). Absorbance levels are related to the number of alive cells. Polystyrene tissue culture (TPS) and untreated ANA were also used as controls. In each group the mean OD of 10 wells was used to measure the percentage of cell viability. The tests were carried out in triplicate.
In situ detection of apoptotic cells
The terminal deoxynucleotidyl transferase (TdT) dUTP-biotin nick end labeling (TUNEL) assay was used to detect in situ apoptotic DNA fragmentation according to the manufacturer’s protocol (In-situ cell death detection kit, Roche). Briefly, AD-MSCs were seeded onto sterilized glass coverslips and cultured in complete medium. After induction of apoptosis, AD-MSCs were fixed in 4% (v/v) paraformaldehyde in PBS (pH 7.4) for 1 h at room temperature. Then, endogenous peroxidase blocked by exposing the cells to methanol containing 0.3% H2O2 at room temperature for 10 min. The cells were incubated in the permeabilization solution containing 0.1% Triton-X 100 and 0.1% sodium citrate for 2 min on ice. Then, the cells were washed and incubated with 50 μL of TdT reaction buffer at 37 °C for 1 h in the dark. Finally, cells were washed with cold PBS for 1 h. For counterstaining, AD-MSCs were exposed to DAPI in the dark for 5 min. The immunostained cells were observed by a fluorescence microscope (Nikon Eclipse TE 2000U). DAPI stain observed with UV excitation (emitting a blue color) and TUNEL signal with blue light excitation (460–490 nm) (emitting a green color). An apoptotic score index (%) was expressed as the ratio of TUNEL-positive cells to total cells. Fifty DAPI positive cells from 10 independent fields per slide counted randomly and determined the percentage of these cells that had green fluorescence (apoptosis). Experiments were done in duplicate for three times, and the percentage of TUNEL-positive cells was determined (Calderón et al. 2020).
Annexin-V/propidine iodide staining
The number of apoptotic AD-MSCs was determined by FITC Annexin V Apoptosis Detection Kit I (BD Bioscience, USA) according to the manufacturer’s protocol. The externalization of phosphatidylserine, which is normally restricted to the inner surface of the plasma membrane is a marker of early-stage apoptosis and can be detected by the annexin V protein conjugated to FITC. Also, membrane damage due to necrosis was detected by the binding of PI to nuclear DNA. Briefly, treated cells were collected and resuspended in 1X binding buffer at a concentration of 1 × 106 cells/ml. Then, 100 µl of solution (1 × 105 cells) transferred to a 5 ml culture tube. Subsequently, the cells were incubated with 5 μl of Annexin V stock solution and 5 μl of propidium iodide (PI) at room temperature for 15 min in the dark. Then, 400 µl of 1X binding buffer added to each tube and immediately analyzed by a BD FACSCalibur flow cytometer and analyzed using CELLQuest software. For each sample, approximately 2 × 104 cells were analyzed were analyzed in each of the samples. Early apoptotic (Annexin V+, PI−), necrotic (Annexin V−, PI+), late apoptotic or secondarily necrotic (Annexin V+, PI+) and healthy (Annexin V−, PI−) cells were expressed as percentages of the 20,000 cells.
Caspase-3 activity assay
The activity of caspase-3 was measured by the corresponding colorimetric assay kit (abcam, USA) according to the manufacturer’s protocol. Briefly, 2 × 106 treated cells were collected and incubated in 50 µl cell lysis buffer for 10 min on ice. The cells were then centrifuged at 10,000 g for 1 min and supernatant was collected. Protein concentrations were measured using the Bradford method and adjust to 100 μg protein per 50 μL Cell Lysis Buffer for each assay. Then, samples were incubated with 2× reaction buffer containing 10 mM DTT and 4 mM labeled substrate (caspase-3:DEVD-p-NA) at 37 °C for 2 h. Finally, absorbance was measured at 405 nm in a microtiter plate reader. The assay was repeated 3 times.
Statistical analysis
All data are reported as the mean ± standard error of mean (SEM). Differences between groups were verified by one-way ANOVA and comparisons between groups were evaluated using Tukey’s test. P-values less than 0.05 were considered statistically significant. All statistical analyses were performed by SPSS version 19.0 software (IBM Corp., Armonk, NY, USA).
Results
Identification of AD-MSCs
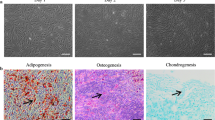
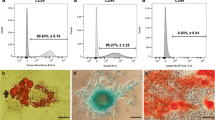
AD-MSCs were identified by morphological observation, flow cytometry and mesodermal differentiation capacity. The phenotype of cultured AD-MSCs at passages 3 had a spindle-like morphology under inverted phase microscopy (Fig. 1b). FACS analysis of surface marker expression was conducted for evaluating the phenotypic characterization of cultured AD-MSCs at passage 3. Results revealed that AD-MSCs expressed a high degree of CD29 (98.6 ± 2.1%) and CD90 (98.4 ± 0.89%), but were negative for endothelial marker CD31 (0.44 ± 0.11%) as well as was negative for markers of the hematopoietic lineage CD45 (1.86 ± 0.1%) and CD11b (1.94 ± 0.3%) (Fig. 1a). These findings coincided with previous studies. Following cultivation of the AD-MSCs in osteogenic medium calcium mineralization was observed by Alizarin Red S staining (Fig. 1c). The accumulation of intracellular lipid vacuole was also observed by the Oil Red O staining after adipogenic differentiation (Fig. 1d).
Flow cytometry characterization and multipotency confirmation of AD-MSCs obtained from adult male Wistar rats. a Flow cytometry results demonstrated that AD-MSCs at passage 3 were uniformly negative for CD11b, CD31 and CD45 and positive for CD29 and CD90 expression. b Isolated AD-MSCs showed fibroblast-like shapes (b), and exhibited multi-differentiation capacity osteogenic and adipogenic differentiation respectively (c and d)
Effects of ALCAR on AD-MSCs proliferation in normal condition
To evaluate the effect of ALCAR itself on AD-MSCs viability, cells were treated with three different concentrations of ALCAR (0.1, 1 and 10 mM) for 96 h under normal conditions. After culturing for 96 h, the colorimetric result of the MTT assay showed that cell viability and proliferation did not differ significantly between ALCAR (0.1, 1 and 10 mM) treated AD-MSCs groups and non- treated AD-MSCs at 24, 48 and 72 h. However, with increasing cultivation time, cell viability and proliferation rate of ALCAR (1 and 10 mM) treated AD-MSCs groups was significantly higher compared with the control group at 96 h. These findings showed that ALCAR did not have any toxic effect on cultured AD-MSCs under normal conditions, after culturing for 96 h (p < 0.05; Fig. 2).
Effect of ALCAR on cell viability under normal condition. The viability and proliferation of AD-MSCs that treated with ALCAR was compared with non-treated AD-MSCs were cultured in 96 well plates using MTT assay. MTT assay show that ALCAR has no toxic effect on cell viability and proliferation under normal culture condition. Data were expressed as mean ± SD (n = 10). *p < 0.05 versus untreated AD-MSCs group at the same time point. The experiments were performed in triplicate
Cytoprotective effects of ALCAR in SGD condition
To evaluate the protective effect of ALCAR against stress induced cell death, the AD-MSCs were incubated under SGD culture conditions in the absence or presence of ALCAR (0.1, 1 and 10 mM) for 8 h. As shown in Fig. 3, MTT assay result showed that under the SGD condition, AD-MSCs viability and proliferation was decreased significantly when compared with the control. The results showed that untreated AD-MSCs survival rate reduced to approximately 56% compared with control after 8 h. On the other hand, ALCAR treatment showed significant protective effects against stress induced cells death and increased AD-MSCs survival rate to 66 and 83% in the concentration of 1 and 10 mM, respectively (p < 0.05; Fig. 3). Nonetheless, there was no significant difference between the control group and 0.1 mM ALCAR treated AD-MSCs group. The data are shown as ratio of the OD of the treated groups to that of the control group.
Effect of ALCAR treatment on AD-MSCs death associated with SGD culture condition. AD-MSCs were exposed to SGD for 8 h in the presence or absence of ALCAR (0.1, 1 and 10 mM). The viability and proliferation of AD-MSCs that treated with ALCAR was compared with non-treated AD-MSCs were cultured in 96 well plates using MTT assay. MTT assay show that ALCAR at concentration of 1 and 10 µM has protective effect on cell viability and proliferation under SGD culture condition. Data were expressed as mean ± SD (n = 10). *p < 0.05 and ***p < 0.001 versus SGD group at the same time point. The experiments were performed in triplicate
Effects of ALCAR on stress-induced DNA fragmentation
TUNEL staining was performed to determine the apoptotic rate of AD-MSCs by detection of DNA fragmentation. TUNEL-positive cells were used to quantify the apoptosis. The results showed that under SGD condition, the TUNEL-positive rate of AD-MSCs was significantly increased to 40.8 ± 6.5% in comparison with control group (3.4 ± 0.9%). In respect to SGD group, ALCAR treatment at a concentrations of 1 and 10 mM decreased the percentage of TUNEL positive cells to 33.1 ± 4.4% and 14.6 ± 5.3%, respectively in a significant manner (p < 0.05; Fig. 4). However, similar to MTT results, ALCAR treatment at concentration of 0.1 mM has no significant effect on percentage of TUNEL positive cells in comparison with SGD group.
Apoptosis detected by TUNEL staining. The cells with green fluorescence (positive reaction) were correlated with DNA fragmentation. Data were expressed as percentages of TUNEL positive-cells to the total cells. The data are shown as mean ± SD. **p < 0.01 and ***p < 0.001 versus SGD group at the same time point. (Color figure online)
Analysis of cell apoptosis by flow cytometry
During ischemia, stress conditions such as deprivation of nutrients and growth factors contribute to cellular death. Therefore, for evaluating the protective effect of ALCAR against stress condition AD-MSCs were exposed to SGD in the presence or absence of acetyl l-carnitine (0.1, 1 and 10 mM) and death was measured by Annexin V/PI staining. Annexin V/PI staining can be used to differentiate the apoptotic and necrotic cells. The apoptotic rate of AD-MSCs was quantified by calculating the percentage of the cells that exhibit the annexin V+/PI− and annexin V+/PI+ phenotype. According to result, the apoptotic ratio was significantly increased in the cells subjected to SGD condition in comparison with control group (p < 0.05; Fig. 5). However, in accordance with MTT assay results ALCAR treatment at concentration of 1 and 10 µM decreased the number of early and late apoptotic cell, significantly (p < 0.05; Fig. 5). This finding suggesting that ALCAR attenuate the apoptotic effects of SGD condition. It is worth mentioning that ALCAR treatment at concentration of 0.1 mM has no significant effect on apoptotic rate of SGD treated AD-MSCs.
Anti-apoptotic effects of ALCAR on AD-MSCs under SGD culture condition. AD-MSCs were exposed to SGD for 8 h in the presence or absence of ALCAR (0.1, 1 and 10 mM) and the apoptotic ratio was quantified by Annexin V/PI assay. The Annexin V+/PI− and Annexin V+/PI+ cells represent early and late apoptosis, respectively, whereas necrotic cells show the Annexin V−/PI+. Data were expressed as percentages of stained cells to the total cells. The data are shown as mean ± SD. **p < 0.01 and ***p < 0.001 versus SGD group at the same time point
Caspase-3 activity
Caspase-3 activity was assessed, because caspase-3 activation is an essential step during the apoptosis process. The results were expressed as the fold changes of caspase-3 activity compared with corresponding control cells. According to results, under SGD conditions the activity of caspase-3 was significantly increased in comparison with control (p < 0.05; Fig. 6). However, findings indicated that cleavage and subsequent activity of caspase-3 was attenuated by ALCAR treatment at concentration of 1 and 10 mM, significantly (p < 0.05; Fig. 6).
Effect of ALCAR treatment in SGD induced caspase-3 activity. AD-MSCs were cultured under SGD condition for 8 h in the presence or absence of ALCAR at concentration of 0.1, 1 and 10 mM. Then, caspae-3 activity was determined by colorimetric assay. Data were expressed as fold-changes relative to SGD group. The data are shown as mean ± SD. **p < 0.01 and ***p < 0.001 versus SGD group at the same time point
Discussion
Despite several advantages, the poor rate of survival and retention of transplanted MSCs decreases their therapeutic potential (Chavakis et al. 2008; Potier et al. 2007). The majority of the transplanted MSCs have been shown to be lost due to the harmful microenvironment of damaged tissues (Shi and Li 2008; Toma et al. 2002). Transplanted MSCs undergo an ischemic microenvironment of hypoxia and serum deficiency (Potier et al. 2007). Metabolic stress is also another cause of limiting the viability and lifespan of transplanted MSCs which limits their therapeutic effectiveness (Chavakis et al. 2008). Therefore, the efficacy of MSC therapy will depend on the possibility of delivering a large number of viable functional cells into injured tissue. In this regards, it is important to enhance MSCs viability and improve their retention. Since stress conditions such as serum and glucose deprivation mainly activate mitochondrial apoptotic pathway (Bialik et al. 1999; Zhu et al. 2006), we hypothesized that mitochondrial protection may be a way to increase AD-MSCs survival under stressful conditions. Therefore, we investigated the pro-survival and anti-apoptotic effect of mitochondrial protective agent, ALCAR on AD-MSCs cultured under SGD conditions. ALCAR, a short chain ester of carnitine l-isomer, plays a crucial role in the long chain transport of fatty acids through the mitochondrial membrane for β-oxidation and ATP synthesis. ALCAR can also protect cells from lipid peroxidation and mitochondrial membrane destruction by its antioxidative function under cell stressful conditions (Gülçin 2006; Zhang et al. 2012). Since the ischemic condition of damaged tissues leads to growth factor withdrawal and nutrient restriction, we cultured AD-MSCs under SGD. SGD condition triggers apoptosis due to lack of trophic factors which are essential for cell survival and metabolism disruption (Kulkarni and McCulloch 1994; Pardo et al. 2002). In this study, the result of MTT assays showed that SGD decreased the viability and proliferation of untreated AD-MSCs. However, our results showed that ALCAR treatment increases survival rate of AD-MSCs at concentration of 1 and 10 mM. Also, annexin V/PI assay and TUNEL staining results indicated that majority of observed mortalities were due to apoptosis. The fragmentation of nuclear DNA is an initial indication of apoptosis and TUNEL assay was performed to detect apoptosis-related DNA-strand breakage. Under our experimental stressful conditions the number of the TUNEL-positive cells significantly increased while ALCAR treatment (1 and 10 mM) significantly decreased TUNEL-positive cell rate. Also, our results indicated that ALCAR treatment significantly decrease the apoptotic ratio (the number of Annexin V+/PI− and Annexin V+/PI+) in a dose-dependent manner. In agreement with these finding, in vitro studies showed that ALCAR has been able to reduce the neuronal mortality rates (Manfridi et al. 1992). Also, some research suggested a protective effect of ALCAR against oxidative stress and apoptosis induced by hypoxia (Barhwal et al. 2007). In another study, Pillich and coworkers showed that ALCAR treatment could attenuate the apoptosis of cultured mouse fibroblast (Pillich et al. 2005). They demonstrated that ALCAR can protect cells from apoptosis by improving the metabolism of the mitochondrion. Furthermore, Fujisawa and colleague reported that l-carnitine suppresses doxorubicin mediated apoptosis in MSCs of the bone marrow. Consistent with these findings, our results revealed that Caspase-3 activity decreased with ALCAR treatment in AD-MSCs cultured under SGD conditions. It seems that, SGD induced apoptosis mainly proceed through the intrinsic or mitochondrial pathway. Growth factors deficiency can disrupt signal transduction and activate intrinsic apoptotic pathway (Li et al. 2014). In the intrinsic pathway, mitochondria release pro-apoptotic factors such as cytochrome c which activate the caspases cascade (Elmore 2007). This pathway leads to activation of caspase-3, a key regulator of apoptosis which involved in apoptotic changes including DNA degradation, chromatin condensation and nuclear fragmentation (Sahara et al. 1999). Some studies showed that ALCAR was able to stimulate the activity of some growth factors such as hepatocyte growth factor (HGF) and nerve growth factor (NGF) (Revoltella et al. 1994; Taglialatela et al. 1991). Previous findings demonstrated that ALCAR treatment improves NGF sensitivity by enhancing neurotrophin receptor expression and affinity to NGF (Manfridi et al. 1992). Growth factors as survival factors can inhibit apoptosis by regulation of signal transduction and gene expression pathways (Collins et al. 1994; Palmerini et al. 2017). Taken together, we concluded that ALCAR may be an important cytoprotective factor, which is able to protect AD-MSCs against SGD condition by probably preservation mitochondrial function and inhibitory effects on intrinsic apoptosis pathway. Nevertheless, the exact mechanism by that ALCAR enhanced the survival of the cells remains to be determined.
Conclusions
In conclusion ALCAR has cytoprotective effects on AD-MSCs against SGD stress. The antiapoptotic effect of ALCAR is probably due to its stabilizing action on mitochondria. Enhance survival and retention capacity of AD-MSCs is important in stem cell therapy and regenerative medicine. Our results may provide an experimental basis for clinical application of ALCAR to increase stem cell therapy efficacy.
Limitations
-
1
We would like to check the neuroprotective effects of rosuvastatin in animal model which will be investigated in future studies of our lab.
-
2
We would like to check the role of ALCAR on marker genes that involve in apoptosis in future studies.
Abbreviations
- MSC:
-
Mesenchymal stem cells
- ALCAR:
-
Acetyl-l-carnitine
- SGD:
-
Serum and glucose deprivation
- AD-MSCs:
-
Adipose-derived mesenchymal stem cells
- ROS:
-
Reactive oxygen species
- PBS:
-
Phosphate buffered saline
- FBS:
-
Fetal bovine serum
- SVF:
-
Stromal-vascular fraction
- DMSO:
-
Dimethyl sulfoxide
- TdT:
-
Terminal deoxynucleotidyl transferase
- TUNEL:
-
dUTP-biotin nick end labeling
- PI:
-
Propidium iodide
- SEM:
-
Standard error of mean
- HGF:
-
Hepatocyte growth factor
- NGF:
-
Nerve growth factor
References
Barhwal K, Singh SB, Hota SK, Jayalakshmi K, Ilavazhagan G (2007) Acetyl-l-carnitine ameliorates hypobaric hypoxic impairment and spatial memory deficits in rats. Eur J Pharmacol 570:97–107
Bialik S, Cryns VL, Drincic A, Miyata S, Wollowick AL, Srinivasan A, Kitsis RN (1999) The mitochondrial apoptotic pathway is activated by serum and glucose deprivation in cardiac myocytes. Circ Res 85:403–414
Brodhun M, Bauer R, Patt S (2004) Potential stem cell therapy and application in neurotrauma. Exp Toxicol Pathol 56:103–112
Calderón EAR, Galarza RA, Faletti AG (2020) 3-Methylcholanthrene impacts on the female germ cells of rats without causing systemic toxicity. Toxicology 429:152328
Chavakis E, Urbich C, Dimmeler S (2008) Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol 45:514–522
Collins MK, Perkins GR, Rodriguez-Tarduchy G, Nieto MA, López-Rivas A (1994) Growth factors as survival factors: regulation of apoptosis. BioEssays 16:133–138
Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED (2006) Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells 24:1054–1064
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Ferreira GC, McKenna MC (2017) l-Carnitine and acetyl-l-carnitine roles and neuroprotection in developing brain. Neurochem Res 42:1661–1675
Fleury C, Mignotte B, Vayssière J-L (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84:131–141
Ghayour MB, Abdolmaleki A, Fereidoni M (2015) Use of stem cells in the regeneration of peripheral nerve injuries: an overview. Neurosci J Shefaye Khatam 3:84–98
Ghayour M-B, Abdolmaleki A, Behnam-Rassouli M, Mahdavi-Shahri N, Moghimi A (2019) Synergistic effects of acetyl-l-carnitine and adipose-derived stromal cells to improving regenerative capacity of acellular nerve allograft in sciatic nerve defect. J Pharmacol Exp Ther 118:254540
Gimble JM, Katz AJ, Bunnell BA (2007) Adipose-derived stem cells for regenerative medicine. Circ Res 100:1249–1260
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309
Gülçin İ (2006) Antioxidant and antiradical activities of l-carnitine. Life Sci 78:803–811
Hollville E, Martin SJ (2016) Measuring apoptosis by microscopy and flow cytometry. Curr Protoc Immunol 112:14–38
Kassem M, Abdallah BM (2008) Human bone-marrow-derived mesenchymal stem cells: biological characteristics and potential role in therapy of degenerative diseases. Cell Tissue Res 331:157–163
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301
Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G (2007) Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol 207:267–274
Kulkarni G, McCulloch C (1994) Serum deprivation induces apoptotic cell death in a subset of Balb/c 3T3 fibroblasts. J Cell Sci 107:1169–1179
Li D, Zhu B, Ding L, Lu W, Xu G, Wu J (2014) Role of the mitochondrial pathway in serum deprivation-induced apoptosis of rat endplate cells. Biochem Biophys Res Commun 452:354–360
Liu Y, Song XD, Liu W, Zhang TY, Zuo J (2003) Glucose deprivation induces mitochondrial dysfunction and oxidative stress in PC12 cell line. J Cell Mol Med 7:49–56
Manfridi A, Forloni G, Arrigoni-Martelli E, Mancia M (1992) Culture of dorsal root ganglion neurons from aged rats: effects of acetyl-l-carnitine and NGF. Int J Dev Neurosci 10:321–329
Martinou J-C, Youle RJ (2011) Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 21:92–101
Palmerini M, Nottola S, Anidito T, Bianchi S, Sato E, Macchiarelli G (2017) A co-culture system supplemented by hormones and growth factors as model to reduce granulosa cell apoptosis in vitro. Int J Reprod Biomed 15:7–8
Pardo OE, Arcaro A, Salerno G, Raguz S, Downward J, Seckl MJ (2002) Fibroblast growth factor-2 induces translational regulation of Bcl-XL and Bcl-2 via a MEK-dependent pathway correlation with resistance to etoposide-induced apoptosis. J Biol Chem 277:12040–12046
Pillich RT, Scarsella G, Risuleo G (2005) Reduction of apoptosis through the mitochondrial pathway by the administration of acetyl-l-carnitine to mouse fibroblasts in culture. Exp Cell Res 306:1–8
Potier E, Ferreira E, Meunier A, Sedel L, Logeart-Avramoglou D, Petite H (2007) Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng 13:1325–1331
Rebouche CJ (2004) Kinetics, pharmacokinetics, and regulation of l-carnitine and acetyl-l-carnitine metabolism. Ann N Y Acad Sci 1033:30–41
Revoltella RP, DalCanto B, Caracciolo L, D’Urso CM (1994) l-carnitine and some of its analogs delay the onset of apoptotic cell death initiated in murine C2. 8 hepatocytic cells after hepatocyte growth factor deprivation. Biochim Biophys Acta (BBA) Mol Cell Res 1224:333–341
Sahara S, Aoto M, Eguchi Y, Imamoto N, Yoneda Y, Tsujimoto Y (1999) Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature 401:168–173
Shi R-Z, Li Q-P (2008) Improving outcome of transplanted mesenchymal stem cells for ischemic heart disease. Biochem Biophys Res Commun 376:247–250
Taglialatela G, Angelucci L, Ramacci M, Werrbach-Perez K, Jackson G, Perez-Polo J (1991) Acetyl-l-carnitine enhances the response of PC12 cells to nerve growth factor. Dev Brain Res 59:221–230
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD (2002) Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105:93–98
Zhang R, Zhang H, Zhang Z, Wang T, Niu J, Cui D, Xu S (2012) Neuroprotective effects of pre-treament with l-carnitine and acetyl-l-carnitine on ischemic injury in vivo and in vitro. Int J Mol Sci 13:2078–2090
Zhu W, Chen J, Cong X, Hu S, Chen X (2006) Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells 24:416–425
Acknowledgments
The authors would like to thank Dr. Ann Paterson for assisting with the English.
Authors' contributions
Participated in research design: Ghayour, Abdolmaleki, Conducted experiments: Ghayour, Abdolmaleki, Behnam, Performed data analysis: Ghayour, Abdolmaleki, Wrote or contributed to the writing of the manuscript: Ghayour, Abdolmaleki, Behnam.
Funding
Research Council of Ferdowsi University of Mashhad.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no Conflict of interest.
Ethical approval
All animal experiments were carried out in accordance with the European Communities Council directive of 24 November 1986(86/609/EEC) and in accordance with local University of Ferdowsi committee for Human and Animal ethics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdolmaleki, A., Ghayour, MB. & Behnam-Rassouli, M. Protective effects of acetyl-l-carnitine against serum and glucose deprivation-induced apoptosis in rat adipose-derived mesenchymal stem cells. Cell Tissue Bank 21, 655–666 (2020). https://doi.org/10.1007/s10561-020-09844-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-020-09844-1