Abstract
The aim of this work is to quantify the total protein and growth factors content in a tissue-suspension obtained from processed human amniotic membrane (hAM). hAM was collected, frozen, freeze dried, powdered and sterilized by γ-irradiation. At each step of the process, samples were characterized for the total protein amounts by a Bradford protein assay and for the growth factor concentrations by ELISA test of the tissue suspensions. Frozen-hAM samples show higher release of total proteins and specific growth factors in the tissue suspension in comparison with freeze-dried hAM. We observed that even if the protein extraction is hindered once the tissue is dried, the powdering process allows a greater release in the tissue suspension of total proteins and growth factors after tissue re-solubilization in comparison with only the freeze-drying process (+91 ± 13% for EGF, +16 ± 4% for HGF, +11 ± 5% for FGF, +16 ± 9% for TGF-β1), and a greater release of EGF (85 ± 10%) in comparison with only the freezing process, because proteins become much readily solubilized in the solution. According with these results, we describe a protocol to obtain a new sterile biological product from hAM tissue, with well-known effects of thermal, mechanical and physical processes on the total protein and grow factors contents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of hAM in ocular surgery was first suggested by Sorsby (Sorsby and Symmons 1946; Sorsby et al. 1947), who examined its role in the management of ocular surface damage. Very good results were reported but, for no evident reason, its use was abandoned or went unreported until recently. The early 1990s were the starting point in tracing the modern history of the use of hAM in ophthalmic surgery, by introducing the now well-accepted method of preservation and storage, by adding considerably to the knowledge of the key components of the membrane and by developing some of the current surgical techniques (Dua et al. 2004).
The functional and structural similarities among different kinds of tissues, such as hAM and cornea, could explain the beneficial effects of hAM transplants in ocular surgery. The mechanisms of action of the membrane are inferred from the composition of the membrane, rather than proven scientifically, in relation to its application in ocular surgery (Dua et al. 2004). Among the properties of hAM, it acts as a substrate or basement membrane for epithelial cell migration and stratification (Azuara-Blanco et al. 1999; Lee and Tseng 1997), as a promoter of epithelialization (Batmanov et al. 1990; Subrahmanyam 1995), as a carrier for ex vivo expansion of corneal epithelial cells (Tseng et al. 2002; Meller et al. 2002), as a suppressor of inflammatory response (Lee et al. 2000), as an inhibitor of angiogenesis (Shao et al. 2004; Jiang et al. 2006), as an anti-microbial agent (Robson and Krizek 1973). Due to these multiple properties, the hAM is currently used in very kind of application in ocular surgery (Chen et al. 2000; Pires et al. 1999; Paridaens et al. 2001; Prabhasawat et al. 1997; Rodriguez-Ares et al. 1999).
In Kim and Tseng 1995 first reported the transplantation of preserved hAM for corneal surface reconstruction in a rabbit model.
More recently, some papers have investigated the potential effects of preservative processes (cryopreservation, freezing, lyophilization, sterilization following lyophilization) on the in vitro properties of hAM. Studies on hAM preserved at −80°C for 1 month revealed the presence of EGF, TGF-α, KGF, HGF, bFGF, TGF-β1, and -β2 by RTPCR for the mRNA and by ELISA for the protein products. TGF-β3 and growth factor receptors KGFR and HGFR were also detected by RT–PCR. A higher level of various growth factors were found in hAM with epithelium than without epithelium indicating an epithelial origin for these growth factors (Koizumi et al. 2000).
Nakamura et al. (2004) analysed the physical, immunohistochemical and morphological characteristics of lyophilized hAM observing similar characteristics in comparison with the cryopreseved hAM; they suggested the lyo-preserved tissue as an useful biomaterials for ocular surface reconstruction bypassing the biological and technical problems of the cryopreservation process.
Rodriguez-Ares et al. (2009) valuated the effects of lyophilization and cryopreservation on hAM in terms of histological characteristics and growth factor levels. The authors observed that lyophilization maintains the histological structure of hAM, even if it seems to cause greater reduction in total protein amount and growth factor concentration than cryopreservation.
These papers reported the effectiveness of sterilized, freeze-dried hAM used for ocular surface reconstruction, investigating effects of preservative processes on the in vitro and in vivo (Nakamura et al. 2004; Nakamura et al. 2006; Sekiyama et al. 2007) properties of hAM.
The idea of a new biological medicinal product for human use, based on the processed hAM, was first developed by Bonci et al. (2005) who investigated the in vivo effectiveness of a suspension made with homogenized hAM, in 21 patients with ocular surface diseases, proposing a new therapy less traumatic than implant. Nevertheless, also the homogenized tissue left some unsolved logistic and biological problems, first of all the homogenized hAM could be stored only for short periods without deterioration; the procedure could not guarantee a completely sterile hAM because of its biological origins; moreover it could be necessary an analysis of the biological properties to be correlated to the in vivo beneficial effects. Therefore, there have been no papers reporting the in vitro characterization of freeze-dried, powdered and sterilized hAM for topical use as a tissue suspension.
The aim of this work is to quantify the total protein amount and growth factors concentrations in a tissue-suspension obtained from processed human amniotic membrane (hAM). The authors describe a protocol to obtain a new sterile biological product from hAM tissue with well-known effects of thermal, mechanical and physical processes on the total protein and grow factors contents. The authors propose testing this new form of processed-tissue, in order to be used as an eyewash, as a substitute or in addition to surgical procedures.
Materials and methods
Isolation of hAM
Human placentae were handled according to the tenets of the Declaration of Helsinki. The research was approved by the institutional human experimentation committee (local ethical committee). Individuals with a history of drug or alcohol abuse and multiple sexual partners were excluded. Informed consent was obtained from all donors after explanation of the nature and possible consequences of the study. Screening for communicable diseases (specifically HIV 1-2, HBV, HCV, TPHA, VDRL, CMV, Toxo) was carried out. HIV 1-2, HBV, HCV tests were performed by molecular quantitative methods (RT–PCR); the tissues were used only if all tests, on both occasions, were negative or non-reactive. Human placenta was collected after caesarean delivery; the placenta was immediately processed under sterile conditions. First, it was washed with sterile saline solution 0.9% to remove blood clots. The hAM was carefully detached from the chorion and rinsed several times with a saline solution 0.9% containing antibiotics, a cocktail to cover Gram-negative and Gram-positive bacteria, and antimycotics (Vancomycin 33 μg/ml, Bramicil/Tobramycin 16 μg/ml, Dalacin/clindamycin 10 μg/ml, Fungizone/amphotericin B 16 μg/ml). Then, hAM was washed again with sterile saline solution 0.9% to remove antibiotics and antimycotics. All the subsequent analysis were performed on hAM with the epithelial layer.
In this work, we totally processed two hAM. From each of them, we obtained 5 patches. Each patch has been subsequently stored by one of the five different preservation processes following described. Thus, for each different preservation process, we analysed two samples prepared from different donors. The number of determinations is two or four per samples. As control, samples of frozen-hAM and frozen for surgical use-hAM were used.
Preparation of frozen for surgical use-hAM sample (FS-hAM)
Normally, patches (4 × 4 cm) of hAM were cut and stored for surgical use. Patches were spread on a nitrocellulose paper, with the epithelial side up, laid in the freezing solution [80% Dulbeco’s modified Eagle Medium without L-glutamin and phenol red (DMEM, Gibco), 10% human albumin 20% solution from human plasma (Kedrion Biopharmaceuticals), 10% DMSO Hibry-max, hybridoma and endotoxin tested (Sigma–Aldrich)] and stored at −80°C into cryovial tubes until use. In this work, the samples were stored at −80°C until processing, at the concentration of 1 g sample/1 ml freezing solution.
Preparation of frozen-hAM sample (F-hAM)
One gram samples of hAM were cut and stored in Dulbecco’s phosphate buffered saline (DPBS, Gibco, Invitrogen) 1× at −80°C until processing, at the concentration of 1 g sample/1 ml DPBS 1×.
Preparation of freeze dried-hAM sample (D-hAM)
One gram samples of F-hAM were freeze-dried by Lio 5Pascal (−45°C, 0.05 mBarr, 14 h). Samples of D-hAM were stored at room temperature until processing into a sterile closed container.
Preparation of freeze dried and powdered-hAM sample (P-hAM)
Samples of D-hAM were powdered (3′ at room temperature) by powder-machine MM440 (Retsch), previously cooled by liquid nitrogenum (5′). Samples of P-hAM were stored at room temperature until processing into a sterile closed container.
Preparation of freeze dryed, powdered and irradiated-hAM sample (I-hAM)
Samples of P-hAM were irradiated by γ-rays (25 KGy) at Gammatom srl, Guanzate (Co-Italy). Samples of I-hAM were stored at room temperature until processing.
Weight of wet and dry samples
We weighed each sample (FS-hAM, F-hAM, D-hAM, P-hAM, I-hAM), in order to measure the weight changes during different preservation processes.
Total protein amount and growth factor concentration
FS-hAM and F-hAM samples were thawed out and incubated at room temperature 40°C in agitation to obtain a homogeneously defrosted tissue. D-hAM, P-hAM and I-hAM samples were resuspended in 1 ml DPBS 1× at room temperature and incubated at room temperature 40’ in agitation to obtain a homogeneous suspension. Then, all samples were centrifuged at 10,000 g for 15 min at 4°C, and the supernatants were isolated to measure total protein amounts and grow factor concentrations.
Total protein amounts were measured in the supernatant from the tissue suspension using the Bradford Protein Assay Kit (Bio-Rad Laboratories, Inc, Hercules, CA). The optical density was read by the DU®530 UV–VIS Life Science spectrophotometer (Beckman Coulter) at 595 nm. Results were expressed as milligrams of total proteins per grams of tissue. The means (from 4 determinations) used for the data presentation came from two independent experiments (on tissues from two donors); data were statistically analysed by two-tailed t test.
The same amount of total proteins was used in all samples to determine growth factor concentrations.
Epidermal growth factor (EGF), hepatocyte growth factor (HGF), keratinocyte growth factor (KGF), fibroblast growth factor basic (bFGF) and transforming growth factor- β1 (TGF-β1) concentrations were measured in the supernatant from the tissue suspension using a commercially available enzyme-linked immunoassay kit (Quantikine enzyme-linked immunosorbent assay kits, DEG00 hEGF, DKG00 hKGF, DHG00 hHGF, DFB50 hFGFbasic, DB100B hTGF-β1- R&D Systems, Minneapolis, Minneapolis, MN, USA). The optical density was read by the microplate photometer Multiskan Ascent 354 (Labsystems); the measurements were performed according to the manufacturer’s instructions. The minimal detection limits for each factor were as follows: EGF 0.84 pg/ml, HGF 2.93 pg/ml, KGF 2.05 pg/ml, bFGF 1.0 pg/ml, TGF-β1 2.20 pg/ml.
The means used for the data presentation were obtained as follows: the number of samples is 2 per test, each of samples is prepared from different donors; the number of determinations is two or four per samples. The concentration of growth factors in the supernatant from the hAM suspension were expressed as picograms of growth factor per gram of wet tissue. Data were statistically analysed by two-tailed t test.
The percentage ratios of growth factors release (% pg each growth factor in D-hAM or P-hAM/pg each growth factor in F-hAM) were expressed as mean of ratios of single determinations, with respective standard deviations, and not as the ratio of the means of single determinations, in order to reduce experimental variability.
Results
In order to quantify how the preservative process (freezing, freeze-drying, powdering and γ-irradiating) affects the final weight of the hAM tissue suspension, we first weighed wet and dry samples after each step of the processing. In Table 1, the results show that the final weight reduction after processing is 94% of wet initial weight, due either to the water elimination from the tissue (91.76%) or to the loss of tissue micro-particles during powdering (2.14%) and transferring into final sterile tubes for irradiation (0.11%).
Total protein amounts in F-hAM, D-hAM and P-hAM samples are given in Table 2. F-hAM samples show the highest release of total proteins in the tissue suspension, revealing statistically significant differences with D-hAM samples and with P-hAM samples (P < 0.01). D-hAM samples show lower total protein release than F-hAM samples (−39%); P-hAM samples show lower total protein release than F-hAM samples (−24%) but higher than D-hAM.
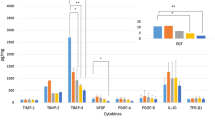
The growth factor concentrations in F-hAM, D-hAM and P-hAM samples are shown in Fig. 1. The highest growth factor release in the three studied groups are for HGF. Statistically relevant differences are found between the groups of analysed samples (P < 0.01) except for differences between F-hAM EGF and D-hAM EGF, and differences between D-hAM KGF and P-hAM KGF. The percentage ratios of growth factors release (% pg each growth factor in D-hAM or P-hAM/F-hAM) are shown in Fig. 2. As with the total protein amounts, D-hAM samples show lower growth factors release in the tissue suspension than F-hAM samples (EGF, HGF, KGF, FGF, TGF-β1). The powdering process allows a slightly increased release of 3 growth factors (+16 ± 4% for HGF, +11 ± 5% for FGF, +16 ± 9% for TGF-β1) after tissue re-solubilization in comparison with only the freeze-drying process and a strongly increased release of EGF in comparison with D-hAM samples (+91 ± 13%) and in comparison with F-hAM samples (+85 ± 10%).
a Table of growth factor concentration (pg growth factor/g fresh tissue, w/w). b Histograms of the distribution of growth factor concentration in the three groups of analysed samples. Data were expressed as mean of at least 2 at most 4 determinations from two independent experiments (on tissues from two donors); the bars represent standard deviations
a Table of the percentage ratio of growth factor concentrations (% pg each growth factor in D-hAM or P-hAM vs pg each growth factor in F-hAM). b Histograms of the percentage ratio of growth factor concentrations in the three groups of analysed samples (F-hAM is fixed to 100, as a reference starting point). The bars represent standard deviations
In order to estimate a quantitative and qualitative comparison between the hAM tissue suspension and the frozen tissue patch (4 × 4 cm) required for surgical use, we performed the following EGF concentration measurements.
In order to exclude that the F-hAM freezing solution (DPBS) could have any effect on the EGF protein, in terms of degradation and ability to recognize a specific epitope, in comparison with the FS-hAM freezing solution [80% Dulbeco’s modified Eagle Medium without L-glutamin and phenol red (DMEM, Gibco), 10% human albumin 20% solution from human plasma (Kedrion Biopharmaceuticals), 10% DMSO Hibry-max, hybridoma and endotoxin tested (Sigma–Aldrich)] we measured EGF concentration in F-hAM and in FS-hAM. In fact, during the preparation of the tissue-suspension, we avoided to use DMSO as a cryoprotective agent to protect biological tissue from damage due to ice formation. Since freeze-drying is a passage from a solid phase (wet frozen tissue) to an other solid phase (dry tissue), we cannot remove DMSO from sample before freeze-drying such as we usually do in FS-hAM, by thawing out the tissue and changing the storage buffer, 24 h before surgical implant. The EGF concentrations in F-hAM and FS-hAM samples are shown in Fig. 3; the percentage ratio of EGF concentrations (% pg EGF F-hAM/FS-hAM) is 110.02 ± 2.81.
a Table of EGF concentration (pg EGF/g fresh tissue, w/w). b Histograms of the distribution of EGF concentration in the two groups of analysed samples (FS-hAM and F-hAM). Data were expressed as mean of at least 2 at most 4 determinations from two independent experiments (on tissues from two donors); the bars represent standard deviations
In order to exclude possible γ-ray irradiation effects on the protein degradation and consequently on the protein content, we measured EGF concentration in P-hAM and in I-hAM (Fig. 4). P-hAM samples showed the same EGF concentrations as I-hAM samples; the percentage ratio of EGF concentrations (% pg EGF I-hAM/P-hAM) is 98.72 ± 0.67.
a Table of EGF concentration (pg EGF/g fresh tissue, w/w). b Histograms of the distribution of EGF concentration in the two groups of analysed samples (P-hAM and I-hAM). Data were expressed as mean of 2 determinations from two independent experiments (on tissues from two donors); the bars represent standard deviations
Comparing quantitatively a patch of amniotic membrane frozen for surgical use with the powdered tissue, processed as here described, a 4 × 4 cm patch normally used for surgical use weighs 374 mg (Table 1) and a dose of 30 mg of I-hAM is prepared from 500 mg of fresh wet tissue. The growth factor reduction (−21 ± 4% HGF; −21 ± 10% KGF; −17 ± 5% FGF; −25 ± 7% TGF- β1;) observed in the powdered product (calculated for the same quantity of starting tissue) could be balanced by the increase of starting tissue amount (500 mg instead of 374 mg, +34%).
Discussion
In this work we examine the properties in terms of weight, total protein amount and growth factors concentrations in a tissue-suspension obtained from processed hAM. These characterization is not an index for predicting hAM’s action in case of in vivo future use, but it represents a starting point in term of composition of these new biological product.
In fact, the biological characterization of sterilized, freeze-dried AM used in ocular surface reconstruction and its effectiveness has been already reported (Nakamura et al. 2004; Nakamura et al. 2006; Sekiyama et al. 2007). This is the first work focused on the biological content of freeze-dried, powdered and sterilized hAM specifically prepared for topical use; any clinical trials on its possible in vivo effectiveness have been already reported.
The only clinical trial on processed hAM produced for topical use studied the effectiveness of a homogenized hAM (Bonci et al. 2005). In that study, the production of the tissue suspension has been performed in sterile conditions but without final sterilization. Moreover, the homogenized hAM could be stored, after thawing out, only for short periods without deterioration and therefore the procedure could not guarantee a convenient use and a long term storage of the tissue suspension. In this work we achieved the goal of a safe and convenient use, by preserving hAM in the dry state and using γ-irradiation for sterilization.
Our quantification of total protein and specific growth factor levels in the freeze-dried, powdered and γ-irradiated hAM were particular interesting. We observed that freeze-drying process causes a reduction in total protein and in specific growth factors release in comparison with only freezing; this reduction in our experimental condition is not so drastic as previously described by Rodriguez-Ares et al. (2009). Understandably, total proteins and specific growth factors re-solubilization is hindered once the tissue is dried.
The powdering process allows a strongly increased release of EGF after tissue re-solubilization compared with only freeze-drying (+91 ± 13%). A drastic effect of powdering on the EGF release in comparison also with only freezing has to be noted (+85 ± 10%). The protein has been powdered so that it became much readily solubilized in the solution, because of the much bigger surface of exposure to the aqueous solvent in the micro-particles of powdered sample. We observed this behaviour in response to the powdering process only for EGF among the other analysed growth factors, supposedly because of the molecular weight and solubility of EGF protein.
In the mammalian eyes, EGF is one of the biologically most potent and best characterized growth factors: it stimulates proliferation, chemotaxis/migration and wound healing of epithelial cells and keratocytes of animal and human species (Imanishi et al. 2000, Hoppenreijs et al. 1996).
The differences in measuring total proteins and grow factors are intrinsically dependent on the methods of preparing hAM; due to these results, we propose to look careful at the powdering process as a way of strongly improving the protein release in the tissue supension.
In order to compare quantitatively and qualitatively a patch of amniotic membrane frozen for surgical use with a tissue-suspension of I-hAM, we checked that the absence of a cryoprotective agent (DMSO) in the freezing buffer and the γ-ray irradiation did not affect the EGF protein levels and its ability to recognize a specific epitope as a target. By the results we exclude any affect on the degradation of the protein.
Kruse and Cursiefen (2008) demonstrated that hAM grafts function primarily as a matrix and not by virtue of transplanted functional cells. Since the viability of the tissue components of the hAM is not essential for its biological effectiveness, we directly compare hAM used for surgical use and the hAM tissue suspension used for topical therapeutic use, because both of them are not viable. On the basis of these results, we have shown that the a specific amount (30 mg) of sterilized powdered freeze-dried hAM retains the characteristics of a patch (4 × 4 cm) of hAM frozen for surgical use.
Moreover, it is known that freeze-dried tissue, because of the water removal, could potentially be stored for longer periods without deterioration in comparison with frozen tissue when thawed out. We suppose that the sterilized powder could be stored dry for long periods without deterioration and could be mixed to an opportune balanced saline solution (BSS) to obtain a tissue re-suspension when necessary.
In conclusion, this is the first study focused on the biological content of freeze-dried, powdered and sterilized new sterile biological obtained a from hAM tissue with well-known effects of thermal, mechanical and physical processes on the total protein and grow factors contents. We propose testing this new form of processed-tissue, to be used as an eyewash, as a substitute or in addition to surgical procedures, because it could be helpful in solving the logistic and biological problems related to hAM preservation methods and in removing or reducing the clinical complications of the its current surgical use. This new kind of topical therapy shows several advantages: the tissue suspension can easily be prepared in eye banks; it is microbiologically safe; it could potentially be stored for a long period and consequently used when necessary; its use is less traumatic than an hAM implantation; it can be used for continuous and periodic topical applications; the simple preparation has a high potential to be used in the treatment of a great number of ocular surface diseases.
References
Azuara-Blanco A, Pillai CT, Dua HS (1999) Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol 83:399–402
Batmanov IE, Egorova KS, Kolesnikova LN (1990) Use of fresh amnion in the treatment of corneal diseases. Vestn oftalmol 106:17–19
Bonci P, Bonci P, Lia A (2005) Suspension made with amniotic membrane: clinical trial. Eur J Ophthalmol 15:441–445
Chen HJ, Pires RT, Tseng SC (2000) Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol 84:826–833
Dua HS, Gomes JAP, King AJ, Maharajan VS (2004) The amniotic membrane in ophthalmology. Surv Ophthalmol 49:51–77
Hoppenreijs VPT, Pels E, Vrensen GFJM, Treffers WF (1996) Corneal endothelium and growth factors. Surv Ophthalmol 41:155–164
Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S (2000) Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res 19:113–129
Jiang A, Li C, Gao Y, Zhang M, Hu J, Kuang W, Hao S, Yang W, Xu C, Gao G, Wang Z, Liu Z (2006) In vivo and in vitro inhibitory of amniotic extraction on neovascularization. Cornea 25(10 Suppl 1):S36–S40
Kim JC, Tseng SC (1995) Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea 14:473–484
Koizumi NJ, Inatomi TJ, Sotozono CJ (2000) Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res 20:173–177
Kruse FE, Cursiefen C (2008) Surgery of the cornea: corneal, limbal stem cell and amniotic membrane transplantation. Dev Ophthalmol 41:159–170
Lee SH, Tseng SC (1997) Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol 123:303–312
Lee SB, Li DQ, Tan DT (2000) Suppression of TGF-beta signalling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res 20:325–334
Meller D, Pires RT, Tseng SC (2002) Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol 86(4):463–471
Nakamura T, Yoshitani M, Rigby H, Fullwood NJ, Ito W, Inatomi T, Sotozono C, Nakamura T, Shimizu Y, Kinoshita S (2004) Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci 45:93–99
Nakamura T, Inatomi T, Sekiyama E, Ang LPK, Yokoi N, Kinoshita S (2006) Novel clinical application of sterilized, freeze-dried amniotic membrane to treat patients with pterigium. Acta Ophthalmol Scand 84:401–405
Paridaens D, Beekhuis H, van Den Bosch W (2001) Amniotic membrane transplantation in the management of conjunctival malignant melanoma and primary acquired melanosis with atypia. Br J Ophthalmol 85:658–661
Pires RT, Tseng SC, Prabhasawat P (1999) Amniotic membrane transplantation for symptomatic bullous keratopathy. Arch Ophthalmol 117:1291–1297
Prabhasawat P, Barton K, Burkett G, Tseng SC (1997) Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology 104:974–985
Robson MC, Krizek TJ (1973) The effect of human amniotic membranes on the bacteria population of infected rat burns. Ann Surg 177:144–149
Rodriguez-Ares MT, Tourino R, Capeans C, Sanchez-Salorio M (1999) Repair of scleral perforation with preserved scleral and amniotic membrane in Marfan’s syndrome. Ophthalmic Surg Lasers 30:485–487
Rodriguez-Ares MT, Lopez-Valladares JM, Tourino R, Vieites B, Gude F, Silva MT, Couceiro J (2009) Effects of lyophilization on human amniotic membrane. Acta Ophthalmol 87(issue 4):396–403. doi:10.1111/j.1755-3768.2008.01261.x
Sekiyama E, Nakamura T, Kurihara E, Cooper LJ, Fullwood NJ, Takaoka M, Hamuro J, Kinoshita S (2007) Novel sutureless transplantation of bioadhesive-coated, freeze-dried amniotic membrane for ocular surface reconstruction. Invest Ophthalmol Vis Sci 48:1528–1534
Shao C, Sima J, Zhang SX, Jin J, Reinach P, Wang Z, Ma JX (2004) Suppression of corneal neovascularization by PEDF release from human amniotic membranes. Invest Ophthalmol Vis Sci 45(6):1758–1762
Sorsby A, Symmons HM (1946) Amniotic membrane grafts in caustic burns of the eye (burns of second degree). Br J Ophthalmol 30:337–345
Sorsby A, Haythorne J, Reed H (1947) Further experience with amniotic membrane grafts in caustic burns of the eye. Br J Ophthalmol 31:409–418. doi:10.1136/bjo.31.7.409
Subrahmanyam M (1995) Amniotic membrane as a cover for microskin grafts. Br J Plast Surg 48(7):477–478
Tseng SC, Meller D, Anderson DF, Touhami A, Pires RT, Grüterich M, Solomon A, Espana E, Sandoval H, Ti SE, Goto E (2002) Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane for treating corneal diseases with total limbal stem cell deficiency. Adv Exp Med Biol 506(Pt B):1323–1334
Acknowledgments
The authors thank Manuela Voltattorni, (MSc, PhD, Interdepartmental Centre of Biotechnological Research (CIRB) of University of Bologna) for assistance during the ELISA procedure and for the precious suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Russo, A., Bonci, P. & Bonci, P. The effects of different preservation processes on the total protein and growth factor content in a new biological product developed from human amniotic membrane. Cell Tissue Bank 13, 353–361 (2012). https://doi.org/10.1007/s10561-011-9261-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-011-9261-5