Abstract
Cerebral glioma is the most common brain tumor as well as one of the top ten malignant tumors in human beings. In spite of the great progress on chemotherapy and radiotherapy as well as the surgery strategies during the past decades, the mortality and morbidity are still high. One of the major challenges is to explore the pathogenesis and invasion of glioma at various “omics” levels (such as proteomics or genomics) and the clinical implications of biomarkers for diagnosis, prognosis or treatment of glioma patients. Establishment of a standardized tissue bank with high quality biospecimens annotated with clinical information is pivotal to the solution of these questions as well as the drug development process and translational research on glioma. Therefore, based on previous experience of tissue banks, standardized protocols for sample collection and storage were developed. We also developed two systems for glioma patient and sample management, a local database for medical records and a local image database for medical images. For future set-up of a regional biobank network in Shanghai, we also founded a centralized database for medical records. Hence we established a standardized glioma tissue bank with sufficient clinical data and medical images in Huashan Hospital. By September, 2013, tissues samples from 1,326 cases were collected. Histological diagnosis revealed that 73 % were astrocytic tumors, 17 % were oligodendroglial tumors, 2 % were oligoastrocytic tumors, 4 % were ependymal tumors and 4 % were other central nervous system neoplasms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As biomolecular technology platforms at various “-omics” levels (such as genomics and proteomics) are making unprecedented advances, cancer research has entered a brand new era toward personalized medicine. However, because tissue specimens are not obtained in a standardized manner and often lack corresponding clinical information (e.g. progression free time, survival time, therapeutic strategy), the reliability and the value of molecular data derived from these platforms is limited. Therefore, establishment of a professional standardized biospecimen bank and clinical database is undoubtedly the cornerstone for translational medicine, which relies on obtaining, preserving and characterizing high-quality biospecimens and systematically collecting clinical data. Urgency is warranted, since the most common malignant brain tumor (Jiang et al. 2011), cerebral glioma is notorious for high invasiveness, high mortality and morbidity. The current hotspots in glioma research lie in investigating the pathogenesis and exploring relevant diagnostic, prognostic and predictive markers. Therefore, it is of immense importance to establish a standardized glioma tissue bank (GTB) with high quality biospecimens annotated with detailed clinical information.
During the past several decades, standardized tissue banks have been established around the world. The National Cancer Institute of USA led the foundation of a national tumor tissue bank, the Cooperative Human Tissue Network (CHTN), which possesses 700,000 specimens and has provided high-quality cases for more than 1,000 scientists and researchers (Qualman et al. 2004). In addition, the TuBaFrost project (http://www.tubafrost.org) has united local frozen tumor banks into a European network that collects and stores tumor tissues in various national and regional institutes according to uniform standardized protocols (Isabelle et al. 2006; Morente et al. 2006; Lopez-Guerrero et al. 2006; Riegman et al. 2006a, b; Teodorovic et al. 2006; van Veen et al. 2006). Several other tissue banks, for example the National Cancer Tissue Resource (NCTR) established in the UK (Kerr 2003; Knox and Kerr 2004), the International Bladder Cancer Network (IBCN) launched in the USA (Goebell et al. 2004, 2005) and the French Liver Cancer Biobank Network (Clement et al. 2009) have also been reported. All of these tumor tissue banks are created on a supra-institutional and even on a supra-national scale, which enlarges tissue availability and accessibility to large amounts of specified or even rare, tumor samples, thereby facilitating large-scale, multi-center studies.
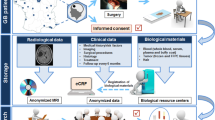
Even though it has been more than 20 years since the establishment of the first tumor tissue bank in western countries, the establishment of standardized tissue banks in China just started. Fortunately, more and more domestic institutes and scholars have realized the importance of standardized tissue banks (Gao and Zhu 2008). As a result, growing numbers of domestic institutes are setting up their own tissue banks, such as the School of Oncology affiliated to Peking University (Ji 2005), Zhujiang Hospital affiliated to Southern Medical University (Jian 2011). However, compared with supra-institutional even supra-national tissue banks in other countries, these banks were built at a single institute level. In order to unite tissue banks collecting frozen tissues on a supra-institutional scale, a regional network of tissue banks (Shanghai Biobank Network) is currently being set up in China. As a branch of the Shanghai Biobank Network, our GTB is designed to establish a standardized single disease (gliomas) tissue bank (Fig. 1b).
Huashan GTB was founded with the support of neurosurgical department of Huashan Hospital affiliated to Fudan University, one of the biggest neurosurgical centers in Asia, whose numerous patient are distributed all over China. In the past 4 years, 881 patients received glioma surgery in 2010 (from April), which increased to 1,289 in 2011, 1,338 in 2012, and 1,068 in 2013 (by September). From January 2003 to December 2008, more than 10,000 nervous system tumor specimens from more than 2,000 cases were collected in Huashan Hospital. However, the samples have some deficiencies for instance, (a) no informed consent or ethical approval; (b) lack of standardization for collection and storage protocols; (c) lack of serum, plasma and lymphocyte samples; (d) lack of quality control; and (e) lack of genetic and other associated clinical data (Lu et al. 2010). Based on these shortcomings, our institute has launched a standard GTB since April 2009 and has been banking specimens since April 2010.
Materials and methods
Ethics and confidentiality
The procedures for the collection, storage, distribution, dissemination and disposal of specimens in the repository were in compliance with all national laws and approved by the Huashan Institutional Review Board. Informed consent was given to each patient when the patient was hospitalized. They agreed to donate their remnant tumor tissues, peritumoral tissues, blood samples, and the associated clinical information to the GTB, on the premise that the pathological diagnosis and clinical treatment was not compromised by collection of the specimens and clinical data as well as permission for the use of the tissue for research purpose. Tissue samples and blood samples will be removed and destroyed when corresponding donors changed their mind after samples collection and storage. Clinical information and related research data will also be deleted. However, published data and anonymized results will be kept. After the donation, donors can inquire information related to sample storage, processing and usage. However, they don’t have access to research data and have no rights to copy or use the samples or clinical information for other purpose.
In addition, the procurement, storage, and use of specimens and associated data were conducted in a way that fully respects the subjects’ privacy. To address the confidentiality, we assigned a unique code for each sample (e.g., N100001T01) when included in GTB, which differed from the patients’ ID used in their medical records. The one-to-one correspondence connection between the unique code and the clinical ID could only be accessed by person authorized by the governing committee of GTB. Two fully trained technicians were responsible for tissue storage and retrieval. The identifying information was not disclosed to requestor or user having access to specimens and data.
Organizational structure and equipment
Under the supervision of the Institutional Review Board, our institute founded the governing committee of GTB which was led by the Department of Neurosurgery, taking responsibility for the construction and maintenance of the GTB (Fig. 1a). A neuropathologist supervised the actual procurement of the tissue. Two fully trained technicians were responsible for tissue preparation, collection, storage and retrieval, and for maintaining the digitalized specimen-related records. Other two staffs were in charge of registering the medical record and obtaining the follow-up data regularly. The image database, which included all medical images during the whole clinical history of patients such as preoperational, intraoperational and postoperational structural as well as functional images, was maintained by two technicians.
From April 2010 until September 2013 to store specimens from 1,326 cases, we had two 61 l liquid nitrogen containers (CB2002, CBS, USA) and five ultra-low temperature freezers upright type (MDF-U53 V, SANYO, Japan) for storage, two of which for backup storage, three full-time technicians, one part-time technician, and a 60 m2 separate room in the Department of Neuropathology (the same floor as the operating room). The alarm was a bi-phase alarm system (Haier, China) with a local visual and acoustic alarm where the storage repository was located and a remote alarm which can automatically sent messages to pre-programmed telephone numbers.
Establishment of standard operating procedures (SOPs)
By referring the best practices issued by the International Society for Biological and Environmental Repositories (ISBER) (Repositories 2012) and learning the experience from many domestic tissue banks, we compiled “Huashan Hospital Glioma Tissue Bank Standard Operating Procedures” on the basis of the characteristics of glioma tissue. The SOPs include sections on “Responsibility of GTB Governing Committee”, “Rules for Access and Use”, “SOP of Blood Sample Collection”, “SOP of Tissue Sample Collection” and so on, which described the management, procurement, storage, and dissemination in detail.
Collections of specimens and data
Specimen dissection and storage
All the cases suspected as cerebral gliomas preoperatively were included after informed consent was given by each patient. After the surgery schedule was settled, technicians responsible for the sample collection were informed. After the excision, tumor tissues were firstly examined by neuropathologists macroscopically and tissue samples for pathology diagnosis purpose was removed. Residual tissues was obtained and processed by our technicians strictly following the SOP of Tissue Sample Collection. If the volume of residual part was not adequate for collection after the typical part of glioma for diagnostic purposes was selected, the case was excluded.
-
After the removal of tissue, the specimens were kept sterile. Firstly, the technicians selected the typical part of glioma for histopathological confirmation of the diagnosis under the supervision of a neuropathologist. Then, the residual specimens were cut into small parts after excess blood or necroses were washed away by phosphate-buffered saline using forceps.
-
RNAlater-submerged tissue: The small slices, less than 0.5 cm thick, were put into the cryogenic vials prefilled with RNAlater reagent. Then the tissues submerged in RNAlater were incubated at 4 °C freezer overnight and then transferred to −80 °C freezer for storage.
-
Snap-frozen tissue: The other small pieces were loaded into 3–5 cryogenic vials and stored in liquid nitrogen containers.
-
OCT-embedded tissue: First, a portion of viable tissue no larger than 1 cm*1 cm and no more than 0.3 cm thick was dissected. Then it was embedded in a disposable mold with OCT (O.C.T. compound, Sakura, USA) and immediately preserved in a −80 °C freezer.
-
Blood samples: The peripheral blood samples were all collected in the morning of the day of surgery. The anticoagulated (plasma/buffy coat/RBC) whole blood and coagulated (serum/clot) blood were all collected in our GTB and both were preserved in a −80 °C freezer.
-
Sample inventory management system: A sample inventory management system (MSI-F1, AvanTech, China) was used to organize the information of samples from cryopreservation vials to cryo-boxes to cryo-box racks and finally to freezers, making the tracking of large amounts of sample information easier. The system makes an inventory of all freezers and tanks and offers information for assigning the positions of samples in the freezer positions, moving samples, and importing and exporting data for statistical purpose. For the purpose of communicating with other tissue banks, we created a sharable and extensible coding system which generated a unique code number for each specimen when it was included. Each code number is linked to the database where specimen-related information was recorded. The following information was included for each sample: (a) identification (gender, age and ID number of the medical record); (b) pathological data (anatomical location of the tumor, pathological diagnosis, tumor grade); (c) type of the specimens; (d) orientation of specimens; and (e) numbers of aliquots prepared and remained. More detailed clinical information was gathered by the linked glioma patient management system and the corresponding imaging information was collected and preserved by the image database.
The specimen type of included cases consisted of peripheral blood samples (plasma, serum and blood cells), tumor and peritumor tissues (frozen tissues, OCT-embedded tissues and paraffin-embedded tissues). The tissue sampling and freezing were done immediately after excision. The lag time between surgical removal and snap freezing was controlled within 30 min. Solid tissues, including tumor and peritumoral tissues, were collected and stored in a priority sequence of RNAlater-submerged (Ambion Company, USA), snap-frozen in liquid nitrogen, and OCT (opti-mum cutting temperature compound, Sakura, USA)-embedded. Paraffin-embedded tissues and slices were included after pathology diagnosis was made and stored in the archive of the Neuropathology department. The flow of collection procedures are displayed in Fig. 2.
Workflow of specimen collection. Informed consent was given to each patient. After the excision, tumor tissues were firstly examined by neuropathologists and tissue samples for pathology diagnosis purpose was removed. Residual tissues was obtained and processed by our technicians strictly following the SOP of Tissue Sample Collection using sample inventory management system. Medical records and follow-up information was added using cerebral glioma patient management system
Data collection—clinical database and image database
To intergrade all data, our cerebral glioma patient management system was upgraded (Fig. 3). This upgraded system contains several important modules, such as users’ authority management, data input, data inquiry and statistical analysis, follow-up and referral, and clinical information management. The authorized technician imports the medical data at computers installed with this program with their unique authorized username and code. All medical records as well as images and follow-up information are stored in the centralized database.
Each case collected in the biospecimens bank had a corresponding record in this clinical database. The period of follow-up differs between low-grade and high-grade glioma. For low grade glioma, the patients were followed up every 3-month in the first year, every 6-month during the second to the fifth year, and every year if the patients survive more than 5 years; the total follow-up duration will last to 10 years. For high-grade glioma, the patients were followed up every 3-month until endpoint.
We also established a local image database in the 3 Tesla intraoperative MRI integrated neurosurgical suite. By setting up a digitalized image-processing workstation which was connected to the picture archiving communication system (PACS) in our hospital and MRI post-processing workstation (Syngo MultiModality Workplace, Siemens AG, Erlangen, Germany), the image database can download associated images from PACS and import the processed images from post-processing workstation. All images were managed by the OSIRIX software tool. Volumetric analysis performed by this software was also a part of image database. The checklists of MRI scan were determined on the basis of tumor grades and locations (Table 1). These series were performed at three time-points—before, during and after the surgery (1, 3, 6 months), which included structural MRI, functional MRI, and metabolism MRI if possible.
Tissue request process
Before requesting a tissue sample, the requestors are all required to fill out a tissue request form (TRF). The TRF includes the following items: principle investigators, their institution (full address and contact information), number and type of samples, approval of local medical ethics committee, description of the research project and experiments to be performed, expected research results and financial support for the project (indicating the financing bodies). The requestors also need to sign the scientific research cooperation agreement with GTB. The samples and data must be used only for research as specified in TRF and the remaining samples after performing the agreed experiments must be returned to GTB. The requestor should bear the costs of preparing as well as of handling and shipping the requested tissue samples. After the submission, the TRF and a copy of research proposal, financial support and medical ethics committee approval documents will be sent to the governing committee of GTB. Final decision will be made in 1 month and a detailed written explanation will be sent to requestors if the sample request is rejected. In addition, this agreement also includes general clauses referring to co-authorship contribution. The requestor should indicate in all the publications resulting from these samples that tissue samples are provided by Glioma Tissue Bank, Huashan Hospital of Fudan University. During the whole process, GTB must protect the privacy of sample donors. The request institute must make no attempt to find the identity of the donors.
Financial sustainability
Establishment of our GTB was funded by the Shanghai Committee of Science and Technology, China (12DZ2295003) (purchasing storage equipment and database computers, equipment maintenance and repairing, staff training). Sample requestors were charged for the storage, handling and shipping of the requested samples. Daily operation of the GTB was also founded by Department of Neurosurgery and several grants of research projects using this tissue collection.
Results
Upgrade of cerebral glioma patient management system and patient centralized medical records
Compared with the former one, our new management system has following advantages:
-
(a)
Genetic information such as isocitrate dehydrogenase (IDH 1 or IDH 2) mutation status, 1p19q co-deletion and MGMT promoter methylation status, were added.
-
(b)
Referral function was added to system. For many patients who received surgical resection in our institute and expected to conduct their chemotherapy or radiotherapy in other medical center, this function would be of great help. Once they are ready for referral, with our system, their basic medical information such as name, age, surgical date and pathological results will be available for the radiotherapy physician or neuro-oncologist. For these doctor once their operate system is connected to the web, they will be informed of referral information before the patients come to their clinic, thus make the therapy plan in advance. Once these patients are admitted, our follow-up staff would also be informed. After finishing these therapies, these doctors can also input the detailed chemotherapy or radiotherapy information in this system, which of course can be seen by our follow-up staff with patients’ approval. This referral function is not only convenient for the patients but also allow us to conduct a better follow-up service for the patients. Once the patient needs a second surgery, their doctor can also make a referral to our institute or other neurosurgery centers. What’s more important, enriched chemotherapy and radiotherapy information combined with our pathology results comprise whole detailed medical records for each patient and each specimen stored in our biobank.
-
(c)
An online medical image database for each patient was added. Using our system, medical images can be upload by our institute or other medical centers to an image database contained in our glioma patient management system with patients approval. These images can be transferred with basic information of patients for chemotherapy and radiotherapy. During follow-up, new medical images can also be uploaded by other doctors.
These upgrades not only helped us to collect more detailed information but also means for each cases, its information was continuously enriched during the whole process, which in turn improved the value of tissues stored in GTB. Referral function ensured medical records could be safely and completely transferred and kept during the whole medical life of each case, which also enhanced the value of correspondent samples and made patient centralized medical records much easier to achieve.
Sample collections
By September 2013, more than 12,000 tissues and blood specimens from 1,326 glioma tissue donors (28.98 % of total glioma surgeries) were collected and stored in the GTB, after inform consents were signed. The number of specimens was steadily growing at a rate of 30–60 cases (about 500 specimens) per month since then. The demographic and clinical characteristics of the 1,326 cases that generated automatically from glioma patient management system was described in Table 2. The distribution of pathology, which was categorized according the 2007 World Health Organization classification of tumors of central nervous system, was shown in Table 3 and Fig. 4.
The distribution of pathology in glioma tissue bank of Huashan Hospital generated from the clinical database automatically. By September, 2013, tissues samples from 1,326 cases were collected. Histological diagnosis reveals that 73 % were astrocytic tumors, 17 % were oligodendroglial tumors, 2 % were oligoastrocytic tumors, 4 % were ependymal tumors and 4 % were other central nervous system neoplasms
Discussion
Currently, most domestic biobanks are solitary organizations. Single a isolated biobank may be able to provide enough amount of samples of a certain high incidence disease. However, the power is too low to significantly contribute to investigating of the distribution and prevalence of different gliomas. In an extremely large nation like China, with a huge population and diverse ethnic minorities, the study result based on samples provided by single isolated biobank will be biased by each institute’s patient selection criteria. At the same time, a single-institute-level biobanks cannot meet the demand of large-scale researches of low incidence disease. What’s more important, the initial purpose of setting up a biobank is not only preserving specimens but also unlocking these treasures, conducting translational researches and improving the prognosis of the patients in the end. Information sharing and cooperation between different biobanks are of vital importance during this whole processes. The goal of Shanghai Biobank Network is to unite different single disease biobanks in different hospitals to set up a regional biobank cooperation network, and in the future, a part of national biobank network. Establishment of our GTB may set an example to other single diseases biobanks.
The establishment of a standardized tissue bank involves the cooperation in multiple departments, such as the departments of surgery, pathology and information technology. The department of surgery or pathology should take a leading role in the establishment and maintenance of a tissue bank. Since the gross morphology is critical for the pathological tumor-node-metastasis (TNM) classification of the body cancers such as gastrointestinal malignancies and esophageal cancer et al., the involvement of pathologists is indispensable in specimen acquisition. Therefore, the pathologist plays an essential role in tissue banking of body tumors. Unlike staging of body tumors, the pathological confirmation of glioma only involves tumor grading which mostly depends on histopathology instead of gross morphology. Accordingly, there is no need to constantly oversee the specimen procurement for neuropathologists. In our experience, the neuropathologists should participate in the collection of specimen and supervise the procedures of sampling at the beginning of establishing GTB. They should train the technicians to select typical glioma tissue. In other words, we could collect the specimen in operating room instead of histopathology department to reduce the lag time from excision of tissue to freezing. On the other hand, the department of neurosurgery could dominate the GTB, and then gain more support from all neurosurgeons. There are more than one hundred neurosurgeons and more than ten surgical teams in our department. The coordination between neurosurgeons and committee of GTB is crucial to the sample collection. Hence, the establishment of a suitable organizational structure ensures an effective and efficient cooperation.
Ideally, snap freezing the tissue should be done as soon as possible after excision to ensure that there is minimal degradation of the specimen. However, there is no consensus on the exact lag time from excision to snap freezing. The TuBaFrost project recommended that the lag time be 30 min, based on the data from the various studies. On account of no studies focus on the lag time on glioma, we accepted the recommendation from TuBaFrost project. Nonetheless, the time varies among different tissues. In our previous study, we detected that the surrounding tissues (edema tissue and normal brain tissue) degraded faster than the glioma tissue. It indicated that the surrounding tissues should be snap frozen in a shorter time. Further research should quantify the effects of lag time on the glioma and surrounding tissues.
It should be noted that our GTB has some limitations. Firstly, although some specimens from GTB have been used successfully in a variety of molecular techniques, the quality of all samples is uncertain. Therefore, it is necessary to establish a quality control system. RNA and DNA should be examined and other molecular techniques should be performed periodically on a random sampling to ensure the quality. What is more important, we need to conduct some translational medicine studies to analyze the quality of our samples. Secondly, even with our new system, the tissue bank in our institute is a separate bank at the moment. It is necessary to set up a sharable network among domestic hospitals. We could share the tissue samples and clinical information with different hospitals and institutes through our newly developed system. However, some domestic hospitals do not have GTBs because of their low patient number. While some other domestic hospitals have GTBs and still hesitate because of the uncertain rights and interests sharing during cooperation. Thirdly, sharing the samples and medical information requires a good protection of patients’ privacy, related guidelines should be established. Fourthly, rights and interests sharing during cooperation and legal issues about intellectual property rights still need more consideration.
Since the biospecimens resource is one of the national strategic resources, our government gradually pays more attention to the establishment and maintenance of biobanks. Recently, taking advantage of rich genetic resources of nervous and mental diseases in Huashan Hospital of Fudan University, Bio-X biomedical research center of Jiaotong University and Shanghai mental health center, a platform aimed to standardize the procedures for the collection, storage, distribution, use and disposal of specimens is under construction. This platform sponsored by Shanghai government plans to collect the biospecimens of nervous and mental diseases, which are stored in major hospitals and centers, searchable and sharable on the Internet. Another program on this platform is the establishment of quality assurance and quality control system to verify that all specimens are handled appropriately and appropriate for the research protocol. Based on this platform, the national standards and biobanks will be promisingly generated. The GTB will be one of the most important parts of national biobanks.
References
Clement B, Chene G, Degos F (2009) A national collection of liver tumours: lessons learnt from 6 years of biobanking in France. Cancer Lett 286(1):140–144. doi:10.1016/j.canlet.2009.04.034
Gao HJ, Zhu MH (2008) Standardization on establishment and application of tumor bank. Zhonghua bing li xue za zhi Chinese J Pathol 37(12):797–798
Goebell PJ, Groshen S, Schmitz-Drager BJ, Sylvester R, Kogevinas M, Malats N, Sauter G, Barton Grossman H, Waldman F, Cote RJ (2004) The International Bladder Cancer Bank: proposal for a new study concept. Urol Oncol 22(4):277–284. doi:10.1016/s1078-1439(03)00175-3
Goebell PJ, Groshen S, Schmitz-Drager BJ, Sylvester R, Kogevinas M, Malats N, Sauter G, Grossman HB, Dinney CPN, Waldman F, Cote RJ (2005) Concepts for banking tissue in urologic oncology: the International Bladder Cancer Bank. Clin Cancer Res 11(2):413–415
Isabelle M, Teodorovic I, Morente MM, Jamine D, Passioukov A, Lejeune S, Therasse P, Dinjens WN, Oosterhuis JW, Lam KH, Oomen MH, Spatz A, Ratcliffe C, Knox K, Mager R, Kerr D, Pezzella F, van de Vijver M, van Boven H, Alonso S, Kerjaschki D, Pammer J, Lopez-Guerrero JA, Llombart Bosch A, Carbone A, Gloghini A, van Veen EB, van Damme B, Riegman PH (2006) TuBaFrost 5: multifunctional central database application for a European tumor bank. Eur J Cancer (Oxford, England: 1990) 42(18):3103–3109. doi:10.1016/j.ejca.2006.04.032
Ji JF (2005) Establishment of tissue bank in Peking University School of Oncology. J Peck Univ (Health Sciences) (chin) 37:329–330
Jian HG-lGYPM-xZ (2011) Establishment and management of hepatocarcinoma tissue bank and data base. J Clin Rehabil Tissue Eng Res 15(18). doi: 10.3969/j.issn.1673-8225.2011.18.030
Jiang T, Tang GF, Lin Y, Peng XX, Zhang X, Zhai XW, Peng X, Yang JQ, Huang HE, Wu NF, Chen XJ, Xing HX, Su TY, Wang ZC (2011) Prevalence estimates for primary brain tumors in China: a multi-center cross-sectional study. Chin Med J 124(17):2578–2583
Kerr C (2003) UK launch “virtual” tumour bank to improve treatment research. Lancet Oncol 4(5):264
Knox K, Kerr DJ (2004) Establishing a national tissue bank for surgically harvested cancer tissue. Br J Surg 91(2):134–136. doi:10.1002/bjs.4486
Lopez-Guerrero JA, Riegman PH, Oosterhuis JW, Lam KH, Oomen MH, Spatz A, Ratcliffe C, Knox K, Mager R, Kerr D, Pezzella F, van Damme B, van de Vijver M, van Boven H, Morente MM, Alonso S, Kerjaschki D, Pammer J, Carbone A, Gloghini A, Teodorovic I, Isabelle M, Passioukov A, Lejeune S, Therasse P, van Veen EB, Dinjens WN, Llombart-Bosch A (2006) TuBaFrost 4: access rules and incentives for a European tumour bank. Eur J Cancer (Oxford, England:1990) 42 (17):2924–2929. doi:10.1016/j.ejca.2006.04.030
Lu JF, Chen H, Wu JS, Mao Y, Zhou LF (2010) Preservation of RNA for glioma tissue bank: comparison of snap-frozen and RNAlater solid tissue storage methods. Chin J Clin Neurosci 18:199–202
Morente MM, Mager R, Alonso S, Pezzella F, Spatz A, Knox K, Kerr D, Dinjens WN, Oosterhuis JW, Lam KH, Oomen MH, van Damme B, van de Vijver M, van Boven H, Kerjaschki D, Pammer J, Lopez-Guerrero JA, Llombart Bosch A, Carbone A, Gloghini A, Teodorovic I, Isabelle M, Passioukov A, Lejeune S, Therasse P, van Veen EB, Ratcliffe C, Riegman PH (2006) TuBaFrost 2: Standardising tissue collection and quality control procedures for a European virtual frozen tissue bank network. Eur J Cancer (Oxford, England:1990) 42(16):2684–2691. doi:10.1016/j.ejca.2006.04.029
Qualman SJ, France M, Grizzle WE, LiVolsi VA, Moskaluk CA, Ramirez NC, Washington MK (2004) Establishing a tumour bank: banking, informatics and ethics. Br J Cancer 90(6):1115–1119. doi:10.1038/sj.bjc.6601678
Repositories ISfBaE (2012) Best practices for repositories: collection, storage, retrieval, and distribution of biological materials for research. Biopreserv Biobank 10(2):82. doi:10.1089/bio.2012.1022
Riegman PH, Dinjens WN, Oomen MH, Spatz A, Ratcliffe C, Knox K, Mager R, Kerr D, Pezzella F, van Damme B, van de Vijver M, van Boven H, Morente MM, Alonso S, Kerjaschki D, Pammer J, Lopez-Guerrero JA, Llombart Bosch A, Carbone A, Gloghini A, Teodorovic I, Isabelle M, Jamine D, Passioukov A, Lejeune S, Therasse P, van Veen EB, Lam KH, Oosterhuis JW (2006a) TuBaFrost 1: Uniting local frozen tumour banks into a European network: an overview. Eur J Cancer (Oxford, England:1990) 42(16):2678–2683. doi:10.1016/j.ejca.2006.04.031
Riegman PH, Oomen MHA, Dinjens WNM, Oosterhuis JW, Lam KH, Spatz A, Ratcliffe C, Knox K, Mager R, Kerr D, Pezzella F, Van Damme B, Van De Vijver M, Van Boven H, Morente MM, Alonso S, Kerjaschki D, Pammer J, Lopez-Guerrero JA, Llombart-Boschi A, Carbone A, Gloghini A, Teodorovic I, Isabelle M, Passioukov A, Lejeune S, Therasse P, Van Veen EB (2006b) Tubafrost: European virtual tumor tissue banking. New trends in cancer for the 21st century, 2nd edn, vol 587, pp 65–74
Teodorovic I, Isabelle M, Carbone A, Passioukov A, Lejeune S, Jamine D, Therasse P, Gloghini A, Dinjens WN, Lam KH, Oomen MH, Spatz A, Ratcliffe C, Knox K, Mager R, Kerr D, Pezzella F, van Damme B, van de Vijver M, van Boven H, Morente MM, Alonso S, Kerjaschki D, Pammer J, Lopez-Guerrero JA, Llombart Bosch A, van Veen EB, Oosterhuis JW, Riegman PH (2006) TuBaFrost 6: virtual microscopy in virtual tumour banking. Eur J Cancer (Oxford, England: 1990) 42 (18):3110–3116. doi:10.1016/j.ejca.2006.04.033
van Veen EB, Riegman PH, Dinjens WN, Lam KH, Oomen MH, Spatz A, Mager R, Ratcliffe C, Knox K, Kerr D, van Damme B, van de Vijver M, van Boven H, Morente MM, Alonso S, Kerjaschki D, Pammer J, Lopez-Guerrero JA, Llombart Bosch A, Carbone A, Gloghini A, Teodorovic I, Isabelle M, Passioukov A, Lejeune S, Therasse P, Oosterhuis JW (2006) TuBaFrost 3: regulatory and ethical issues on the exchange of residual tissue for research across Europe. Eur J Cancer (Oxford, England:1990) 42 (17):2914–2923. doi:10.1016/j.ejca.2006.04.028
Acknowledgments
The authors would like to thank all the nurses of operating rooms and wards for their cooperation during the collection; Jian-bing Shi and Zhong Yang for the management and collection of image database; Qiong-ji Zhu for the collection of tissue; Zhen-xiao Wang and Yan Chen for the input of clinical information.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Abudumijiti Aibaidula and Jun-feng Lu contributed equally to this study
Rights and permissions
About this article
Cite this article
Aibaidula, A., Lu, Jf., Wu, Js. et al. Establishment and maintenance of a standardized glioma tissue bank: Huashan experience. Cell Tissue Bank 16, 271–281 (2015). https://doi.org/10.1007/s10561-014-9459-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-014-9459-4