Abstract
Mesenchymal stem cells (MSCs) have vast potential in cell therapy, and are experimentally used in the clinic. Therefore, it is critical to find a serum- and xeno-free cryopreservation method. The aim of this study was to compare two serum- and xeno-free cryoprotectants for MSCs. Adipose tissue MSCs (Ad-MSCs) and bone marrow MSCs (BM-MSCs) were cryopreserved in two cryoprotectants: the defined serum- and xeno-free STEM-CELLBANKER™ (CB) and 10 % dimethyl sulfoxide (DMSO) in a xeno-free serum replacement cell culture medium and compared to non-cryopreserved MSCs. MSCs cryopreserved in CB or DMSO had similar morphology and surface marker expression compared to their respective non-cryopreserved MSC. Ad-MSCs and BM-MSC in both cryoprotectant media exhibited reduced mean fluorescence intensity (MFI) for CD105, BM-MSCs for CD73 and Ad-MSC increased MFI for HLA class I compared to non-cryopreserved MSCs. Population doubling time of CB cryopreserved and non-cryopreserved Ad-MSCs was similar (38.1 ± 13.6 and 36.8 ± 12.1 h), but somewhat higher when cryopreserved in DMSO (42.2 ± 10.8 h). BM-MSCs had higher population doubling time (CB 47.7 ± 11.4 and DMSO 62.3 ± 32.9 h respectively, p < 0.05) compared to Ad-MSCs. The viability of Ad-MSCs was significantly higher after cryopreservation in CB compared to DMSO (90.4 ± 4.5 % vs. 79.9 ± 3.8 % respectively). Ad-MSCs and BM-MSCs retained their mesodermal differentiation potential when cryopreserved in both cryoprotectants. The characteristics of Ad-MSCs post-thawing are better preserved by CB than by DMSO in serum- and xeno-free medium. Furthermore, Ad-MSCs and BM-MSCs are differently affected by the cryoprotectants, which may have implications for cell therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stem cells (MSCs) are regarded as promising in the field of regenerative medicine. MSCs are characterized by their ability to adhere to plastic, exhibiting a specific surface marker expression and multilineage differentiation potential into bone, cartilage and fat (Dominici et al. 2006).
Adipose-derived MSCs (Ad-MSCs) were isolated in 2001 from liposuction material (Zuk et al. 2001). Since then, the interest in Ad-MSCs has increased due to their therapeutic potential in cellular therapies in the near future (Dicker et al. 2005). They are regarded as an equivalent or even better alternative to the commonly used bone marrow-derived MSCs (BM-MSCs) for several reasons: adipose tissue can easily be obtained in large volumes with minor donor morbidity, they are present in the adipose tissue regardless of the donor age, they can be isolated in high numbers since 1 g of adipose tissue yield up to 5,000 Ad-MSCs, whereas 1 ml bone marrow yield up to 100–1000 BM-MSCs, and they have higher proliferative potential compared to BM-MSCs (Al-Saqi et al. 2014, Basu et al. 2011; Casteilla et al. 2011; Strem et al. 2005; Witkowska-Zimny and Walenko 2011; Zuk et al. 2001). Like MSCs from other tissues, the beneficial effects of Ad-MSCs in terms of differentiation and self-renewal are believed to be mediated through their ability to produce soluble cytokines and growth factors (Kim et al. 2009). Similarly as BM-MSC, Ad-MSCs have immunosuppressive, anti-inflammatory, anti-apoptotic and pro-angiogenic properties (Lin et al. 2011, 2012).
It is of critical value to find an effective cryopreservation medium and an optimized protocol to preserve human Ad-MSCs in general and for potential clinical use in particular. Optimized cryopreservation is needed to facilitate expansion and validation of the cells and for transport between laboratories and clinics and to have a stored backup if repeated transplantations are necessary.
An ideal cryoprotectant should be non-toxic and non-immunogenic for both the cells and the recipient, maximally preserve cell viability and characteristics, be chemically inert and highly water soluble in low temperature, induce minimal ice-crystal formation, have a low molecular weight and be affordable (Janz Fde et al. 2012; Naaldjik et al. 2012; Pegg 2007). Different types of cryopreservation solutions have been evaluated to cryopreserve human MSCs. So far, dimethyl sulfoxide (DMSO) in combination with serum is the most commonly used cryoprotectant. DMSO can certainly be used for cryopreservation of MSCs, but used alone it increases extracellular sodium, calcium and potassium and may have negative effects on the ion transport pumps (Qi et al. 2008). A combination of the extracellular cryoprotectant hydroxethyl starch and the intracellular cryoprotectant DMSO for cryopreservation of unfractionated bone marrow (Stiff et al. 1987) and xeno-free cryoprotectants (CryoStor medium) have been tried to cryopreserve BM-MSCs (Ginis et al. 2012), with promising results in short term. STEM-CELLBANKER™ (CB) is a new defined serum- and xeno-free formulation having 3 cryoprotectants; 10 % DMSO, glucose and high polymer anhydrous dextrose (Saliem et al. 2012). CB has been demonstrated to successfully cryopreserve hepatocytes and human embryonic and induced pluripotent stem cells (Holm et al. 2010; Saliem et al. 2012).
In a previous study, we compared Ad-MSCs and BM-MSCs expanded in a defined serum-free or serum-containing culture system and found that the serum-free medium was superior for Ad-MSCs (Al-Saqi et al. 2014). Therefore it is now of value to find the best serum-free cryopreservation medium in order to cryopreserve Ad-MSCs for clinical use. In the present study, we compared a defined serum- and xeno-free formulation (STEM-CELLBANKER™, CB) for cryopreservation of Ad-MSCs and BM-MSCs cultured in a defined serum-free medium (Mesencult®-XF), to DMSO in defined serum- and xeno-free culture medium (Mesencult®-XF). Post-thaw viability, growth potential, cell surface marker expression and mesodermal differentiation capability was determined and compared to non-cryopreserved cells. Our results show that CB enhanced the preservation of MSC viability, growth and mesodermal differentiation potential when compared to DMSO in Mesencult®-XF. Furthermore, the CB cryopreservation medium was better for Ad-MSCs compared to BM-MSCs.
Materials and methods
Sample collection
Two types of human MSCs were used; Ad-MSCs and BM-MSCs. Subcutaneous adipose tissue samples from the lower abdomen were obtained at elective caesarean sections from 5 female patients with mean age of 33 years (range 27–42 years). Bone marrow samples were obtained from the iliac crest of 5 healthy volunteers with a mean age of 22.6 years (range 5–47 years, the young donor was a clinical bone marrow donor for a sibling). The bone marrow donors were 40:60 female:male. The study was approved by the Regional Ethics Committee, Stockholm, Sweden. Informed oral and written consent were obtained from all participants. Written and oral informed consent was obtained from guardians on behalf of the children.
Isolation of Ad-MSCs and BM-MSCs
Adipose tissue-derived MSCs were isolated as previously described with few modifications (Halvorsen et al. 2001). In brief, samples were collected and transferred to the laboratory in sterile Hanks Balanced Salt Solution (HBSS) (Lonza, Vervviers, Belgium) and processed within 2–3 h. The samples were washed several times with HBSS until the eluent was clear, and then cut into small pieces with a scalpel. The tissue pieces were then treated with an equal volume of 1 mg/ml collagenase type II (GIBCO, Grand Island, USA) for 45 min at 37 °C with manual shaking every 5 min to digest the extracellular matrix. When the majority of the tissue was digested, collagenase activity was inhibited by adding an equal volume of Mesencult®-XF medium (Stem Cell Technologies, Vancouver, Canada) and centrifuged at 300×g for 10 min at 4 °C. Three layers were then apparent; an upper yellow layer (fat), a middle red layer (blood cells and medium) and a cell pellet. Both upper layers were discarded, and the cell pellet was resuspended in medium as described below.
MSCs from BM were prepared as described before (Le Blanc et al. 2003). Briefly, heparinized bone marrow was mixed with double volumes of phosphate-buffered saline (PBS) and centrifuged for 10 min at 900×g at room temperature. The cell pellet was resuspended and brought to 107 cells/ml PBS. The cells were layered on Percoll (Amersham, Biosciences, Sweden) and centrifuged at 500×g for 30 min at room temperature without brakes. The interface was collected and washed with PBS at 500×g for 10 min at room temperature and resuspended in medium as described below.
Ex vivo cell expansion
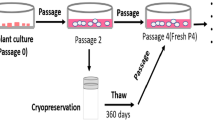
All cultures were performed in triplicates with Ad-MSCs and BM-MSCs. MSCs were cultured according to the manufactures instructions up till passage 5 in Mesencult®-XF (Stem Cell Technologies), which is a defined serum- and xeno-free medium. Mesencult™-SF attachment substrate was used to pre-coat the culture plates overnight at 4 °C or for 2 h at room temperature. After isolation, MSCs were plated at 1–1.5 × 105 cells/cm2 and maintained at 37 °C in a humidified environment containing 5 % CO2. Non-adherent cells were discarded after 2 days and the medium replaced and there after changed every 3–4 days. MSCs were passaged when they reached 70–80 % confluence using Mesencult®-ACF Dissociation Kit (Stem Cell Technologies). All samples were counted in triplicates at all passages using trypan blue exclusion in a hemocytometer. MSCs were thereafter re-plated at a density of 4 × 103 cells/cm2 at each passage and cultured until the 3rd passage when they were cryopreserved as described below. After cryopreservation and subsequent thawing, MSCs were propagated for two more passages (passage 4–5) at a density of 4 × 103 cells/cm2.
Cell morphology was documented by photography using an inverted microscope with a XM10 camera (Olympus IX81, Tokyo, Japan).
Cryopreservation
Ad-MSCs and BM-MSCs harvested from the 3rd passage were cryopreserved using either 10 % DMSO in Mesencult®-XF or STEM-CELLBANKER™ (CB) (ZENOAQ, Fukushima, Japan). CB is a new defined serum- and xeno-free formulation including 3 cryoprotectants; 10 % DMSO, glucose and high polymer anhydrous dextrose (dextran). It also contains NaCl, KCL, NaHPO4, HK2PO4 and NaHCO3. All ingredients are of United States, European and Japanese Pharmacopeia grade. Cold cryoprotectant (4 °C) was added directly to the cell pellet and cells were transferred to pre-cooled CryoTubes®, (Nunc®, Sigma-Aldrich, Stockholm, Sweden) on ice and moved directly to −80 °C in a polystyrene cryopreservation box, a protocol optimized in our laboratory. The cooling rate was −1 °C per minute. The cells were frozen at a concentration of 0.5–1 × 106 in 1 ml. After 24 h the cells were transferred to liquid nitrogen.
Cell thawing
The cells were thawed by incubating the cryo tubes at 37 °C for 1–2 min in a water bath. The tubes were removed from the water bath while there were still ice crystals in the medium. The cells were transferred to a 15 ml Falcon tube, suspended in 5 ml medium and centrifuged at 300×g for 5 min. Cells that were cryopreserved in CB were suspended in CELLOTION™ cell washing solution (ZENOAQ), which is a chemically defined cell washing solution that optimizes cell recovery. It contains no serum, proteins or sugars. The cells that were cryopreserved in DMSO were suspended in Mesencult®-XF.
Cell viability
Viability of Ad-MSC and BM-MSCs was assessed using a fluorescence-based live/dead assay (LDA) (Molecular Probes, Eugene, USA) immediately post-thawing to determine the percentage of live to dead cells according to manufacturer’s instructions. Live cells show green fluorescence in the cytoplasm due to the conversion of non-fluorescent calceinAM to green-fluorescent calcein after hydrolysis by intracellular esterases. The cell nuclei in dead cells stain red by Ethidium-1, which enters into the intracellular compartment through damaged cell membranes. All experiments were performed in triplicates, stained cells were photographed and 3 fields per sample were blindly quantified by counting live and dead cells. Percent viability was calculated by dividing the number of live cells with the total number (live + dead) of cell in the same field.
Cell viability was also analyzed by flow cytometry at early passage (passage 1–2) and at late passage (passage 5) using Propidium Iodide (PI) (Molecular Probes, Invitrogen, Paisley, UK). PI was added immediately before the analysis, and 10,000 events were recorded. Trypan blue exclusion method was used for viability analysis during passaging and plating.
The percent cell recovery was calculated using the equation [100 × (total number of cells recovered after thawing × % ‘live’ cells)/(total number of cells cryopreserved)].
Population doubling time
Ad-MSCs and BM-MSCs cultured in Mesencult®-XF were plated at a density of 4x103 cell/cm2 in triplicates. Cells were harvested when they reached 70–80 % confluence. Population doubling time (PDT) was estimated by dividing hours in culture by the logarithm of number of cells harvested divided by number of cells plated. PDT after cryopreservation was compared to PDT of freshly expanded non-cryopreserved Ad-MSCs and BM-MSCs at the 4th and 5th passage.
β-Galactosidase staining
Beta-Galactosidase staining was performed using Senescence Cells Histochemical Staining Kit (SIGMA, Saint Louis, USA) to stain cells at passage 5 according to manufacture’s instructions. The staining is based on histochemical assay of Beta-Galactosidase activity in senescent cells at pH 6. The staining was performed by washing MSCs with PBS and 0.5 ml (12-well plate) fixation buffer was added and incubated for 7 min at room temperature. The fixation buffer was removed and MSCs were rinsed with PBS 3 times. Staining mixture was added and incubated at 37 °C without CO2 overnight. MSCs were viewed under an inverted microscope with XM10 camera (Olympus IX81, Tokyo, Japan). Beta-Galactosidase positive MSCs stained blue. The total cell number and blue-stained cells were counted. The percent of Beta-Galactosidase positive MSCs were calculated from a mean of 5 fields photographed from 3 samples.
Phenotype of Ad-MSCs and BM-MSCs
The surface marker expression by Ad-MSCs and BM-MSCs frozen in CB or DMSO was characterized by flow cytometry (FACS Calibur, Becton–Dickinson, Mountain View, USA). The analysis was performed at passage 5 (2 passages after cryopreservation). Surface marker expression on cryopreserved cells was compared to same-passage cells that had never been cryopreserved. The following surface antigens were analyzed using monoclonal FITC or PE conjugated antibodies: CD3, CD14, CD31, CD34, CD45, CD73, CD80, CD90 (Becton–Dickinson), CD105 (Ancell, Bayport, USA), HLA-I (A, B, C) and HLA-II (DR) (DakoCytomation, Glostrup, Denmark). Isotype antibodies (γ1 FITC and γ2 PE, Becton–Dickinson) were used as negative control. Ad-MSCs and BM-MSCs were harvested as described above and 1 × 106 cells were resuspended in PBS, incubated with the antibodies at 4 °C for 30 min in the dark and washed with PBS. The data composition and analysis was performed with CELLQuest Pro v 5.2 software (Becton–Dickinson). Viability of the cells was evaluated by staining with Propidium Iodide (Molecular Probes, Invitrogen, Paisley, UK) immediately before the analysis and for dead cell exclusion. In each sample, a minimum of 10,000 events were recorded in the analysis region of viable cells using log amplified fluorescence and linearly amplified side- and forward scatter signals.
To estimate changes in expression of each surface markers, the mean fluorescence intensity (MFI) values were calculated for cryopreserved and non-cryopreserved MSCs. This was done by dividing the expression value with antigen specific antibody by the MFI value for the same cells stained with isotype matched control Ig [MFI non-cryopreserved, cryopreserved in CB, cryopreserved in DMSO/MFI isotype control].
Differentiation of Ad-MSCs and BM-MSCs
Ad-MSCs and BM-MSCs were cryopreserved at the 3rd passage, thawed and cultured for another 2 passages. The differentiation ability of the cells towards osteogenic and adipogenic lineages was evaluated in duplicates at passage 5 as described before (Santos et al. 2002). Non-cryopreserved MSCs at the same passage was added as comparison. Adipogenic differentiation was started at 70 % cell confluency by changing to adipogenic induction medium and thereafter the medium was replaced every 3–4 days for 3 cycles alternating between induction and supportive media. Adipogenic induction medium consisted of Dulbecco’s Modified Eagles Medium–High Glucose (DMEM-HG) (GIBCO), 50 U/ml penicillin, 50 µg/ml streptomycin, 10 % fetal bovine serum (FBS), 1.0 μM dexamethasone, 0.2 mM indomethacin, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) (all from Sigma Aldrich, Stockholm, Sweden) and 0.01 mg/ml insulin (GIBCO). Adipogenic supportive medium consisted of DMEM-HG, 50 U/ml penicillin, 50 µg/ml streptomycin, 10 % FBS and 0.01 mg/ml insulin. Cells cultured in medium made of DMEM-HG, 50 U/ml penicillin, 50 µg/ml streptomycin, and 10 % FBS served as negative control. Lipid droplets in the cells were identified by Oil Red O staining, as described before (Le Blanc et al. 2003). Quantification of the staining was performed by eluting the Oil Red O dye using 100 % isopropanol and measuring the optical density at 500 nm by a spectrophotometer. Background values for negative controls were multiplied by differentiation measurements.
Osteogenic differentiation was initiated at 70 % cell confluency by culturing the cells in bone induction medium made from DMEM-Low Glucose (DMEM-LG), 50 U/ml penicillin, 50 µg/ml streptomycin, 10 % FBS, 10 nM dexamethasone, 0.05 mM ascorbic acid, 10 mM glycerophosphate (all from Sigma Aldrich). The negative control was cultured in DMEM-LG, 50 U/ml penicillin, 50 µg/ml streptomycin and 10 % FBS. The cultures were maintained for 17 days and the media was replenished every 3 to 4 days. Extracellular calcium deposition was visualized using Alizarin red S and Von Kossa staining as described before (Le Blanc et al. 2003). Quantification of the Alizarin Red S staining was performed by eluting the dye with 10 % cetylpyridinium chloride and measuring the optical density at 562 nm by a spectrophotometer. Background values for the negative controls were multiplied by differentiation measurements.
Statistics
All data is presented as mean ± SD. Analysis of variance (ANOVA) and paired Student t test was performed. Results were considered statistically significant when p values were < 0.05. Regression analysis was performed to identify differences between the individual groups when the ANOVA analysis showed significant values.
Results
Cell culture and morphology
All Ad-MSCs and BM-MSCs (n = 5 of each) were successfully isolated and expanded in Mesencult®-XF and cryopreserved in CB and DMSO at passage 3. After thawing, MSCs were expanded for 2 more passages. At passage 5, Ad-MSCs and BM-MSCs had a homogenous morphology with the typical fibroblast-like spindle-shaped morphology, but with thin and long cytoplasmic extensions more prominent in BM-MSCs (Fig. 1). There was no gross change in the morphology of Ad-MSCs before and after cryopreservation (Fig. 1A I–III). Intra-cytoplasmic vacuoles were apparent in the cytoplasm of BM-MSCs (indicated by arrows) at passage 4 to 5 regardless of the cryopreservation condition (Fig. 1A IV–VI).
Morphology of Ad-MSCs and BM-MSCs at passage 5. A Ad-MSCs and BM-MSCs (n = 5 of each) were isolated and cultured for 3 passages, cryopreserved in CB and DMSO and thereafter thawed and cultured for another 2 passages. MSCs from the same donor that had never been cryopreserved (fresh) were used as a control. Representative pictures at passage 5 are shown of: Ad-MSCs non-cryopreserved (I); CB (II) and DMSO (III); BM-MSCs non-cryopreserved (IV); CB (V) and DMSO (VI). Arrows indicate intra-cytoplasmic vacuoles. B Cells were stained for Beta-Galactosidase at passage 5. Ad-MSCs in CB (I); Ad-MSCs in DMSO (II) and BM-MSCs in CB (III); BM-MSCs in DMSO (IV). Blue staining indicates Beta-Galactosidase positive cells. CB STEM-CELLBANKER™, DMSO dimethyl sulfoxide. Original magnification ×10. (Color figure online)
β-Galactosidase staining
Only BM-MSCs cryopreserved in both cryoprotectants revealed positive Beta-Galactosidase activity by blue staining of the cells (Fig. 1B III&IV). Percent Beta-Galactosidase-positive BM-MSCs were 15 ± 2.5 % in both groups (CB and DMSO). Ad-MSCs were negative for Beta-Galactosidase (Fig. 1B I&II).
Viability
Viability of Ad-MSCs and BM-MSCs cryopreserved in either CB or DMSO was assessed by live/dead assay (LDA) immediately post thawing. There was a significant difference in viability between Ad-MSCs cryopreserved in CB compared to DMSO (90.4 ± 4.5 % vs. 79.9 ± 3.8 %, p < 0.024, Fig. 2A, B), while there was no difference in BM-MSCs cryopreserved in CB compared to DMSO (85.6 ± 5.7 % vs. 86.0 ± 4.7 %) (Fig. 2A, B). However, there was no significant differences in viability assessed by flow cytometry at passage 5 (after the cryopreserved cells had been propagated for two more passages post thawing) both in the inter- and intra-groups of Ad-MSCs and BM-MSCs freshly propagated or cryopreserved in CB or DMSO (Fig. 2C).
Cell viability. The viability of MSCs was determined at passage 5 after cryopreservation in either CB or DMSO. A The amount of live and dead Ad-MSCs and BM-MSCs after cryopreservation was determined using Live/Dead assay. Green staining in the cytoplasm depicts live cells and red staining of the nucleus depicts dead cells. Representative pictures are shown of: Ad-MSCs in CB (I), Ad-MSCs in DMSO (II), BM-MSCs in CB (III) and BM-MSCs in DMSO (IV). Original magnification ×20. B Quantification of percentage live and dead Ad-MSCs and BM-MSCs after cryopreservation in CB or DMSO. All samples were performed in triplicates and 3 photos of each sample were blindly quantified. The percent viability was calculated by dividing the number of live cells by the total number of cells (live and dead) in the same field. *p < 0.05. C The viability of Ad-MSCs and BM-MSCs (n = 5 of each) cryopreserved in CB or DMSO at passage 5 was evaluated by flow cytometry at passage 5 and compared to non-cryopreserved (fresh) MSCs. Propidium Iodide was added to the cell suspension immediately before running the experiment. CB STEM-CELLBANKER™, DMSO dimethyl sulfoxide. (Color figure online)
We calculated the percent cell recovery after cryopreservation and thawing in each group. We found that the recovery of Ad-MSCs cryopreserved in CB was higher compared to Ad-MSCs cryopreserved in DMSO (87.8 ± 15.4 % vs. 65.4 ± 26.7 %, non-significant difference). For BM-MSCs the recovery was lower, although statistically non-significant, in both groups compared to Ad-MSCs with no significant differences between BM-MSCs cryopreserved in CB (56.7 ± 16.1 %) or in DMSO (54.9 ± 19.1 %).
Growth kinetics
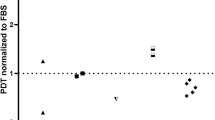
The population doubling time (PDT) of cryopreserved Ad-MSCs and BM-MSCs was evaluated at passage 4–5 and compared to non-cryopreserved freshly propagated Ad-MSCs and BM-MSCs at the same passage. None of the cryopreservation media significantly affected the PDT of Ad-MSCs. There was a trend for a difference in PDT between non-cryopreserved BM-MSCs and DMSO-cryopreserved BM-MSCs (43.0 ± 12.2 vs. 62.3 ± 32.9 h) (p < 0.069) (Fig. 3). Ad-MSCs cryopreserved in CB or DMSO had significantly shorter PDT (38.1 ± 13.6 and 42.2 ± 10.8 h, respectively) compared to BM-MSCs (47.7 ± 11.4 and 62.3 ± 32.9 h respectively) (p < 0.013) (Fig. 3).
Population doubling time at passage 4-5. Triplicates of Ad-MSCs and BM-MSCs (n = 5 of each) were cryopreserved at passage 3, thawed, plated at 4 × 103 cell/cm2 and passaged at 70–80 % confluency. Non-cryopreserved MSCs (fresh) at the same passage were included as control. The cells were counted using Trypan blue exclusion and the population doubling time (PDT) calculated over passage 4–5. CB STEM-CELLBANKER™, DMSO dimethyl sulfoxide. *p < 0.05
Phenotype
Cell surface marker expression was analyzed by flow cytometry before and after cryopreservation of Ad-MSCs and BM-MSCs at passage 3 and after subsequent culture for 2 more passages. Cryopreserved and non-cryopreserved Ad-MSCs showed a stable expression of the classical MSC surface markers at passage 5 with cells being positive (>95 %) for CD73, CD90, CD105, HLA-I and negative (<5 %) for CD3, CD14, CD34, CD45, CD80 and HLA-II (Fig. 4A, data not shown for passage 1–2 before cryopreservation). Cryopreserved and non-cryopreserved BM-MSCs (Fig. 4A) showed a significant down regulation of CD90 at passage 5 compared to Ad-MSCs (54.6 ± 19.2 % vs. 97.1 ± 3.5 % respectively, p < 0.00015), and a significant up regulation of HLA class II (11.6 ± 12.3 % vs. 0.2 ± 0.3 %, p < 0.003), which we previously have attributed to the Mesencult®-XF media (Al-Saqi et al. 2014). BM-MSCs at passage 2 also displayed the same features, but to a lower extent (data not shown). There were no significant changes in the mean fluorescence intensity in any of the markers between non-cryopreserved and cryopreserved MSCs (Fig. 4B).
Surface marker expression at passage 5. MSCs were cryopreserved at passage 3 and thereafter cultured for 2 passages and surface marker expression evaluated by flow cytometry. A Ad-MSCs and BM-MSCs cryopreserved in CB or DMSO or non-cryopreserved (fresh). B Mean fluorescence intensity (MFI) of positive (>95 %) surface marker expression on Ad-MSCs and BM-MSCs in non-cropreserved (fresh) and cryopreserved in CB and DMSO. CB STEM-CELLBANKER™, DMSO dimethyl sulfoxide. *p < 0.05. (Color figure online)
Differentiation ability
Ad-MSCs and BM-MSCs cryopreserved in either CB or DMSO were induced towards adipogenic and osteogenic lineages at passage 5. Non-cryopreserved Ad-MSCs and BM-MSCs at the same passage were included for comparison (results not shown). Ad-MSCs cryopreserved in CB showed a trend for higher adipogenic differentiation compared to BM-MSCs cryopreserved in CB, p = 0.06 (Fig. 5A, B). On the contrary, both Ad-MSCs and BM-MSCs displayed similar osteogenic differentiation potential in both cryoprotectant media (Fig. 6A, B).
Adipogenic differentiation of cryopreserved MSCs. In vitro adipogenic differentiation potential by Ad-MSCs and BM-MSCs that had been cryopreserved in CB or DMSO at passage 3 and then cultured for 2 passages was analyzed. A The cells were stained with Oil Red O to visualize lipid droplets (red color) after 3 cycles of induction. All samples were performed in duplicates. Representative pictures are shown of: Ad-MSCs in CB (I), Ad-MSCs in DMSO (II), BM-MSCs in CB (III), BM-MSCs in DMSO (IV). Original magnification ×20. B Quantification of the Oil Red O stain was performed by eluting the dye using 100 % isopropanol and measuring the optical density at 500 nm. Negative control values have been multiplied by differentiation values. CB STEM-CELLBANKER™, DMSO Dimethyl sulfoxide. (Color figure online)
Osteogenicdifferentiationof cryopreserved MSCs. Osteogenic differentiation ability of Ad-MSCs and BM-MSCs cryopreserved in CB or DMSO at passage 3 and then cultured for 2 passages was determined by in vitro culture for 17 days. A Alizarin Red S (red staining) and Von Kossa (black staining) were used to stain extracellular calcium deposits. All samples were performed in duplicates. Typical pictures are shown of: Ad-MSCs in CB with Alizarin Red Stain (I), Von Kossa stain (II), Ad-MSCs in DMSO with Alizarin Red stain (III), Von Kossa stain (IV), BM-MSCs in CB with Alizarin Red Stain (V), Von Kossa stain (VI), BM-MSCs in DMSO with Alizarin Red stain (VII), Von Kossa stain (VIII). Magnification ×20. B Quantification of Alizarin Red S staining was performed by eluting the dye using 10 % cetylpyridinium chloride and measuring the optical density at 562 nm. Negative control values have been multiplied by the differentiation values. CB STEM-CELLBANKER™, DMSO Dimethyl sulfoxide. (Color figure online)
Discussion
Most cellular therapies include cryopreservation, whereby the demand for an efficient clinical grade cryopreservation solution is increasing. In this study, we compared a chemically defined serum- and xeno-free cryopreservation medium to the traditional DMSO-based cryopreservation in serum- and xeno-free medium (Mesencult®-XF) using MSCs as the model system.
The morphology of Ad-MSCs and BM-MSCs cryopreserved in CB or DMSO were comparable with typical fibroblast-like cells with thin and long cytoplasmic extensions before and after cryopreservation, which is typical for MSCs cultured in Mesencult®-XF (Al-Saqi et al. 2014, Bray et al. 2012, Miwa et al. 2012). BM-MSCs cryopreserved in either CB or DMSO showed signs of senescence at passage 4–5 confirmed by positive Beta-Galactosidase stain. However, we have previously reported that BM-MSCs expanded in Mesencult®-XF in comparison to DMEM with FBS underwent earlier senescence compared to Ad-MSCs in the same culture medium (Al-Saqi et al. 2014). Hence, these results should be accounted to the expansion medium rather than cryopreservation medium. Of note is also that if the culture medium had been optimized for BM-MSCs, it may have positively influenced the cell recovery after thawing.
Regardless of the cryopreservation media, Ad-MSCs displayed shorter PDT compared to BM-MSCs, which others and we have reported before (Al-Saqi et al. 2014; Ikegame et al. 2011). Interestingly, BM-MSCs cryopreserved in DMSO had longer PDT compared to non-cryopreserved BM-MSCs, showing that the cryopreservation medium indeed can influence PDT as long as 2 passages after thawing. It can be explained by delayed cell death of cells cryopreserved in DMSO compared to CB or that BM-MSCs require longer recovery time after cryopreservation in DMSO compared to CB. The effect on PDT seems to be source specific since it was not apparent in the more highly proliferating Ad-MSCs.
The viability of Ad-MSCs immediately post thawing was significantly higher when cryopreserved in CB than in DMSO and BM-MSCs cryopreserved in either cryopreservation medium. An even more prominent increase in viability was seen in embryonic and induced pluripotent stem cell cryopreserved in CB. In this study the cells had a viability of more than 90 % in CB compared to 50 % in DMSO (Holm et al. 2010). Similarly, hepatocytes cryopreserved in CB showed higher viability than those cryopreserved in DMSO (46.4 vs. 31.3 % respectively) (Saliem et al. 2012). However, all these cells have previously been difficult to cryopreserve. Our results also showed better recovery of Ad-MSCs cryopreserved in CB compared to Ad-MSCs in DMSO and BM-MSCs in any cryopreservation media, although not statistically significant. The difference between Ad-MSCs cryopreserved in CB or DMSO might be explained by less cryo-damage to the cell membrane thanks to the CB formulation, as it is composed of both intra- and extra-cellular cryoprotectants.
Expression of surface markers by Ad-MSCs and BM-MSCs was examined 2 passages after cryopreservation and compared with the same sample of non-cryopreserved MSCs. The expression of CD90 and HLA class II by BM-MSCs deviated from the normal expression pattern of MSCs, which previously have been attributed to the cell culture medium (Al-Saqi et al. 2014; Bray et al. 2012; Campioni et al. 2008; Pal et al. 2009). One study that examined cryopreservation of MSCs in a cryopreservation solution made of human serum, 10 % DMSO and 20 % hydroxyethyl starch (Plasmin ® 460/0.7, 6 % solution; HalexIstar) showed alterations in surface expression of CD73 and CD146 (de Lima Prata et al. 2012), which our and others reports did not demonstrate (Naaldjik et al. 2012). However, even when more than 95 % of the MSCs express HLA class I on the surface, the cells do not activate the immune system in miss-matched transplantations (Le Blanc 2006).
In this study we showed that both CB and DMSO preserve MSCs ability to differentiate to mesodermal lineages. Similar findings were reported in other studies where BM-MSCs (Ginis et al. 2012; Naaldjik et al. 2012) and Ad-MSCs (Zeisberger et al. 2011) were cryopreserved using combinations of DMSO and hydroxyl ethyl starch. Osteogenic differentiation was similar over all groups, whereas there was trend for increased adipogenic differentiation for Ad-MSCs cryopreserved in CB. Hence, the differentiative capacity of MSCs may be affected by the cryoprotectant formulation. It may also have diverse actions depending on the cell source or gender as all Ad-MSC donors were female compared to 40 % female bone marrow donors.
Both CB and DMSO in Mesencult®-XF are chemically defined, serum- and xeno-free cryoprotectant media. However, CB is simple, it does not need preparation by adding medium as with DMSO. Cryopreservation in CB results in significantly higher cell viability and proliferation compared to when DMSO in Mesencult®-XF is used and it maintains MSC multi-potentiality. Both CB and DMSO in Mesencult®-XF, are safe cryoprotectants with medical graded ingredients with no risk for animal product contamination or serum batch-to-batch variability. In addition, the CB formulation has both intra- and extra-cellular cryoprotectants, which prevents cell dehydration and cell swelling ultimately minimizing the risk of cell damage. In contrast, the DMSO formulation has no extracellular cryoprotectant (Motta et al. 2014). The higher post-cryopreservation viability and functionality of Ad-MSCs in CB may be due to the presence of both intra and extra-cellular cryoprotectants. However, CB is more expensive, approximately the double price, when compared to DMSO.
We conclude from this study that both CB and DMSO in Mesencult®-XF are good alternative for MSCs storage. However, we prefer CB to DMSO in Mesencult®-XF as a first choice for Ad-MSCs cryopreservation. This is based on that Ad-MSCs cryopreserved in CB retain higher viability and better growth potential than when cryopreserved in DMSO in Mesencult®-XF. CB cryopreservation medium can be applied in many fields of MSCs research and regenerative medicine. Our aim is to use CB in the cryopreservation of Mesencult®-XF expanded Ad-MSCs for cellular therapy in urogenital atrophy and urogynecology.
Change history
05 April 2019
In the original article, Fig.��1A was by mistakenly duplicated. The corrected image is provided in this correction article.
05 April 2019
In the original article, Fig.��1A was by mistakenly duplicated. The corrected image is provided in this correction article.
Abbreviations
- MSCs:
-
Mesenchymal stem cells
- Ad-MSCs:
-
Adipose tissue-derived MSCs
- CB:
-
STEM-CELLBANKER™
- DMSO:
-
Dimethyl sulfoxide
- BM-MSCs:
-
Bone marrow-derived MSCs
- HBSS:
-
Hanks balanced salt solution
- PBS:
-
Phosphate-buffered saline
- LDA:
-
Live/dead assay
- PI:
-
Propidium iodide
- PDT:
-
Population doubling time
- DMEM-HG:
-
Dulbecco’s modified eagles medium-high glucose
- FBS:
-
Fetal bovine serum
- IBMX:
-
Isobutyl-1-methylxanthine
References
Al-Saqi SH, Saliem M, Asikainen S, Quezada HC, Hovatta O, Le Blanc K et al (2014) Defined serum-free medium for in vitro expansion of adipose derived mesenchymal stem cells. Cytotherapy. doi:10.1016/j.jcyt.2014.02.006
Basu J, Genheimer CW, Guthrie KI, Sangha N, Quinlan SF, Bruce AT et al (2011) Expansion of the human adipose-derived stromal vascular cell fraction yields a population of smooth muscle-like cells with markedly distinct phenotypic and functional properties relative to mesenchymal stem cells. Tissue Eng Part C Methods 17(8):843–860 Epub 2011/05/21
Bray LJ, Heazlewood CF, Atkinson K, Hutmacher DW, Harkin DG (2012) Evaluation of methods for cultivating limbal mesenchymal stromal cells. Cytotherapy 14(8):936–947 Epub 2012/05/17
Campioni D, Lanza F, Moretti S, Ferrari L, Cuneo A (2008) Loss of Thy-1 (CD90) antigen expression on mesenchymal stromal cells from hematologic malignancies is induced by in vitro angiogenic stimuli and is associated with peculiar functional and phenotypic characteristics. Cytotherapy 10(1):69–82 Epub 2008/01/19
Casteilla L, Planat-Benard V, Laharrague P, Cousin B (2011) Adipose-derived stromal cells: their identity and uses in clinical trials, an update. World J Stem Cells 3(4):25–33 Epub 2011/05/25
de Lima Prata K, de Santis GC, Orellana MD, Palma PV, Brassesco MS, Covas DT (2012) Cryopreservation of umbilical cord mesenchymal cells in xenofree conditions. Cytotherapy 14(6):694–700 Epub 2012/04/24
Dicker A, Le Blanc K, Astrom G, van Harmelen V, Gotherstrom C, Blomqvist L et al (2005) Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res 308(2):283–290 Epub 2005/06/01
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317 Epub 2006/08/23
Ginis I, Grinblat B, Shirvan MH (2012) Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng Part C Methods 18(6):453–463 Epub 2011/12/27
Halvorsen YD, Bond A, Sen A, Franklin DM, Lea-Currie YR, Sujkowski D et al (2001) Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: biochemical, cellular, and molecular analysis. Metabolism 50(4):407–413 Epub 2001/04/05
Holm F, Strom S, Inzunza J, Baker D, Stromberg AM, Rozell B et al (2010) An effective serum- and xeno-free chemically defined freezing procedure for human embryonic and induced pluripotent stem cells. Hum Reprod 25(5):1271–1279 Epub 2010/03/09
Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F et al (2011) Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 13(6):675–685 Epub 2011/01/15
Janz Fde L, Debes Ade A, Cavaglieri Rde C, Duarte SA, Romao CM, Moron AF, et al (2012) Evaluation of distinct freezing methods and cryoprotectants for human amniotic fluid stem cells cryopreservation. J Biomed Biotechnol 2012:649353. Epub 2012/06/06
Kim WS, Park BS, Sung JH (2009) Protective role of adipose-derived stem cells and their soluble factors in photoaging. Arch Dermatol Res 301(5):329–336 Epub 2009/04/28
Le Blanc K (2006) Mesenchymal stromal cells: tissue repair and immune modulation. Cytotherapy 8(6):559–561 Epub 2006/12/07
Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O (2003) Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 57(1):11–20 Epub 2003/01/25
Lin YT, Chern Y, Shen CK, Wen HL, Chang YC, Li H et al (2011) Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington’s disease mouse models. PLoS ONE 6(8):e22924 Epub 2011/08/19
Lin RZ, Moreno-Luna R, Zhou B, Pu WT, Melero-Martin JM (2012) Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis 15(3):443–455 Epub 2012/04/25
Miwa H, Hashimoto Y, Tensho K, Wakitani S, Takagi M (2012) Xeno-free proliferation of human bone marrow mesenchymal stem cells. Cytotechnology 64(3):301–308. doi:10.1007/s10616-011-9400-7
Motta JPR, Paraguassú-Braga FH, Bouzas LF, Porto LC (2014) Evaluation of intracellular and extracellular trehalose as a cryoprotectant of stem cells obtained from umbilical cord blood. Cryobiology 68(3):343–348. doi:10.1016/j.cryobiol.2014.04.007
Naaldjik Y, Staude M, Federova V, Stolzing A (2012) Effect of different freezing rates during cryopreservation of rat mesenchymal stem cells using combinations of hydroxyethyl starch and dimethylsulfoxide. BMC Biotechnol 12(1):49 Epub 2012/08/15
Pal R, Hanwate M, Jan M, Totey S (2009) Phenotypic and functional comparison of optimum culture conditions for upscaling of bone marrow-derived mesenchymal stem cells. J Tissue Eng Regen Med 3(3):163–174 Epub 2009/02/21
Pegg DE (2007) Principles of cryopreservation. Methods Mol Biol 368:39–57 Epub 2007/12/18
Qi W, Ding D, Salvi RJ (2008) Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res 236(1–2):52–60 Epub 2008/01/22
Saliem M, Holm F, Bergström Tengzelius R, Jorns C, Nilsson L-M, Ericzon B-G et al (2012) Improved cryopreservation of human hepatocytes using a new xeno free cryoprotectant solution. World J Hepatol 4(5):176
Santos NC, Figueira-Coelho J, Saldanha C, Martins-Silva J (2002) Biochemical, biophysical and haemorheological effects of dimethylsulphoxide on human erythrocyte calcium loading. Cell Calcium 31(4):183–188 Epub 2002/05/25
Stiff PJ, Koester AR, Weidner MK, Dvorak K, Fisher RI (1987) Autologous bone marrow transplantation using unfractionated cells cryopreserved in dimethylsulfoxide and hydroxyethyl starch without controlled-rate freezing. Blood 70(4):974–978 Epub 1987/10/01
Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE et al (2005) Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 54(3):132–141 Epub 2005/10/21
Witkowska-Zimny M, Walenko K (2011) Stem cells from adipose tissue. Cell Mol Biol Lett 16(2):236–257 Epub 2011/03/12
Zeisberger SM, Schulz JC, Mairhofer M, Ponsaerts P, Wouters G, Doerr D et al (2011) Biological and physicochemical characterization of a serum- and xeno-free chemically defined cryopreservation procedure for adult human progenitor cells. Cell Transpl 20(8):1241–1257 Epub 2010/12/24
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228 Epub 2001/04/17
Acknowledgments
This study has been supported by Karolinska Institutet foundation. We thank ZENOAQ for supplying the CB and CELLOTION media.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Saqi, S.H., Saliem, M., Quezada, H.C. et al. Defined serum- and xeno-free cryopreservation of mesenchymal stem cells. Cell Tissue Bank 16, 181–193 (2015). https://doi.org/10.1007/s10561-014-9463-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-014-9463-8