Abstract
The aim of this study is to evaluate the beneficial effect of Myoinositol (MYO) supplement in freezing media on the post thaw sperm quality. Semen samples from 40 normozoospermic men were divided into two aliquots and frozen with simple or 2 mg/mL MYO supplemented freezing medium. Post thaw process including, computer-assissted sperm analysis was used to analyze sperm motility and morphology. Reactive oxygen species was evaluated by the fluorometry of DCFH-DA, as well as total antioxidant capacity and lipid peroxidation were measured based on colorimetric assay by ELISA reader. Eventually, DNA fragmentation was assessed using TUNEL staining. MYO significantly improved progressive motility and normal morphology in treated samples (p < 0.05). Lipid peroxidation (malondialdehyde level) can be diminished in samples were frozen by MYO supplemented freezing media (p < 0.05). While MYO did not affect the amount of ROS (p > 0.05), it was associated with high values of total antioxidant capacity (p < 0.05). DNA integrity was significantly affected by MYO, as in MYO treated samples, DNA fragmentation was decreased compared to control ones (p < 0.001). The findings support the use of 2 mg/mL myoinositol supplemented freezing media in sperm cryopreservation to increase sperm quality after freezing–thawing procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last years, the field of cryobiology has undergone a great improvement. In the context of the cryobiology of reproductive cells and tissues, sperm cryopreservation has the longest history and is the most widely used in human reproductive medicine, due to their higher cryoresistance, large numbers and straight protocols (Nagy et al. 2017). Sperm banking following cryopreservation is embraced to preserve the future fertility of men who temporarily or permanently lose their fertility due to a variety of reasons, including cancer, lupus, multiple sclerosis and ulcerative colitis (Gupta et al. 2014; Hamada et al. 2012). Sperm cryopreservation is often recommended to patients with urological diseases and surgical procedures, including varicocele, testicular torsion and bilateral vasectomy (Anger et al. 2003; Hamada et al. 2012). Healthy men with occupational exposure to toxic chemicals, ionizing radiation or biological contaminants, moreover males undergoing gender reassignment may also be a potential target population (Anger et al. 2003; Gupta et al. 2010). Ultimately, cryopreservation is widely used to store spermatozoa retrieved from oligozoospermic and azoospermic patients, men with ejaculatory dysfunction or spinal cord injury and in cases of testicular sperm extraction or percutaneous epididymal sperm aspiration to avoid the need for repeated biopsies and aspiration (Varghese et al. 2010).
Despite the general exploitation of cryopreservation in assisted reproductive technology, it is clear that cryopreservation still can cause extensive damage to membranes, alter the functional and metabolic status, disturb the bioenergetics processes by damaging the mitochondria and cause damage to the integrity of genetic material in reproductive cells (Johnston et al. 2012; Kopeika et al. 2014). Reactive Oxygen Species (ROS) in physiological level is necessary for sperm function, but in pathological level should be scavenge immediately by the cumulative sum of the enzymatic and non-enzymatic antioxidants called total antioxidant capacity (TAC). Nevertheless, Sperm cells are sensitive to oxidative stress due to lack of cytoplasmic defenses and the polyunsaturated fatty acids richness of their plasma membrane, which are vulnerable to ROS attack. It is established that cryopreservation can induce oxidative stress (OS) because of imbalance between ROS production and TAC level (Agarwal et al. 2005). It is known that oxidative stress can damage a variety of biomolecules such as carbohydrates, lipids, proteins, and DNA, and thus can negatively affect membrane integrity, viability, motility, morphology and fertility potential of the cryopreserved sperms (Aitken et al. 2010; Kao et al. 2008; Vatannejad et al. 2017). High levels of ROS causing damage to the mitochondrial membrane and may thus compromise mitochondrial membrane potential (MMP), ATP production and consequently sperm function, on the other hand, the damaged mitochondrial membrane causing increased ROS production (Alizadeh et al. 2016; Wang et al. 2003). Moreover it has been shown that malondialdehyde (MDA) as a product of lipid peroxidation induced by oxidative stress, negatively correlate with sperm viability, motility and morphology (Benedetti et al. 2012; Das et al. 2009).
In order to achieve the optimal sperm cryopreservation outcomes, many factors including composition of cryopreservation medium, the way of packaging the samples, freezing and thawing duration and etc. have been assessed (Ansari et al. 2011; Meamar et al. 2012; Pavlovych et al. 2016). According to studies, one of the effective strategies for overcoming the sperm cryopreservation problems, is sperm freezing media supplementation with antioxidants agents such as vitamin E, vitamin C, taurine, selenium and glutathione (Branco et al. 2010; Brugnon et al. 2013; Ghorbani, et al. 2016; Kalthur et al. 2011; Rezaeian et al. 2016).
Myoinositol (MYO), the most important form of inositol in nature, belongs to the vitamin B complex group1 and it is produced by the human body. MYO plays a crucial role in cell morphogenesis and cytogenesis, involved in cell membrane formation, lipid synthesis and cell growth (Eisenberg Jr and Parthasarathy 1987). MYO is a precursor of second messengers in the cellular signal transduction system and consequently participates in the regulation of calcium intracellular concentration (Marat and Haucke 2016). Therefore, it plays crucial role in insulin sensitization, metabolic alterations and particularly reproduction (Santamaria et al. 2012, 2016). MYO concentration is significantly higher in the seminiferous tubules than in the serum (Chauvin and Griswold 2004). In male reproductive organs, MYO is mainly produced by Sertoli cells in response to follicle-stimulating hormone (FSH) and is involved in processes that include the regulation of maturation, motility, capacitation and acrosome reaction of sperm cells (Bevilacqua et al. 2015). It has been suggested that MYO may play a role in the osmoregulation of seminal fluid (Chauvin and Griswold 2004). In addition, it acts in the human sperm chemotaxis and thermotaxis through activation of phospholipase C, resulting in the production of InsP3 and opening of calcium channels. In this regard, MYO causes an increase of cytosolic calcium concentration and consequently an increase of mitochondrial Ca2+ that stimulates the oxidative mechanism and the ATP production, improving mitochondrial function of spermatozoa, preventing apoptosis, and facilitating chromatin compactness (Condorelli et al. 2011). Several in vivo/vitro studies tested myoinositol as possible antioxidant agent for the treatment of male infertility, in order to improve spermatozoa quality and subsequently fertilization (Korosi et al. 2017; Poverini et al. 2014; Rubino et al. 2015).
However, there are no literature publications on the effects of MYO on optimization of human sperm cryopreservation outcomes. This research aimed to evaluate the effect of myoinositol on different bio functional parameters of human sperm after freezing–thawing procedures.
Materials and methods
Patient selection
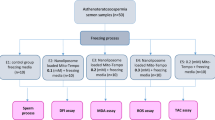
This study was approved by the Ethical Committee of Iran University of Medical Sciences and informed consent was obtained from all individual participants included in the study. Forty healthy men, aged 25–40 years, who have been referred to Akbarabadi and Mehr IVF clinic, were enrolled in this study. Men with history positive for cryptorchidism or varicocele, microrchidism, systemic diseases, accessory gland infection, cigarette smoke, alcohol intake and recent hormonal treatment were excluded.
Semen samples collection and freezing–thawing procedures
Semen samples were collected after 3–5 days of sexual abstinence and after liquefaction were analyzed according to the World Health Organization criteria 2010 (Organization 2010). Samples with normal semen analysis divided into two aliquots: First, frozen semen sample with treatment by 2 mg/mL MYO, according to Condorelli (2011), Second, frozen semen sample without treatment as a control. Both samples were cryopreserved by sperm slow freezing method (Anger et al. 2003). After one month, the specimens were thawed at room temperature and different biofunctional parameters were assessed.
Computer assisted semen analysis (CASA)
The CASA system was used in this study. Sperm parameters including motility and morphology were calculated according to WHO guidelines (2010) after thawing (Organization 2010).
Measurement of ROS
Total production of ROS was measured by detecting Dichlorofluorescin diacetate (DCFH-DA, D6883.SIGMA, Sigma-Aldrich, St. Louis, Missouri, United States) fluorescence in semen that had undergone freezing–thawing process. A 100 mM DCFH-DA was prepared in DMSO. 100 µL of the working solution of DCFH-DA was added to 300 µL of sperm samples. Samples were incubated at 25 °C for 40 min and then analyzed using a fluorometer equipped with 488 nm laser as a light source (Agarwal et al. 2009).
Total antioxidant (TAC) assay
ZellBio TAC assay kit (ZB-TAC-96A, ZellBio GmbH, Germany) was used to quantitative assay Antioxidant Capacity on the basis of the oxidation reduction colorimetric assay. According to manufacturer’s instructions with minor changes, after centrifuging seminal plasma at 600 g for 10 min, 10 µL samples or 10 µL reconstituted Trolox in different concentrations as standard, were mixed with 190 µL prepared working chromogen reagent at each well of microplate. The plate was covered and incubated for 2 min at room temperature. Eventually the absorbance was read at 490 nm using a plate ELISA reader (Agarwal et al. 2009).
Measurement of malondialdehyde (MDA)
Seminal MDA levels were analyzed by ZellBio MAD assay kit (ZB-MDA-96A, ZellBio GmbH, Germany) according to manufacturer’s instructions. Briefly, a 50 µL reagent 4 was added to 50 µL semen samples or different concentrated standards. After adding 1 µL ready chromogen solution in the microtubes, the mixtures were heated for one hour in boiling water bath and cooled in ice bath. Then each microtube was centrifuged for 10 min at 3000–4000 g and the supernatant absorbance was read with microplate reader/ELISA reader at 535 nm (Agarwal et al. 2009).
Sperm DNA Fragmentation (SDF)
The terminal deoxynucleotidyl transferase-mediated fluorescein-dUTP nick end labeling (TUNEL) assay using the In Situ Cell Death Detection Kit (11684795910ROCHE, Roche, Mannheim, Germany) was applied for SDF detection in freezing–thawing semen.
According to the manufacturer’s instruction, semen samples were washed in phosphate-buffered saline (PBS). 100 µL of each sample were resuspended in 100 µL 4% paraformaldehyde and incubated 60 min in 15–25 °C. After centrifuging for 10 min at 300 g for removing fixative, the samples were washed with 200 µL PBS. Thereafter sperm pellets were resuspended in 50 µL of TUNEL reaction mixture for 60 min at 37 °C. The specimens were washed in twice in PBS. The analysis of SDF was performed by Becton–Dickinson FACScan flow cytometer (excitation, 488 nm). SDF was reported as the percentage of spermatozoa emitting green fluorescence at 515–555 nm using the FL‐1 detector. The software used to analyze the data was flowJo software and results presented as the percentage of fluorescent spermatozoa (Mahfouz et al. 2009).
Statistical analysis
Shapiro–Wilk test was used to assess the normality of the variables. The data were compared using the independent Student’s t test and showed as the mean ± SE. Statistical significance was defined as p ≤ 0.05.
Results
Effect of MYO on sperm parameters
MYO in frozen/thawed samples could improve significantly the total and progressive sperm motility compared to control (p < 0.05). The result of sperm morphology revealed that the MYO increased the normal sperm morphology in comparison to the group without MYO (p < 0.05) (Table 1).
Assessment the effect of MYO on TAC and ROS levels
As illustrated in Fig. 1a, the result of TAC level revealed that the MYO could improve total antioxidant capacity in the treated group in comparison to the control (p < 0.001). However, based on Fig. 1b, the level of ROS was attenuated in the treated group with MYO compared to control group, however this attenuation was not significant.
The effect of MYO supplementation on Lipid peroxidation
MDA as a marker of spermatozoa lipid peroxidation was evaluated in samples frozen without/whit 2 mg/mL MYO. Results showed a significant decrease in the MDA production with adding 2 mg/mL MYO to sperm freezing media (p < 0.001, Fig. 2).
Evaluation the effect of MYO on DNA damage
The percentage of sperm DNA fragmentation between the control and treatment group was different, so that TUNEL levels in the control group was significantly higher than the samples were frozen with MYO supplemented freezing media (p = 0.001, Fig. 3a). Representative fluorometric analysis for the SDF levels in both groups are presented in Fig. 3b.
a TUNEL levels in the frozen/thawed samples with simple freezing media or MYO supplemented freezing media. The level of statistical significance was set at p < 0.05 was shown with*, b Fluorometric analysis for the SDF levels in the frozen/thawed samples with simple freezing media or MYO supplemented freezing media
Discussion
It has been proven that freezing–thawing procedure can impress the different parameters of sperm (O’connell et al. 2002). For the first time, present study investigated the effect of myoinositol supplement in freezing media on the post thaw sperm quality and showed that using MYO can optimize sperm parameters.
This study revealed that samples frozen by MYO supplemented freezing media had significantly higher progressive motility and normal morphology than control samples. Condorelli et al. (2012) showed that incubation the fresh semen samples with 2 mg/mL MYO increases significantly progressive and total motility in both normozoospermic men and patients with oligo-astheno-teratozoospermia. Palmieri et al. (2016) confirmed the ameliorant effect of myoinositol on the sperm total and progressive motility in both fresh and thawed samples. Montanino Oliva et al. (2016) found that Andrositol, which contains myoinositol as principal compound, improves sperm motility and morphology in asthenospermic males with metabolic syndrome. MYO plays a role in the chemiotaxis and human sperm thermotaxis through the activation of PLC. It results in production of InsP3 and calcium channels opening leading to an increase in Ca2+ intracellular concentrations in the flagellum and consequently regulates sperm motility (Bahat and Eisenbach 2010). On the other hand, MYO amends semen samples quality in OAT patients by reduction of amorphous fibrous material around spermatozoa removing the amorphous material around spermatozoa and improved morphology of mitochondrial cristae (Calogero et al. 2015).
It is shown that oxidative stress as a result of an inappropriate balance between oxidants and antioxidants, increases during the freezing and thawing process (Chatterjee and Gagnon 2001; Memon et al. 2012). In this study, although the reduction in ROS production was not significant by MYO, total antioxidant capacity level in MYO supplemented cryopreserved samples was higher than control samples. It is proven that due to the possible effect of myoinositol on the cytoplasmic and mitochondrial inner membranes, MYO plays an antioxidant role in sperms of patients with OAT (Colone et al. 2010), men with metabolic syndrome (Montanino Oliva et al. 2016) and erythrocytes of PCOS patients (Donà et al. 2012).
Malondialdehyde as one of the reactive and mutagenic aldehyde products of lipid peroxidation in seminal plasma (Shang et al. 2004) was measured in present study. The results suggest that MYO supplementation in semen freezing media can affect negatively the level of lipid peroxidation of spermatozoa. Sperm plasma membrane is susceptible to lipid peroxidation in oxidative stress condition, due to the high concentration of polyunsaturated fatty acids, consequently lipid peroxidation can lead to loss of membrane fluidity and integrity (Duru et al. 2000). The inverse relationship between antioxidant levels and lipid peroxidation has been proven (Atig et al. 2012; Colagar et al. 2013). Geva et al. (1996) determined that treatment of fertile normospermic men with low fertilization rates by vitamin E, may improve the fertilization rate of them after 1 month of treatment, possibly by reducing the MDA levels. Asadpour, et al. (2012) in their study showed that production of malondialdehyde decreases by addition of 100 IU superoxide dismutase/mL, 0.5 and 1 mM butylated hydroxytoluene to semen extender in chilled bull spermatozoa. The antioxidant effect of MYO on the MDA production in fresh or cryopreserved spermatozoa has not been investigated to date.
Oxidative stress due to freezing–thawing procedure can negatively influence DNA integrity through DNA strand breaks (Kalthur et al. 2011; Meamar et al. 2012; Zandieh et al. 2017). Kalthur et al. (2011) suggested that that supplementation of vitamin E (5 mM) significantly improves the post-thaw motility and DNA integrity in normozoospermic and asthenozoospermic semen samples. Also Branco, et al. indicated that the addition of ascorbic acid as an antioxidant to semen samples of infertile men before cryopreservation can reduces DNA damage. In present study the effect of MYO on DNA fragmentation was assessed by TUNEL test and results revealed that myoinositol may protect DNA fragmentation in frozen-thawed semen samples. Since sperm DNA susceptibility to increased oxidative stress because of poor DNA repair mechanisms and low levels of cytoplasmic antioxidant enzymes (Agarwal et al. 2008; Prakash et al. 2014), MYO as an antioxidant agent, may protects DNA against ROS induced damages (Condorelli et al. 2017; Montanino Oliva et al. 2016).
In conclusion, this study showed that MYO is able to ameliorate the frozen-thawed sperm quality in men with normal sperm parameters. If these results are confirmed by other studies, possible use of MYO supplementation in freezing media for the optimization of sperm cryopreservation success is recommended.
References
Agarwal A, Prabakaran SA, Said TM (2005) Prevention of oxidative stress injury to sperm. J Androl 26(6):654–660
Agarwal A, Makker K, Sharma R (2008) Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol 59(1):2–11
Agarwal A, Varghese AC, Sharma RK (2009) Markers of oxidative stress and sperm chromatin integrity Mol Endocrinol (pp 377–402): Springer
Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI (2010) Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod 25(10):2415–2426
Alizadeh R, Navid S, Abbasi N, Yari A, Mazaheri Z, Daneshi E, Abbasi M (2016) The effect of aminoguanidine on sperm motility and mitochondrial membrane potential in varicocelized rats. Iran J Basic Med Sci 19(12):1279
Anger JT, Gilbert BR, Goldstein M (2003) Cryopreservation of sperm: indications, methods and results. J Urol 170(4):1079–1084
Ansari MS, Rakha BA, Andrabi SM, Akhter S (2011) Effect of straw size and thawing time on quality of cryopreserved buffalo (Bubalus bubalis) semen. Reprod Biol 11(1):49–54
Asadpour R, Jafari R, Tayefi-Nasrabadi H (2012) The effect of antioxidant supplementation in semen extenders on semen quality and lipid peroxidation of chilled bull spermatozoa. Iran J Vet Res 13(3):246–249
Atig F, Raffa M, Ali HB, Abdelhamid K, Saad A, Ajina M (2012) Altered antioxidant status and increased lipid per-oxidation in seminal plasma of tunisian infertile men. Int J Biol Sci 8(1):139
Bahat A, Eisenbach M (2010) Human sperm thermotaxis is mediated by phospholipase C and inositol trisphosphate receptor Ca2 + channel. Biol Reprod 82(3):606–616
Benedetti S, Tagliamonte MC, Catalani S, Primiterra M, Canestrari F, De Stefani S, Bulletti C (2012) Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod Biomed Online 25(3):300–306
Bevilacqua A, Carlomagno G, Gerli S, Montanino Oliva M, Devroey P, Lanzone A, Bizzarri M (2015) Results from the international consensus conference on myo-inositol and D-chiro-inositol. Gynecol Endocrinol 31(6):441–446
Branco CS, Garcez ME, Pasqualotto FF, Erdtman B, Salvador M (2010) Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology 60(2):235–237
Brugnon F, Ouchchane L, Pons-Rejraji H, Artonne C, Farigoule M, Janny L (2013) Density gradient centrifugation prior to cryopreservation and hypotaurine supplementation improve post-thaw quality of sperm from infertile men with oligoasthenoteratozoospermia. Hum Reprod 28(8):2045–2057
Calogero A, Gullo G, La Vignera S, Condorelli R, Vaiarelli A (2015) Myoinositol improves sperm parameters and serum reproductive hormones in patients with idiopathic infertility: a prospective double-blind randomized placebo-controlled study. Andrology 3(3):491–495
Chatterjee S, Gagnon C (2001) Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol Reprod Dev 59(4):451–458
Chauvin TR, Griswold MD (2004) Characterization of the expression and regulation of genes necessary for myo-inositol biosynthesis and transport in the seminiferous epithelium. Biol Reprod 70(3):744–751
Colagar AH, Karimi F, Jorsaraei SGA (2013) Correlation of sperm parameters with semen lipid peroxidation and total antioxidants levels in astheno-and oligoasheno-teratospermic men. Iran Red Crescent Med J 15(9):780
Colone M, Marelli G, Unfer V, Bozzuto G, Molinari A, Stringaro A (2010) Inositol activity in oligoasthenoteratospermia–an in vitro study. Eur Rev Med Pharmacol Sci 14(10):891–896
Condorelli R, La Vignera S, Di Bari F, Unfer V, Calogero A (2011) Effects of myoinositol on sperm mitochondrial function in-vitro. Eur Rev Med Pharmacol Sci 15(2):129–134
Condorelli RA, La Vignera S, Bellanca S, Vicari E, Calogero AE (2012) Myoinositol: does it improve sperm mitochondrial function and sperm motility? Urology 79(6):1290–1295
Condorelli R, La Vignera S, Mongioi L, Vitale S, Lagana A, Cimino L, Calogero A (2017) Myo-inositol as a male fertility molecule: speed them up! Eur Rev Med Pharmacol Sci 21(2 Suppl):30–35
Das P, Choudhari A, Singh A, Singh R (2009) Correlation among routine semen parameters, sperm viabilty and malondialdehyde levels in human subjects with different fertility potential
Donà G, Sabbadin C, Fiore C, Bragadin M, Giorgino FL, Ragazzi E, Armanini D (2012) Inositol administration reduces oxidative stress in erythrocytes of patients with polycystic ovary syndrome. Eur J Endocrinol 166(4):703–710
Duru NK, Morshedi M, Oehninger S (2000) Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril 74(6):1200–1207
Eisenberg F Jr, Parthasarathy R (1987) Measurement of biosynthesis of myo-inositol from glucose 6-phosphate. Methods Enzymol 141:127–143
Geva E, Bartoov B, Zabludovsky N, Lessing JB, Lerner-Geva L, Amit A (1996) The effect of antioxidant treatment on human spermatozoa and fertilization rate in an in vitro fertilization program. Fertil Steril 66(3):430–434
Ghorbani M, Vatannejad A, Khodadadi I, Amiri I, Tavilani H (2016) Protective effects of glutathione supplementation against oxidative stress during cryopreservation of human spermatozoa. CryoLetters 37(1):34–40
Gupta S, AA, Sharma R, Ahmady A (2014) Sperm banking via cryopreservation Rizk Botros RMB, Aziz N, Agarwal A, Sabanegh E Medical and Surgical Management of Male Infertility. New Delhi Jaypee Brothers Medical Publishers Ltd
Gupta S, Agarwal A, Sharma R, Ahmady A (2010) Recovery, preparation, storage and utilization of spermatozoa for fertility preservation in cancer patients and sub-fertile men. J Reprod Stem Cell Biotechnol 1(2):150–168
Hamada A, Wasik M, Gupta S, Agarwal A (2012) Sperm banking: indications and regulations. Infertility: diagnosis, management and IVF. Jaypee Brothers Medical Publishing, New Delhi, pp 409–434
Johnston S, Satake N, Zee Y, López-Fernández C, Holt WV, Gosalvez J (2012) Osmotic stress and cryoinjury of koala sperm: an integrative study of the plasma membrane, chromatin stability and mitochondrial function. Reproduction 143(6):787–797
Kalthur G, Raj S, Thiyagarajan A, Kumar S, Kumar P, Adiga SK (2011) Vitamin E supplementation in semen-freezing medium improves the motility and protects sperm from freeze-thaw–induced DNA damage. Fertil Steril 95(3):1149–1151
Kao S-H, Chao H-T, Chen H-W, Hwang TI, Liao T-L, Wei Y-H (2008) Increase of oxidative stress in human sperm with lower motility. Fertil Steril 89(5):1183–1190
Kopeika J, Thornhill A, Khalaf Y (2014) The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update 21(2):209–227
Korosi T, Barta C, Rokob K, Torok T (2017) Physiological Intra-Cytoplasmic Sperm Injection (PICSI) outcomes after oral pretreatment and semen incubation with myo-inositol in oligoasthenoteratozoospermic men: results from a prospective, randomized controlled trial. Eur Rev Med Pharmacol Sci 21(2 Suppl):66–72
Mahfouz RZ, Sharma RK, Said TM, Erenpreiss J, Agarwal A (2009) Association of sperm apoptosis and DNA ploidy with sperm chromatin quality in human spermatozoa. Fertil Steril 91(4):1110–1118
Marat AL, Haucke V (2016) Phosphatidylinositol 3‐phosphates—at the interface between cell signalling and membrane traffic. EMBO J e201593564
Meamar M, Zribi N, Cambi M, Tamburrino L, Marchiani S, Filimberti E, Forti G (2012) Sperm DNA fragmentation induced by cryopreservation: new insights and effect of a natural extract from Opuntia ficus-indica. Fertil Steril 98(2):326–333
Memon AA, Wahid H, Rosnina Y, Goh Y, Ebrahimi M, Nadia F (2012) Effect of antioxidants on post thaw microscopic, oxidative stress parameter and fertility of Boer goat spermatozoa in Tris egg yolk glycerol extender. Anim Reprod Sci 136(1–2):55–60
Montanino Oliva M, Minutolo E, Lippa A, Iaconianni P, Vaiarelli A (2016) Effect of myoinositol and antioxidants on sperm quality in men with metabolic syndrome. Int J Endocrinol 2016. https://doi.org/10.1155/2016/1674950
Nagy ZP, Varghese AC, Agarwal A (2017) Cryopreservation of Mammalian Gametes and Embryos. Methods Mol Biol 1568
O’connell M, McClure N, Lewis S (2002) The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod 17(3):704–709
Organization WH (2010) WHO laboratory manual for the examination and processing of human semen
Palmieri M, Papale P, Della Ragione A, Quaranta G, Russo G, Russo S (2016) In vitro antioxidant treatment of semen samples in assisted reproductive technology: effects of myo-inositol on nemaspermic parameters. Int J Endocrinol 2016. https://doi.org/10.1155/2016/2839041
Pavlovych O, Revenko O, Gapon G (2016) Optimization of thawing regimen for cryopreserved human sperm at normo-and pathospermia. Probl Cryobiol Cryomed 26(1):45–52
Poverini R, Carlomagno G, Lisi R, Lisi F, Oliva MM (2014) Improving IUI outcomes by adding myo-inositol to the semen preparation procedure. Fertil Steril 102(3):e334
Prakash S, Prithiviraj E, Suresh S, Lakshmi NV, Ganesh MK, Anuradha M, Dinesh P (2014) Morphological diversity of sperm: a mini review. Iran J Reprod Med 12(4):239
Rezaeian Z, Yazdekhasti H, Nasri S, Rajabi Z, Fallahi P, Amidi F (2016) Effect of selenium on human sperm parameters after freezing and thawing procedures. Asian Pac J Reprod 5(6):462–466
Rubino P, Palini S, Chigioni S, Carlomagno G, Quagliariello A, De Stefani S, Bulletti C (2015) Improving fertilization rate in ICSI cycles by adding myoinositol to the semen preparation procedures: a prospective, bicentric, randomized trial on sibling oocytes. J Assist Reprod Genet 32(3):387–394
Santamaria A, Giordano D, Corrado F, Pintaudi B, Interdonato M, Vieste GD, D’Anna R (2012) One-year effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome. Climacteric 15(5):490–495
Santamaria A, Di Benedetto A, Petrella E, Pintaudi B, Corrado F, D’Anna R, Facchinetti F (2016) Myo-inositol may prevent gestational diabetes onset in overweight women: a randomized, controlled trial. J Maternal Fetal Neonatal Med 29(19):3234–3237
Shang X-J, Li K, Ye Z-Q, Chen Y-G, Yu X, Huang Y-F (2004) Analysis of lipid peroxidative levels in seminal plasma of infertile men by high-performance liquid chromatography. Arch Androl 50(6):411–416
Varghese AC, Nandi P, Mahfouz R, Athayde KS, Agarwal A (2010) Human sperm cryopreservation Rao AR, Agarwal A, Srinivas MS. Andrology laboratory manual. Jaypee Brothers Medical Publishers Ltd., New Delhi
Vatannejad A, Tavilani H, Sadeghi MR, Amanpour S, Shapourizadeh S, Doosti M (2017) Evaluation of ROS-TAC score and DNA damage in fertile normozoospermic and infertile asthenozoospermic males. Urol J 14(1):2973–2978
Wang X, Sharma RK, Gupta A, George V, Thomas AJ, Falcone T, Agarwal A (2003) Alterations in mitochondria membrane potential and oxidative stress in infertile men: a prospective observational study. Fertil Steril 80:844–850
Zandieh Z, Vatannejad A, Doosti M, Zabihzadeh S, Haddadi M, Bajelan L, Amanpour S (2017) Comparing reactive oxygen species and DNA fragmentation in semen samples of unexplained infertile and healthy fertile men. Irish J Med Sci 187:1–6
Acknowledgements
We wish to thank Dr.Joghataei for his kind courtesy and attempt to improve the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the national research committee.
Financial support
This research was supported by Shahid Akbarabadi Clinical Research Unit, Iran University Of Medical Sciences.
Rights and permissions
About this article
Cite this article
Mohammadi, F., Varanloo, N., Heydari Nasrabadi, M. et al. Supplementation of sperm freezing medium with myoinositol improve human sperm parameters and protects it against DNA fragmentation and apoptosis. Cell Tissue Bank 20, 77–86 (2019). https://doi.org/10.1007/s10561-018-9731-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-018-9731-0