Abstract
Direct application of amnion has greater risk of immunological rejection and infection. Decellularization is an effective method to lower the risk of immune complications and infections. The bioreactor assembly with multiple cassettes was designed for decellurization of multiple amnions with different cell types simultaneously in single run. A detergent-based protocol was modified to remove all cellular components from amnion and diminish the DNA content to render it non-immunogenic. Amnion (n = 10) were treated with 2% sodium dodecyl sulphate (SDS), 5% dimethyl sulfoxide (DMSO) and 2% sodium deoxycholeate (SD). Decellularized amnion samples were analyzed by haematoxylin–eosin staining (HE), Alcian blue pH 1 (AB-pH-1), 4,6-diamnionidino-2-phenylindol (DAPI), Massion’s trichrome stain, DNA quantification, mechanical testing and scanning electron microscopy (SEM). Histological analysis showed complete removal of cellular components and the histoarchitecture of scaffold remained intact. Amnion scaffold activated with platelet rich plasma (PRP) and calcium chloride composition supported better adherence to the wound than amnion alone. Only single application showed good healing. In vivo assessment of activated amnion revealed stable dressing. It has good promising outcome. At day 7, histologically the wounds treated with activated amnion were almost closed without scarring and showed well differentiated epidermis, proliferation of keratinocytes, hair follicles and basement membrane as compared to controls and silver nitrate gel dressings in a mouse (Mus musculus). Cryopreservation had no adverse effect on the mechanical properties of the amnion scaffold. Cryopreservation of decellularized amnion by Dulbecco’s modified eagle medium (DMEM) was expected to prepare off-the-shelf skin substitutes and preserve them to be immediately available upon request of patients’ needs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amnion is the thin innermost semitransparent membrane separated from the chorion, devoid of blood vessels, lymphatic, nerves and biological viable which is ideal for creating scaffold (Augustine et al. 2014; Niknejad et al. 2008). It can produce growth factors (Zhang et al. 2013) and has anti-inflammatory (Kesting et al. 2014), anti-microbial, anti-fibrosis, anti-scarring (Niknejad et al. 2008) and analgesic properties (Sheikh et al. 2014). Furthermore, in animal models it shows inhibition of scar formation (Zhang et al. 2016) and low immunogenicity (Castellanos et al. 2016). In major burn cases, an impaired epidermal barrier combined with reduced immunity can result in bacterial sepsis (Plichta et al. 2017). Most wound dressing treatments aim to facilitate regeneration and repair to reduce size of damaged area by providing a moist environment and protecting against bacterial sepsis that would perturb normal healing (Bo et al. 2010; Kuna et al. 2016). In the extensive burn patients, the limited donor site is a constant problem. Wounds usually covered with biological tissue such as amnion, stretched meshed skin graft or synthetic covers or cadaver skin to prevent fluid loss and microbial contamination (Kuna et al. 2016; Maguire and Yarmush 2011). Applicability of these skin substitutes has many limitations such as bacterial sepsis, transmission of infectious diseases, greater graft contraction, delayed healing, scar tissue formation. These substitutes are not easily available; healing rate remains below 50% and need for immunosuppressive drugs to prevent immune rejection (Markeson et al. 2013; Macneil 2007). However these methods require regular dressing which leads to the frequent wound opening. Infection still remains the most commonly reported complication of widely accepted artificial biological dermal substitute like skin grafts, Integra TM (Wen et al. 2016; Aponte et al. 2013; Lohana et al. 2014). These limitations have thus led to the develop a non cellular dermal substitutes which will accelerate the tissue healing, risk free substitute to reduce frequent dressing to manage a burn wound. Various attempts were made to remove all cellular components from amnion to produce a biological substrate and enhance its properties (Ehrenreich and Ruszczak 2006). Fresh amnion scaffold have a short life span (Kraus and Kirker 2006; Amer et al. 2015; Mokbel et al. 2011).Footnote 1 With this background, we invented a bioreactor assembly to remove all cellular components from amnion in effective way within a very short time period. The novel principle behind scaffold activated with PRP and calcium chloride composition is that PRP inhibits the infection and complications (Robiony et al. 2002), deliver cytokines and release multiple growth factors like interferon a, b, interleukins 1, 6, 8 and platelet derived growth factors (PDGF) to the wound bed which can be considered as a effective bioactive dressing help to alleviate inflammatory response and augment healing process. The present work aimed to develop a bioreactor assembly to prepare multiple amnion scaffolds in single run and to study the efficiency of a cryopreserved AM scaffold loaded with PRP and calcium chloride composition in the management of experimentally induced burn wound in a Mus Musculus mouse. Thus the cryopreservation method in DMEM allows storage of amnion for longer period of time, effectively preserve histological, biochemical, and functional properties of the amnion scaffold and sustain its beneficial properties and make it potentially suitable candidate to use it in long run. Therefore clinical need of burn wound treatment will be efficient, easily applicable and available on demand.
Materials and methods
Amnion retrieval
The use of specimens from human subjects was approved by Institutional Review Board (Ref.- 6/IAEC/2017). During 2014 and 2015, the human placenta of five patients was harvested at the time of elective lower segment caesarian section (L.S.C.S.) within 24 h under sterile conditions by obtaining informed consent. All donors had negative serologic tests for HIV, HBV, HCV and syphilis. All routine blood investigations were normal. Placenta was collected in normal saline containing antibiotics (penicillin 1000 U/ml, streptomycin 20 µg/ml, antifungal–amphotericin B 2.5 µg/ml in concentration). Under sterile conditions, the amnion was separated from the chorion by blunt dissection under a laminar air hood. Separated amnion was washed for 2–3 times to remove all blood clots and blood.
Bioreactor assembly (Fig. 1)
The bioreactor was designed to decellularize the amnion. Bioreactor was made of acrylic material, which can be autoclaved. These two different colored cassettes will be helpful for recellurization of amnion with two types of cells one on each side. Each cassette measuring (19 × 14 × 0.3) cm fixed with next cassette (Fig. 1A) by holding an amnion in between these two frames. This assembly was kept in acrylic container so that multiple amnions can be decellularize simultaneously (Fig. 1B, C) and cryopreserved.
Bioreactor assembly. A Bioreactor was made up of pairs of yellow (female holding plate) and pink colored (male holding plate) cassettes (B, C). Cassettes placed in acrylic container over one another. D Under aseptic conditions, the prepared amnion scaffolds of size 19 cm × 14 cm were cryopreserved with cassette. (Color figure online)
Decellularization of amnion
Amnions were decellularized using in-house developed protocols. Amnions were properly fixed on the cassettes of bioreactor of (19 × 14 × 0.3) cm. A detergent-based protocol was modified to remove all cellular components from amnion to make it non-immunogenic. Decellularization of amnion consisted 8–10 cycles carried out by four methods. The processes of decellularization were checked at every 5th cycle with Haematoxylin–Eosin staining (HE) and 4,6-diamnionidino-2-phenylindol (DAPI) staining to confirm cell removal. Freshly collected amnion is used as a control. In method 1, amnion was transferred to a 2% sodium dodecyl sulphate (SDS) (w/v), Sisco Research Laboratories Pvt. Ltd. India, with gentle agitation on shaker (REMI RX-12R-DX) at 180 rpm for 12 h with shaking at room temperature. In method 2, amnion was transferred to 2% sodium deoxycholeate (SD) (w/v), Hi media, India, for 6 h followed by 2% SDS for 6 h. Method 3 was performed using 5% Dimethyl sulfoxide (DMSO) (v/v) Hi media, India for 6 h followed by 2% SDS (w/v) for 6 h. In above three methods, after 12 h amnion was transferred into deionized distilled water and stored in deep freezer overnight. It was thawed next morning at room temperature and cycle was repeated again. In method 4, amnion was treated with 5% DMSO by keeping it overnight at − 40 °C for 12 h. It was thawed next morning at room temperature followed by 6 h wash in distilled water with constant shaking on shaker. After decellularization, it is stored in distilled water containing antibiotics at − 40 °C.
Histological examination
Control and decellularized amnions were investigated at regular interval of cycle. Fresh amnions were used as a control. Specimens were fixed with 10% (w/v) neutral-buffered formalin, dehydrated through alcohol grades and embedded in paraffin wax. The sections of 4 µ were taken by using high profile blades (Thermo scientific, US) with microtome (Leica RM 2235) and stained for extracellular matrix using HE. Sections were treated with DAPI (Invitrogen, CA, USA) for 1 min and mounted in fluorescent mounting medium (Dako, Denmark). Stained sections were examined under fluorescent microscope (Nikon Ti-S, Eclips, Japan). An estimation of collagen content was made by Massion’s trichrome stain. Sections were stained with Weigert’s iron hematoxylin working solution and Biebrich scarlet − 1% acid fuchsin solution, differentiated in phosphomolybdic–phosphotungstic acid solution and transferred directly (without rinse) to aniline blue solution. GAG content of samples was determined using Alcian blue pH (AB pH-1) staining.
Scanning electron microscopy
Rectangular shaped full-thickness decellularised and control amnion of 2 mm width and 5 mm length, were obtained for the SEM. Native amnion and decellularized scaffolds were processed for SEM. Samples were fixed in 2.5% glutaraldehyde in phosphate buffered saline (PBS) 0.06 mol (pH 7.3). The specimens for SEM were dehydrated in oven at 37 °C and coated with gold. The samples were fixed on stage with double sided tape. The height of the sample stage adjusted with sample preparation tool before placement of the specimen and stage into the SEM. Proper height adjustment was important to make sure the stage and sample cleared the ceiling of the sample feed and was high enough for the SEM to bring an image of the amnion surface into focus. SEM was conducted by using a JEOL scanning electron microscope (JEOL JSM-6360 model). Several sets of images were taken of each sample at the scales of 1, 2, 10, 20, 50, 100 and 200 µm.
DNA quantification
Residual DNA was quantified in fresh and decellularized amnion using UV Spectrophotometer (Shimadzu corp.) at 260 nm. Samples of control and decellularized amnion by all four methods were collected at concentration of 1 mg/ml and allowed to freeze at − 20 °C. After 15 min, all samples were crushed with mortar pastel and D/W was added to it. This DNA solution was poured in 2 ml micro centrifuge tubes which was used to obtain DNA content. Prepared 40 µl DNA solution of solution was taken using Hamilton syringe in 4 ml cuvette to which 3.96 ml of D/W was added. At most care was taken to ensure that solution is air bubble free. Solution was kept for 10 min to ensure the complete diffusion of DNA throughout the solution. This represents a 1:100 dilution of the standards and DNA samples. The spectrophotometer was set at 260 nm and readings were noted down.
Mechanical testing
Mechanical testing was conducted using a displacement-controlled setup, with two horizontal hydraulic actuators. Control and decellularized amnion was cut precisely into cylindrical-shaped strips with a surgical scalpel to obtain the required dimensions of specimens of 3 cm length for mechanical analysis. Tissue samples were kept at − 20 °C for 4 h in order to minimize dehydration. Samples were tested within 4 h of cold ischemia after retrieval. Tensile properties were performed using uniaxial testing rig on a tensometer. Burst pressure was determined using a modified syringe pump to increase pressure on the 3 cm long scaffolds until failure. The both end of scaffold was sealed with a pressure transducer to determine the ultimate pressure at failure. The sandpaper was used to improve the grip of the tissue samples. Each test was digitally recorded to measure amnion distention at a given pressure using Image J software. In mechanical testing, force threshold was automatically detected by force sensors. Amnion decellularised by each method was tested according to a monotonic uniaxial tension protocol till it ruptures. The clamping jigs in which the amnions were hold, were displaced with the axis displacements.
Cryopreservation of scaffold
The major drawback regarding biological scaffold is preserving and storing. Maintaining large number of scaffolds is an important issue. To ensure a steady supply of amnion scaffolds and unpredictable demand, cryopreservation of scaffold is needed (Haddad et al. 2017). The scaffolds with opaque, transparent white colour, free from tears and holes, not having any unpleasant odour and discolouration were selected for cryopreservation. In a laboratory, in biosafety cabinet, under aseptic conditions, the prepared amnion scaffolds of size 19 cm × 14 cm were cryopreserved with cassette (Fig. 1D). The preservation media consists 5% DMSO in Dulbecco Modified Eagle Medium (DMEM) along with 50 units/ml penicillin, 0.5 mg/ml amphotericin B, 25 μg/ml streptomycin and stored at − 80 °C until required.
Stability of cryopreserved scaffolds
Cryopreserved amnions were allowed to thaw completely at room temperature to prevent the damage. The DMSO media was removed by washing with normal saline. To ensure the further condition of the cryopreserved amnion, infectious screening and mechanical testing was carried out as explained above.
PRP preparation
PRP was prepared from autologous blood by a modified method. PRP is prepared from fresh whole blood collected from a peripheral vein, stored in anticoagulant EDTA and processed to separate the components of blood (Yamaguchi et al. 2012; Amable et al. 2013). 15 ml of venous blood was drawn from the syringe pre-filled with 5 ml of EDTA and centrifuged immediately at 200 g for 10–15 min. The blood gets separated into three layers: red blood cells at the bottom, a buffy coat layer in middle and cellular plasma in the supernatant. The plasma layer and buffy coat were transferred into another tube and re-centrifuged at 500×g for 10 min and PRP was obtained (Chang et al. 2015).
Animal study
Cryopreserved scaffolds activated with PRP and calcium chloride. Biocompatibility of these scaffolds was assessed by burn wound dressing of mouse. Six month old female mice (Mus Musculus) weighing between 50 and 60 g were used for experiments. The animal study was approved by Institutional Animal Ethical Committee (IAEC). They were kept according to principles of laboratory animal care and water and food adlibitum. The dorsum of each mouse was shaved from base of neck to base of tail and skin cleaned with betadine solution and then washed with sterile solution. For burn injury experiments, 3 mice were anesthetized with subcutaneous, extra-peritoneal injection of Thiosol 1 g/vial, (Neon laboratories Ltd.) at a dose of 12 units/gm of body weight. A 1 cm × 0.5 cm burn injury was done at three places on dorsum by heating end of spatula on spirit lamp for 1 min and placing it on dorsum of mice for 15 s and removed it immediately. The frame was purposely applied to inhibit the mouse wound healing by contraction and enable the wound closure mainly by cell migration from the surrounding skin. Wound dressing was performed with activated amnion scaffold, silver nitrate gel (0.2% w/v, Virchow Biotech Ltd., India) and one burn wound was kept without dressing for natural healing. Dressing were accomplished every third day according to requirement. Evaluation of wound was undertaken by photography. Animals were sacrificed by cervical dislocation on day 7. The status of scar formation, inflammation and infection of burn wound after dressing was evaluated by two investigators with score none (0), mediate (1) and intense (2).
Biopsy samples were taken for analysis of epithelization. Histological analysis of wound healing was done by HE staining and histochemical analysis were done by Massion’s trichrome staining and AB-pH 1 staining technique as described previously. Skin biopsies were fixed in 10% formalin for 24 h. All samples were then dehydrated with ethanol solutions of increasing gradient concentrations and then cleared in xylene. The dehydrated samples were embedded in paraffin wax and sectioned. Sections of 4 μm in thickness were then deparaffinized and stained using hematoxylin and eosin and Massion trichrome stain.
Results
Histological and histochemical comparison of fresh and decellularized amnion
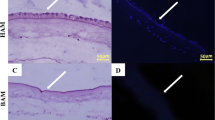
A histological assessment of the Control and decellularized amnion was done by HE, AB pH-1, DAPI, Massion’s trichrome to establish whether the treatment successfully removed cellular components and restored the histoarchitecture of scaffold (Fig. 2).
Histochemical comparison of fresh and decellularized amnion. A HE stained decellularized amnion in all methods showed no cells or cell fragments. HE staining in Control showed better organized and differentiated layer of epithelial stratification lining with cuboid to columnar, compact alignment. B DAPI staining in control showed intact nucleus primarily located at the apical border of amnion. Method 2 showed complete decellularization earlier than other methods. In method 2, decellularization is observed after 10th cycle. C Massion trichrome staining in control showed intact appearance of collagen and elastin with no obvious disruptions to collagen and elastin. The distribution and quantity of collagen remain unchanged after decellularization in all methods. D AB pH-1 staining demonstrated that the GAG have been conserved and its distribution appeared to remain unchanged after decellularization as compared to AB pH-1 staining in fresh amnion. E SEM analysis of decellularized amnion by all four methods revealed not much apparent changes or loss in density of ECM and collagen but amnion decellularized by method 2 showed better ECM including parallel arrangement of collagen
Fresh and decellularized amnion was stained using HE stain to establish whether the treatment successfully removed cellular components. Control amnion showed differentiated layer with clear epithelial stratification lining at day 0. The epithelium was better organized in the control amnion; cells were cuboid to columnar, smaller, more compact, and aligned with clear demarcation. HE staining confirmed that the process was successful and no cells or cell fragments were remains in decellularized amnion. The cells had decreased considerably by day 15 in all methods. The amnion treated by the SD (6 h)-SDS (6 h)-D/W get decellularized earlier as compared to the other methods. In this method, nucleus disappeared almost completely by 10th cycle (Fig. 2A).
DAPI analysis showed that nucleus was primarily located at the apical border of amnion and appeared to be intact in control. It was observed that complete decellularization was achieved by 15th cycle in all methods. Complete decellularization was observed by method 2 after 10th cycle (Fig. 2B).
In control, Massion’s trichrome staining showed intact appearance of collagen and elastin. Massion’s trichrome staining demonstrated no obvious disruptions to overall histoarchitecture of amnion following treatment. Collagen and elastin appeared to have been conserved and appeared to be intact. The distribution and quantity of collagen remain unchanged after decellularization (Fig. 2C).
AB pH-1 staining demonstrated no obvious disruption to the overall GAG’s following treatment. The GAG, major structural components of amnion appeared to have been conserved. The distribution and quantity of GAG’s appeared to remain unchanged after decellularization in fresh and decellularized amnion. GAG concentration suggests that the scaffold provides a proper environment for cellular migration and proliferation, facilitating extensive ECM remodeling (Fig. 2D).
SEM was used to assess damage to collagen fibers and density of ECM present in amnion. Analysis revealed no apparent changes or loss were noted in density of ECM and collagen in decellularized amnion. All ECM and collage were observed as similar as to that of collagen fibers in control and decellularized amnion. SEM of Method 2 showed preserved ECM including parallel arrangement of collagen. In Method 3, ultra structure of the ECM appeared to be well-preserved following decellularization with bundles of fibrous collagen; at higher magnification nano-fibrous collagen with minimal damage to the matrix during decellularized protocol was observed. Method 4 showed fine collagen network architecture and parallel arranged collagen fibers (Fig. 2E).
Quantification of residual DNA
The UV Spectrophotometer (Shimadzu corp.) at 260 nm was used to quantify residual DNA present in the matrix after decellularization. The DNA content of amnion in control was found to be 0.054 ug/ul. During the decellularization process, DNA content was reduced in each cycle. DNA content of amnion decellularized by method 2 showed less DNA content as compared to other decellularization methods (Table 1). Method 1 and method 3 showed almost same results. After decellularization, a significant drop to 0.0035 ug/ul was observed (P < 0.05; ANOVA) (Fig. 3).
Mechanical testing
Elasticity, elongation, fracture toughness, resistance, stress rupture, and fatigue limit of amnion is confirmed by mechanical testing. Thickness measurements of the amnion were taken to standardize the tensile loading. The mechanical testing analysis resulted that the ultimate tensile strength, ultimate circumferential stress, elasticity and burst pressure for the fresh and decellularized amnion was not significantly different. Amnions decellularized by method 2 and method 3 showed there was not much significant difference in ultimate strain and elasticity but method 1 and method 4 showed significant loss of ultimate strain and elasticity (Fig. 4).
Mechanical analysis. The ultimate circumferential stress, ultimate tensile strength, elasticity and burst pressure for control and scaffold was not significantly different. Ultimate strain and elasticity behavior in method 2 and method 3 were almost same. Method 1 and method 4 showed significant loss of ultimate strain and elasticity. The overall decellularization process and detergents used had no adverse effect on the ultimate stress, strain, ultimate tensile strength, elasticity and burst pressure. These results of control and scaffold indicate that there is no measurable influence in elasticity, elongation, fracture toughness, resistance, stress rupture, and fatigue limit
This indicates that the decellularization process had no adverse effect on the overall strength of the amnion scaffold. The process of decellularization and detergents used did not compromise the strength of the prepared scaffold. There was no significant difference in transition strain, transition stress or failure strain of decellularized amnion scaffold from those of fresh samples of amnion. Values obtained of mechanical testing are drawn into Table 2.
Cryopreservation of scaffold
Cryopreservation of decellularized amnion reduces the burden of burn patients and to fulfill the need of clinical demand of artificial skin. In our present study, we preserved the scaffold for 6 months. Experimental techniques, mechanical testing and screening for infections were carried out at every month. Cryopreserved scaffold showed effective functional properties and no major difference in accordance to previous tests. The study of cryopreserved scaffolds was evaluated for 6 months. The cryopreservation process did not alter the properties of scaffolds.
Mechanical testing of scaffold after cryopreservation
The cryopreservation of amnion effectively preserved mechanical and functional properties of the amnion. The cryopreserved amnion exerted good anti-inflammatory and anti-scarring effects. In the present study, we have preserved and analyzed the amnion scaffolds in − 80. The scaffolds were tested for mechanical analysis. The values of mechanical testing of amnion after cryopreservation resulted in no statistically significant differences for Young’s modulus, ultimate circumferential stress, elasticity and burst pressure summarized in Table 3.
Animal study
To evaluate the status of wound healing after dressing clinical signs of infection, inflammation and scar formation estimated by two investigators with score none (0), mediate (1) and intense (2). Wound evaluation showed lowest score over activated amnion treated wounds and significant increase in silver nitrate treated and control wound. This reflects a positive outcome as a low score and negative outcome as high score (Table 4). Wound healing was evaluated visually for re-epithelization, wound contraction and achievement of skin (Fig. 5).
Macroscopic evaluation of wound healing and wound contraction. Wound healing evaluated for re-epithelization, wound contraction and achievement of skin. Activated amnion treated group presented better outcome than supported wound treated with silver nitrate gel concerning wound contraction. No any local inflammation and infection was associated with wound and without any disturbance to general condition of animals. Contraction of wound treated with activated amnion scaffold is markly faster than the burn wound treated with routine traditional methods like silver nitrate gel application and control wound. This visual evaluation proves that the activated amnion accelerates the wound healing
Amnion activated with PRP and calcium chloride showed better adherence to wound as compared to unactivated decellularized amnion. The PRP with calcium chloride composition release cytokines growth factors (GFs) including vascular endothelial growth factor (VEGF), transform-ing growth factor (TGF), platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) which increase the bioactive properties of PRP and thus amnion scaffold. Concerning wound contraction activated amnion treated group presented better outcome than supported wound treated with silver nitrate gel. No any local inflammation and infection was associated with wound and without any disturbance to general condition of animals. Activated amnions showed the keratinocyte growth. Amnion scaffold accelerated wound healing and facilitate efficient cell migration growth and skin regeneration. This shows the biocompatibility of a scaffold. This study explores the scaffold’s functionality for better cell growth and accelerates the wound healing. In addition to antibacterial properties of amnion activated by PRP and calcium chloride composition, it has reported the possible pro-healing properties. The use of amnion in the past has been restrained by the need to produce it as bacteria free, microbial free thereby increasing the potential side effects. Decellurization has provided a way of producing pure amnion. Activated amnion also markedly increases the rate of healing as compared to other traditional methods. Herein we report that activated amnion can promote wound healing and reduce scar formation. Furthermore, our studies show that activated amnion act by decreasing inflammation. Decellularized amnion supported the fibroblast and keratinocyte growth. In amnion treated wound, most of the cells are spindle like. Hair follicles were prominently visualized. Burn wound treated with amnion showed a well differentiated epidermis and basement membrane. It also showed an even surface and a pink coloration. The potential benefits of such type of amnion in burn wounds can therefore be enormous. The epidermis was well stratified with columnar basal cells along with many small blood vessels. It indicates better cell recruitment from the surrounding healthy tissue (Fig. 6).
Histological analysis of wound healing. In HE, SN gel treated wounds showed good cell proliferation at 7th day. SN gel accelerated the keratinocyte growth, epidermis was well striatified. But at 14th day, hair follicles were not visualized. Wound treated with amnion scaffold accelerated the regeneration of skin. At 7th day, activated amnion supported the fibroblast and keratinocyte growth. Most of the cells were spindle shaped. The epidermis was well stratified with columnar basal cells along with many small blood vessels. At 14th day, activated amnion dressing showed well differentiated epidermis and basement membrane. Hair follicles were prominently visualized. It also showed an even surface and a pink colouration at wound site. In amnion treated wound, massion trichrome staining showed the dense collagen fiber networks and fibroblast layers better than SN treated wound. The activated amnion dressing provided a structural support for the attachment and migration of cells and further induces the cell proliferation. This shows the biocompatibility of a scaffold
In massion Trichrome, SN gel treated wound showed intact appearance of collagen and elastin with no obvious disruptions to collagen and elastin. The distribution and quantity of collagen remain unchanged after decellularization. Activated amnion scaffold treated wound explored efficient cell migration and well differentiated hair follicles. These results explore the activated amnion’s functionality for better cell growth and acceleration of the wound healing.
Discussion
In the present study, we standardized a bioreactor assembly to decellularize the multiple amnions in single run within very short time period, multiple decellularization methods and cryopreservation method for storing amnion scaffold and innovative concept of its activation with PRP-growth factors before its application on burn wounds. Treating amnion by antibiotic solution and its direct application on wound have a greater risk of bacterial infection thereby increasing the potential side effects. The decellularized scaffold is an effective biological dressing provides an antibacterial and antimicrobial effect and has no risk of immune rejection. A number of methods for the removal of cells from amnion have been investigated with varying degrees of success. We used four methods for the removal the cells from human amniotic membrane which gave a varying degree of success. These cell-extraction methods have ranged from using detergents such as SDS to chemicals such as DMSO and SD are very useful for decellularization. Decellularization processes which we used removed major immunogenic cellular components which are most important for preventing the immune response. Among all four methods which we used for decellurization, SD (6 h)-SDS (6 h)-D/W method is best as compared to other processes. It gave required results earlier as compared to other methods. HE staining demonstrated removal of cells by the absence of cells. Further DAPI staining gave confirmation results of decellurization as it showed no nuclear staining. These results showed that the all immunogenic cellular components were removed. Massion’s trichrome staining; GAG’s and SEM showed that only collagen remained intact after decellurization process. These methods showed that collagen fibers remained intact and in an ordered fashion. Presence of collagen fibers indicated that they remained unaffected after detergent and chemical treatments. The intact scaffold which is essential for the cell culture, seeding, cell attachment, proliferation, and ultimately skin modeling.
Unactivated amnion scaffold requires multilayer application and frequent dressing after regular interval. More than one dressing of amnion scaffold was needed as it acted as a temporary covering for burn wounds. It showed difficulty in adherence properly to the burn wounds. But amnion scaffolds activated with PRP and calcium chloride showed better adherence keeping them in a place. It required only single dressing that is a main advantage of this amnion which markedly increases the rate of healing by decreasing inflammation and reduces scar formation as compared to its direct application without activation. PRP releases the transforming growth factor beta (TGF-β), Fibroblast growth factors (FGFs), insulin-like growth factor 1, insulin-like growth factor 2, vascular endothelial growth factor, epidermal growth factor, Interleukin 8, keratinocyte growth factor, connective tissue growth factor so that activated amnion with PRP and calcium chloride adheres and accelerates the process of wound healing naturally. This minimizes the need of recurrent dressings and decreases plasma oozing, fluid loss and heat loss at affected area. Single dressing showed complete wound healing without any scarring in animal study. It interacts with burn wound very effectively providing good adherence of cells to the affected area and maintaining the moist environment while healing thus eliminates the itching and irritation at the affected area. It significantly accelerates the blood vessel formation during healing, reduces inflammation and helps to enhance epithelialization.
Mechanical testing showed that a scaffold is suitable for applications as its elasticity, tensile strength, elongation, hardness, fracture toughness, impact resistance, stress rupture, and fatigue limit is appropriate for clinical applications. The mechanical testing analysis after cryopreservation resulted in no significant differences for ultimate circumferential stress, elasticity and burst pressure (Table 3). Decellularization of amnion provides a source of scaffold for the treatment of burn cases in human. Traditional methods of autologous skin grafting have a greater risk of bacterial infection and may be problematic because suitable tissue will not be available in excess burn cases. Allogenic skin grafts have also been used of grafts but it has many problems. Immunological rejection is a greater risk. But application of tissue engineered decellularized scaffolds activated with platelet rich amnion is ideal in these chronic cases. It is non immunogenic and does not causes the additional trauma to the patients and no chance of further any infections and injury to the affected patients. It is biocompatible and ideal source owing to widespread availability and low risk of disease transmission. In this study, we come to know that keratinocytes spread and well proliferated on decellularized amnion. Wound treated with amnion showed good recovery and formation of hair follicles as compared to the wound treated with silver nitrate gel. Silver nitrate gel recovery showed a good keratinization and growth of sebaceous glands but markly less as compared to the wound treated with amnion. Anti-scaring, anti-inflammation, good mechanical properties with no immunogenicity were important characteristics observed in decellularized amnion which are considered to be important for use of scaffold in clinical use. This study demonstrated that decellularized amnion compared with other well known skin substitute reduces wound contraction, enhances the recellularization. This scaffold can be cryopreserved and used when required by seeding the patient’s cells. Now a day, there is no ideal skin substitute for burn wounds currently available. Rapid progress in tissue engineering and design such type of scaffold gives us hope to regenerate autologous skin for the treatfment of extensively burned patients. Production of tissue engineered skin scaffold must move quickly from laboratory to clinical use.
Conclusion
Burn wounds are serious trauma and prone to many infections hence major problem posing social and economic burden to patients. A burn wound causes the loss of body fluids like water, proteins and electrolytes. Autografts can be problematic because of a lack of availability of suitable tissue (especially in pediatric patients) and additional trauma to the patient.
The amnion is low immunogenic, anti-inflammatory, anti-fibrotic, anti-scaring, anti-microbial, easily accepted by patient, eliminate the risk of post-operative infection. These scaffolds will not show tumourogenicity upon transplantation as it is acellular. It can be an effective biological dressing in human application. Many of its characteristics will make it potentially suitable for use in human burn wounds in clinical use. It can be applied when required as it is ready to hand. It diminishes the oozing of plasma, bacterial count, and fluid, protein and heat loss in the affected area, and possesses various growth factors and bio-macromolecules important for wound healing. Frequent dressing is not necessary in activated amnion application. Activated amnion scaffold is less expensive, available off the shelf. It has a long shelf life, free from infections. As it is durable and flexible, cover the irregular surfaces. It provides a bacterial barrier and helps to reduce water loss to a greater extent and doesn’t show any hypertrophic changes. Thus the scaffold activated with PRP and calcium chloride successfully interfere the immune barrier and decreases the chances of rejection. In addition, other advantages of CAS that suggest it is an excellent source of re-epithelization. The ECM components of the amnion are important for overlying cell growth and hence have a great role in cell adhesion. However, the amnion is a biological material so its uses should be carefully carried out. In addition, the quality of wound healing will be negatively affected due to infections in burn wounds. It is important to use of an efficient and an effective burn wound healing strategy for preventing infections. CAS will be suitable skin dressing of burn wounds due to its promising ability to heal burns and protect against bacterial infections. In this study, we found that amnion scaffold was able to inhibit the bacterial growth in animal study. The future of cryopreserved amnion scaffold (CAS) applications in human burn wound is very exciting. Further research is needed to determine its full potential for its uses in human use.
Therefore, the ideal solution would be a tissue-engineered amnion. CAS grafting will protect burn wounds against nosocomial infections in clinical application of amnion scaffold in human. As per author’s knowledge, no any biological dressing activated with growth factors have been used successfully on burn wounds. This would generate a reliable source of readily available source as a skin substitute and ready to implant activated amnion scaffold will greatly reduce the incapacitation time of patients.
Notes
Homing and efficacy of intra-articular injection of autologous.pdf.
References
Amable PR et al (2013) Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Therapy 4:1–13
Amer MS, Shamaa A et al (2015) The efficacy of cryopreserved amniotic membrane seeded with mesenchymal stem cells for management of bone defect in a canine model. Res J Pharm Biol Chem Sci 6(3):1620–1631
Aponte PM, Schlatt IIS, Luiz III, Franca LRD (2013) Biotechnological approaches to the treatment of aspermatogenic men. Clinics. https://doi.org/10.6061/clinics/2013(sup01)18
Augustine R, Kalarikkal N, Thomas S (2014) Advancement of wound care from grafts to bioengineered smart skin substitutes. Prog Biomater 3:103–113. https://doi.org/10.1007/s40204-014-0030-y
Bo S, Biedermann T, Reichmann E (2010) Tissue engineering of skin. Burns 36:450–460
Castellanos G, Garcia AB, Garcia C, Pinero A, Moraleda JM et al (2016) The use of amniotic membrane in the management of complex chronic wounds. In: Alexandrescu VA (ed) Wound healing - new insights into ancient challenges. InTech. https://doi.org/10.5772/64491
Chang Y et al (2015) Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med 8(1):1286–1290
Ehrenreich M, Ruszczak Z (2006) Update on tissue-engineered biological dressings. Tissue Eng 12:2407–2424
Haddad AG, Giatsidis G, Orgill DP, Halvorson EG (2017) Skin substitutes and bioscaffolds temporary and permanent coverage skin substitutes bioscaffolds allografts xenografts dermal templates. Clin Plast Surg. https://doi.org/10.1016/j.cps.2017.02.019
Kesting MR, Wolff K, Nobis CP, Rohleder NH (2014) Amniotic membrane in oral and maxillofacial surgery. Oral Maxillofac Surg 18:153–164. https://doi.org/10.1007/s10006-012-0382-1
Kraus KH, Kirker C (2006) Mesenchymal stem cells and bone regeneration. Vet Surg 35(3):232–242
Kuna VK et al (2016) Significantly accelerated wound healing of full-thickness skin using a novel composite gel of porcine acellular dermal matrix and human peripheral blood cells. Cell Transplant. https://doi.org/10.3727/096368916x692690
Lohana P, Hassan S, Watson SB (2014) Integra™ in burns reconstruction: our experience and report of an unusual immunological reaction. Ann Burns Fire Disasters XXVII:17–21
Macneil S (2007) Progress and opportunities for tissue-engineered skin. Nature 445:874
Maguire TJ, Yarmush ML (2011) Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. https://doi.org/10.1146/annurev-chembioeng-061010-114257
Markeson D, Pleat JM, Sharpe JR, Harris AL, Seifalian AM (2013) Scarring, stem cells, scaffolds and skin repair. J Tissue Eng Regen Med 9(6):649–668
Mokbel A, El-Tookhy O, Shamaa AA, Sabry D, Rashed L, Mostafa A (2011) Homing and efficacy of intra-articular injection of autologous mesenchymal stem cells in experimental chondral defects in dogs. Clin Exp Rheumatol 29:275–284
Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM (2008) Properties of the amniotic membrane for potential use in tissue engineering. Eur Cells Mater 15:88–99
Plichta JK, Holmes CJ, Gamelli RL, Radek KA (2017) Local burn injury promotes defects in the epidermal lipid and antimicrobial peptide barriers in human autograft skin and burn margin: implications for burn wound healing and graft survival. J Burn Care Res. https://doi.org/10.1097/bcr.0000000000000357
Robiony M, Polini F, Costa F (2002) Osteogenesis distraction and platelet-rich plasma for bone restoration of the severely atrophic mandible. J Oral Maxillofac Surg. https://doi.org/10.1053/joms.2002.33107
Sheikh S, Hamid A, Kamalia N (2014) Scanning electron microscopic assessment on surface morphology of preserved human amniotic membrane after gamma sterilisation. Cell Tissue Bank 15:15–24. https://doi.org/10.1007/s10561-012-9353-x
Wen A et al (2016) Skin tissue engineering advances in severe burns: review and therapeutic applications. Burn Trauma. https://doi.org/10.1186/s41038-016-0027-y
Yamaguchi R et al (2012) effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J Surg Res 173:258–266
Zhang T et al (2013) The effect of amniotic membrane de-epithelialization method on its biological properties and ability to promote limbal epithelial cell culture. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.12-10805
Zhang L, Zou D, Li S, Wang J, Qu Y (2016) An ultra-thin amniotic membrane as carrier in corneal epithelium. Nat Publ Gr. https://doi.org/10.1038/srep21021
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare no potential conflict of interest.
Human and animals rights
The inform consent were taken and the study was approved form Institutional Review Board (Ref.- 6/IAEC/2017). All animals experiments are performed after due approval from animal ethics committee of the university.
Rights and permissions
About this article
Cite this article
Kshersagar, J., Kshirsagar, R., Desai, S. et al. Decellularized amnion scaffold with activated PRP: a new paradigm dressing material for burn wound healing. Cell Tissue Bank 19, 423–436 (2018). https://doi.org/10.1007/s10561-018-9688-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-018-9688-z