Abstract
Contrast-induced acute kidney injury (CI-AKI) is defined as an abrupt deterioration in renal function associated with the administration of iodinated contrast media. This type of acute kidney injury is frequently encountered as a complication of percutaneous coronary intervention (PCI) and is associated with adverse short- and long-term outcomes including mainly mortality, cardiovascular morbidity and prolongation of hospitalization. The incidence of CI-AKI after PCI ranges from 2 to 20 % according to baseline kidney function. It may also range according to the clinical setting, being higher after emergency PCI. The primary manifestation is a small decline in kidney function, occurring 1 to 3 days after the procedure. Kidney function usually returns to preexisting levels within 7 days. Incidence of acute renal failure requiring dialysis following PCI is rare (<1 %). The present article aims to review up-to-date published data concerning diagnosis, definition, epidemiology and prognosis of this novel in-hospital epidemic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Contrast-induced acute kidney injury (CI-AKI) is defined as an abrupt deterioration in renal function associated with the administration of iodinated contrast media, in the absence of another etiology [1]. This type of acute kidney injury is frequently encountered as a complication of percutaneous coronary intervention (PCI) and is associated with adverse short- and long-term outcomes [2–6]. Radio-contrast associated kidney injury is the third leading cause of acute kidney injury in hospitalized patients [7].
The incidence of acute renal insufficiency after PCI ranges from 2 % in those patients with normal baseline renal function to as high as 20–30 % in those patients with a baseline creatinine > 2 mg/dL) prior to PCI [6, 8]. It may also range according to the clinical setting, as the incidence of CI-AKI has been found to be higher after emergency PCI compared to elective PCI [9]. Nash et al. [10] reported that 11 % of hospital-acquired renal insufficiency cases are due to contrast media (CM), with coronary angiograms and PCI being the leading cause. The incidence of acute renal failure requiring dialysis following PCI, however, is fortunately rare and is < 1 % [11]. Having in mind that PCI is the treatment of choice in patients with coronary artery disease (CAD) and that advancement in technology and resources allowed the application of this treatment modality in increasing number of patients, it is logical to hypothesize that this novel pathologic entity could reach epidemic proportions. Of interest, it is estimated that 500,000 patients undergo PCI in the United States every year [12]. In addition, the continuous aging of the world population results in a higher prevalence of CI-AKI risk factors (such as renal impairment or diabetes mellitus). This fact may also cause the overall importance of CI-AKI as a clinical entity to be augmented in the future [13].
The primary manifestation of CI-AKI is a small decline in kidney function, occurring 1 to 3 days after the procedure [14]. Kidney function usually returns to preexisting levels within 7 days [15], and acute kidney injury after radio-contrast administration rarely requires acute dialysis treatment [14]. Despite these small declines in kidney function, several studies have suggested that CI-AKI is associated with short- and long-term adverse clinical outcomes including in-hospital morbidity, longer hospitalizations, subsequent cardiovascular events, progression to chronic kidney disease (CKD) and increased mortality [4–6, 16]. The observation that CI-AKI affects patients with a greater burden of co-morbidities such as pre-existing renal insufficiency, diabetes mellitus, heart failure or advanced age, suggested that adverse outcomes in patients who develop CI-AKI also reflects this burden. On the other hand, the findings of prospective studies, in which patients with CI-AKI had a 20-fold increase in mortality during their hospital stay, raise the possibility that CI-AKI may independently contribute to an increased risk of early morbidity and mortality [5, 6, 17]. Even after adjusting for co-morbidities, in-hospital mortality is about five-fold higher in CI-AKI patients than in patients who received CM but did not develop CI-AKI, and 1-year and 5-year mortality rates are about four-fold higher [6, 18, 19].
The present article aims to review up-to-date published data concerning pathophysiology, diagnosis, definition, epidemiology and prognosis of this novel in-hospital epidemic. Furthermore, we highlight some skeptical commentaries regarding our knowledge on CI-AKI and we present in brief the available guidelines concerning CI-AKI. A discussion of the various measures used to prevent CI-AKI falls outside the scope of this review article.
Pathophysiology
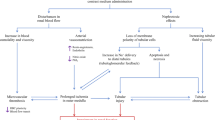
The pathophysiology of CI-AKI is very complex and incompletely understood. Some important information regarding the pathophysiology of CI-AKI are presented here, as this subject is reviewed in detail elsewhere [20, 21]. The development of CI-AKI seems to be based on a combination of mechanisms. CM administration causes renal hypoperfusion/hypoxia but has also been shown to exhibit direct toxicity on tubular epithelial cells. In support of this, the clinical course of CI-AKI, lasting several days, could reflect the progress of both CM clearance and tubular epithelial cell recovery [22].
Renal medulla represents the anatomic region of the kidney mostly affected by hypoxia, due to a combination of low vascular blood supply and high metabolic rate in this area [23]. Some decades ago, hypertonic contrast media were shown to decrease renal blood flow and glomerular filtration rate (GFR) in some classical animal studies [24–26]. In the years that followed, it was reported by various studies that adenosine is an important factor involved in the hemodynamic response of the kidney to CM administration. The concentration of adenosine in the urine is increased after administration of CM and this increase is proportional to the osmolality of the latter [27]. Using an adenosine receptor antagonist, such as theophylline, can attenuate renal function impairment in both animals and humans, whereas administration of an adenosine uptake inhibitor can lead to further impairment [27–29]. Adenosine is connected to the generation of ROS, which in turn are known to contribute to the modulation of renal vasoconstriction [30]. Although the mechanisms are not very clear, it is speculated that ROS could act by mediating the actions of various vasoconstrictors, such as angiotensin II, thromboxane A2, endothelin (ET) and adenosine itself, thus creating an extremely complex interplay among all these mediators [21]. ET seems to play some role in the pathophysiology of CI-AKI, since a selective blockage of ET-A receptors has been shown to attenuate renal medullary hypoxia in rats [31]. Noteworthy, blockade of both ET-A and ET-B receptors in human individuals undergoing coronary angiography exacerbated renal function compared to hydration alone [32]. The importance of ROS in orchestrating the CM-induced nephrotoxicity is highlighted by the fact that, after inhibiting generation of ROS using allopurinol, CM-induced reduction of GFR has been shown to be reduced in both animals [33] and humans [34].
Apart from the established effects of CM on renal blood flow, which lead to a decreased GFR, and although medullary hypoxia itself can also lead to extensive DNA damage in epithelial cells, as shown in animal models [35], it would be rational to assume that CM also exhibit some kind of direct cytotoxic effect on renal tubular epithelial cells, taken into account that CM are primarily excreted in the urine. The extent of this direct cytotoxic damage is related to the duration of the exposure of these cells to the CM [36]. Using a porcine tubule cell line, it was shown that CM can reduce cell proliferation and reversibly alter mitochondrial function [22]. This effect on mitochondria was relative to CM ionicity, molecular structure and osmolality. Regarding the latter, low-osmolar CM affected mitochondrial function to a lesser extent compared to iso-οsmolar and high-osmolar CM [22]. In addition, induction of cell apoptosis by CM also seems to play a role in direct tubular cell toxicity, even in the absence of hypoxia, especially in the hypertonic environment of the renal medulla [37].
Finally, increased resistance to flow is another mechanism implicated in the pathophysiology of CI-AKI, mainly when iso-osmolar CM are used. Iso-osmolar CM may harm renal function to a greater extent compared to low-osmolar CM [21]. Iso-osmolality dimers, used in iso-osmolar CM can increase the viscosity of renal ultrafiltrate and, thus, also increase resistance to flow in renal tubules [21, 38].
Contrast-Induced Acute Kidney Injury Diagnosis
CI-AKI is an impairment of renal function resulting from the administration of CM in the absence of an alternative etiology [17, 36]. According to the Contrast-Induced Nephropathy Consensus Working Panel, CI-AKI is defined as either an absolute increase in serum creatinine (sCr) concentration of 0.5 mg/dL (or 44.2 μM/L) or a 25 % relative increase in creatinine from baseline [5, 18]. The European Society of Urogenital Radiology defines CI-AKI as impairment in renal function (an increase in serum creatinine by >0.5 mg/dL or >25 % within 3 days after intravascular administration of CM, without an alternative etiology) [36, 39]. The Acute Kidney Injury Network definition includes a rise in serum creatinine ≥0.3 mg/dL with oliguria, which may be a new standard to follow [36]. The European Renal Best Practise (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) guidelines has recently proposed a new definition of CI-AKI, that of an increase by ≥50 % or by ≥ 0.3 mg/dL at 48 h [40]. Regarding the use of absolute or relative increase in creatinine levels the Contrast-Induced Nephropathy Consensus Working Panel has recommended using the relative increase in creatinine to define CI-AKI [41].
There is a considerable number of alternative definitions and cut-off values for sCr used to define CI-AKI in various published clinical trials and guidelines (Table 1, also see Supplementary Table 2). The varying definitions of CI-AKI produced a great amount of confusion since CI-AKI incidence and its association with clinical outcomes varied depending on the definition used [36, 39–49]. Furthermore, the effect of preventive treatment also varied according to the definition used [48, 49]. This controversy led a part of the medical community to argue regarding the true clinical entity of CI-AKI [50]. The wide variation in CI-AKI incidence and its relative association with adverse outcomes underscore the need for a standardized, clinically relevant definition. A possible consensus is possible as it has been shown that more strict definitions (i.e., an absolute increase of creatinine > 0.5 mg/dL) are associated with lower incidence although with more robust association with clinical outcome [43, 47].
Despite poor CI-AKI definition, CI-AKI clinical course is very well described. CI-AKI typically manifests within 1-3 days of CM administration, peaks within 3–5 days and resolves within 10– 21 days [36, 51]. In rare occasions, sustained or permanent kidney injury occurs warranting the use of dialysis. Therefore, to monitor for CI-AKI, it is recommended that serum creatinine follow-up should be obtained at not less than 24 h or more than 72 h following contrast exposure [41].
Problems of Using Serum Creatinine Levels
Quantification of sCr levels remains the main tool the clinicians use for the diagnosis of acute kidney injury (AKI). However, it certainly has some important disadvantages, due to which AKI diagnosis remains a problem that is often challenging to diagnose and manage. A significant impairment in renal function leads to an increase in sCr levels. However, it may take several days in order for the new steady state of creatinine levels to be reached [52–54] and, during this period, the calculated GFR (eGFR incorporating sCr levels) does not correspond to the actual creatinine clearance. Additionally, the rate of sCr rise depends not only on the new, lower level of renal clearance, but also on the rate of creatinine production and the volume of creatinine distribution [53]. Because these two latter parameters often do not remain unchanged and also have considerable inter-individual variation, diagnosing AKI using sCr levels may be misleading.
The inherent drawbacks of sCr measurement seem to become of greater importance in patients with an already impaired renal function, particularly when relative increases in sCr over baseline are used as the definition of AKI. Using percentage increases as the diagnostic criterion for AKI can lead to a delayed diagnosis in patients with CKD [54]. On the other side, patients with low sCr levels at baseline (such as pregnant women or patients with cirrhosis) may not be effectively diagnosed as having AKI by using an absolute definition [54]. Another drawback of sCr as a biomarker in those patients with an elevated sCr level at baseline, is the high variability of sCr measurement, which is of both laboratory and biological origin [55]. This can lead to high false-positive rates in the diagnosis of AKI and result in an AKI misclassification of patients with CKD [55].
As far as AKI due to CM administration is concerned, sCr increase in CI-AKI manifests 1-3 days after CM administration, as already discussed. This delayed increase can be a reason both for overlooking CI-AKI and also for prolonging hospitalization in the majority of patients who will not finally develop CI-AKI [56].
The aforementioned drawbacks of sCr levels as a diagnostic means for AKI have created the need for novel biomarkers, which would help diagnose AKI timely and efficiently and thus improve management and overall prognosis of patients with AKI. This topic will be covered in a later section of this review article.
Contrast-Induced Acute Kidney Injury Epidemiology
It is undoubtedly difficult to unravel the details of CI-AKI epidemiology, because of the vast number of factors that may affect the incidence values reported by published studies. These factors may be patient-related (diabetes mellitus or other co-morbidities) or study-design-related (inclusion/exclusion criteria, CI-AKI definition etc.). Of course, intervention-related factors, such as the choice of contrast agent, the volume of agent used and also CM administration route may also strongly affect CI-AKI incidence. Sometimes, CI-AKI incidence can be affected by a mixture of factors belonging to all three of the aforementioned categories, making any direct comparisons among studies confusing or even impossible. For example, both the selection of contrast agent and the patient characteristics are often the result of a particular study design.
As already discussed, CI-AKI incidence widely varies depending on the presence (or not) of an impaired renal function before diagnostic or interventional cardiac catheterization. These two factors are possibly the most important ones defining CI-AKI incidence, although no such definite conclusion can be safely reached. Table 2 attempts to summarize the various incidence levels reported in the literature presented in this review article, categorized based on the factors affecting incidence that are present in each study or patient subset. It should be taken into account that the majority of studies do not report incidence rates in patient subsets defined by various patient characteristics and, therefore, this table is not exhaustive.
Another interesting issue to address is the number of factors that can function as independent predictors of the development of CI-AKI. Once more, various patient- and procedure-related factors that lead to a greater risk for CI-AKI have been proposed over the years, such as an impaired renal function, diabetes mellitus (DM), old age, administration of nephrotoxic drugs and CM volume [57]. Although the effects of patient-related risk factors have been generally confirmed, there is considerable heterogeneity in the findings of various studies regarding the factors that can function as independent predictors for CI-AKI development. For example, there is a discussion on whether DM or age is a risk factor or it actually functions as one, due to the fact that diabetic or elderly individuals very often have an impaired renal function [58]. This means that the role of confounders in the establishment or renal dysfunction could be of major importance, thus making CM administration a contributor rather than a cause of CI-AKI. Many of the proposed risk factors could actually serve as markers for coexisting conditions [14]. Despite this confusion, there is a general “consensus” that an impaired renal function prior to CM administration plays a major role, among other risk factors, in the development of CI-AKI [58]. In Table 3 we provide a list of independent risk factors often appearing in the literature, derived from multivariate analyses adjusting for cofounders.
The volume and type used during coronary angiography or PCI has also been shown to affect CI-AKI epidemiology. Higher CM volume (CMV) has been shown to be associated with increased rates of CI-AKI and also increased mortality [59, 60]. The association of CMV with CI-AKI incidence is dose-dependent and the risk of CI-AKI development almost doubles with every 20 ml of CM that is administered [60]. Additionally, the same relatively low volume of CM may have a tremendous effect on the risk for CI-AKI development in a patient with comorbidities such as diabetes mellitus and CKD [61].
The matter of the importance of CMV in defining the risk for development of CI-AKI has been addressed by various studies [62]. There are some indices that take into account the volume used during coronary angiography and/or PCI, that have been proposed to function as predictors of a high risk for CI-AKI (Supplementary Table 1). Maximal acceptable contrast dose (MACD) has been defined using the formula 5 ml of contrast × body weight (kg) (maximum value is 300 ml)/baseline SCr (mg/dl) [63]. This formula has been applied in a registry of 16,592 patients undergoing PCI and was found to be the strongest predictor of nephropathy requiring dialysis after PCI [64]. In addition, it has been associated with increased mortality in a set of 561 patients with STEMI undergoing primary PCI [59]. The CMV/eGFR index is another tool used to calculate the risk for CI-AKI development, based on CMV, which has been also associated with high 1-month mortality in patients undergoing primary PCI for STEMI [65]. The risk for CI-AKI increases particularly when the index exceeds a value of 3.7 [65]. An important publication by Gurm et al. concluded that this index is superior to MACD in defining patients at high risk for CI-AKI. A ratio of under 2 is associated with a low incidence of CI-AKI, whereas a ratio higher than 3 is associated with an increased risk of CI-AKI and need for dialysis [66]. In a recent study including patients with ACS it was shown that every one-tenth increase in CMV/eGFR ratio is associated with a 4.9 % increase in the risk for CI-AKI, whereas using a volume higher than the maximal acceptable contrast dose with a 19-fold increase [67]. The same investigators proposed the following two-step algorithm: if CMV exceeds the MACD, the risk is considered high whereas if CMV does not exceed MACD, the CMV/eGFR ratio should be calculated. A score < 2 represents a low risk and a score > 2 a moderate risk. This algorithm was found to effectively identify ACS patients who will develop CI-AKI [67]. Interestingly, the CMV/eGFR index predicted CI-AKI better when eGFR was not normalized according to body surface area (BSA), i.e., when raw eGFR was used [68]. Finally, the ratio of iodine quantity (in grams) to eGFR (g-I/eGFR) has been also proposed as another relevant index that can act as an useful indicator for determining CM dose [69, 70].
As far as the type of the CM is concerned, osmolality and viscosity are the two main physical properties of the CM that can influence the possibility of CI-AKI development. A categorization of available CM into iso-, low- and high-osmolar according to Azzalini et al. [71] can be found in Table 4. A lower osmolality results in a better absorbance of x-rays and a better visualization of the vessel [72]. On the other side, high-osmolar CM have been associated with a higher nephrotoxic potential [20, 72]. There is therefore a tendency towards prefering low- and iso-osmolar CM in clinical practice and this fact is also reflected in the new European Society of Cardiology guidelines for myocardial revascularization [67, 71–73]. The issue of the “best CM” remains controversial, because the lower the osmolality of CM, the higher the viscosity (viscosity increases when shifting from high- to low- and iso-osmolar CM [71]). Administering a CM with high viscosity results to an increase in the viscosity of renal ultrafiltrate and, thus, also to an increased resistance to flow in renal tubules, ultimately leading to tubular damage [21, 38].
Finally, anemia due to periprocedural bleeding is another procedure-related variable that can affect the risk for CI-AKI development. It has been retrospectively shown that periprocedural bleeding in patients treated with PCI is significantly associated with CI-AKI [74]. The incidence of CI-AKI correlated with bleeding severity and rose from 6.2 % in patients with a < 1 g/dl decrease in hemoglobin levels to 26.2 % in patients with > 3 g/dl decrease [74].
Contrast-Induced Acute Kidney Injury in the Clinical Setting
CI-AKI and Mortality
CI-AKI has been shown to significantly affect the prognosis and clinical outcome in patients undergoing not only coronary angiography and PCI [75], but also other diagnostic procedures utilizing CM, such as CM-enhanced CT [76]. Many studies published over the years have established an association of CI-AKI with higher in-hospital and long-term mortality rates. Supplementary Table 2 offers a summary of relevant studies. Noteworthy, results among studies investigating the association of CI-AKI with mortality vary considerably. This can be attributed to differences in study design or data analysis. For example, it should be taken into account that many of these studies report values that are not adjusted for confounders, which makes the comparison among their results difficult and confusing. The patient selection criteria (inclusion/exclusion criteria) and the CI-AKI definition used in each study may also confound the extracted values.
James and co-workers published a very interesting meta-analysis that investigated the connection between CI-AKI after coronary angiography and adverse clinical outcomes [77]. Not all studies included in this meta-analysis used the same CI-AKI definition, however the association between CI-AKI and mortality remained similar. Of the 34 included studies examining mortality, 33 reported significantly increased mortality rates in patients who developed CI-AKI after coronary angiography. From 23 studies adjusting for confounders, the pooled adjusted risk ratio for mortality and cardiovascular events was 2.39; (95 % CI, 1.98-2.90) and 1.98 (95 % CI, 1.52-2.59), respectively. A significant statistical heterogeneity among the studies was reported by the authors for both mortality and cardiovascular events rates. Noteworthy, a publication bias was also reported for mortality rates. The authors concluded that the results of these observational studies are rather weak to prove a causal relationship between CI-AKI and mortality, due to the potential residual that remains, even after adjustment. The development of CI-AKI was also associated with a greater length of hospital stay and a higher probability of progression to end-stage renal disease (ESRD).
As previously discussed, impaired renal function is an independent predictor for the development of CI-AKI. Noteworthy, the strength of the association between CI-AKI and mortality seems to also depend on baseline renal function. A low eGFR before the procedure is associated with incrementally higher mortality [78], in particular when sCr levels at baseline are higher than 1.5 mg/dl [79].
Despite the fact that the majority of studies have shown that the development of CI-AKI is associated with higher in-hospital, short-term and long-term mortality (Supplementary Table 2), sometimes associating even small absolute or relative changes in sCr with a worse outcome [80], there are reports that propose that no association exists between CI-AKI and increased mortality in patients without CKD [81] and that these patients follow a rather benign clinical course [82].
The strength of the association of CI-AKI and mortality seems to also depend on whether PCI is elective or emergent. As already mentioned, patients with an ACS are at higher risk of developing CI-AKI. Noteworthy, CI-AKI development in those patients is associated with a higher resultant mortality [9]. In particular, patients presenting with STEMI have been shown to exhibit a more complicated course compared to NSTEMI [83]. An interesting prospective cohort study conducted in 181 patients with baseline CKD undergoing non-emergent PCI found CI-AKI to be a low-frequency event with a less robust association with adverse outcomes compared to other studies [84].
CI-AKI and Length of Hospital Stay
The development of CI-AKI after administration of CM is associated with a more complicated clinical course which, among other factors, also results to a prolongation of hospitalization. A relatively small number of studies have examined the association between CI-AKI and length of hospital stay. However, all of them seem to conclude that patients that develop CI-AKI after coronary angiography or PCI tend to remain longer in the hospital. The results of some representative studies are summarized in Supplementary Table 2.
The meta-analysis published by James et al. [77], after analyzing 19,674 participants in 10 studies, reported an unadjusted mean length of hospital stay ranging from 0.5 to 8.3 days longer for patients with CI-AKI compared to patients without CI-AKI, although there was a lot of heterogeneity among studies.
CI-AKI and Chronic Loss of Kidney Function
The progression to CKD is an important potential outcome of CM administration, which can strongly affect prognosis and mortality. In various published studies, the rate of progression to end-stage renal disease requiring dialysis post-PCI varies from < 1 % in the general population [8, 64] to 7 % in patients with CKD [85]. It is thus considered a relatively rare complication, but with a poor prognosis, given that patients who develop end-stage renal disease have an in-hospital mortality rate of almost 40 % [64].
The association of CI-AKI with progression to ESRD is based on a much smaller number of studies compared to its association with mortality (Supplementary Table 2). The meta-analysis by James et al. regarding progression to ESRD showed a pooled risk ratio of 8.07 (95 % CI, 3.23-20.19) [77]. Studies examining the long term outcomes of CM administration have established a novel aspect in the discussion of the importance of CI-AKI as a predictor of permanent kidney damage.
One such study was recently published by Nemoto et al. and included patients with ACS who underwent PCI [86]. They were divided into two groups, namely with and without persistently impaired renal function (defined as a > 0.5 mg/dl or > 25 % increase of sCr levels 6-8 months after PCI). Interestingly, the group with persistently impaired renal function comprised 16 % of total patients included in the study. This group had higher rates of CI-AKI post-PCI and also higher 5-year mortality rates compared to the group who had no persistent impairment in their renal function. CI-AKI was an independent predictor for the development of persistent impairment of renal function. About 40 % of patients who developed CI-AKI had a persistent impairment of renal function, whereas this percentage in the control group was 11 %.
The importance of CI-AKI for the development of ESRD was recently challenged by a study that enrolled patients with impaired renal function (eGFR 30-60 mL/min/1.73 m2) undergoing elective coronary angiography [87]. This study examined the composite long-term outcome of death or need for dialysis after a mean follow-up period of 34.5 months in patients undergoing elective coronary angiography in comparison to a control group of patients with no CM exposure (CKD patients presenting for a regular follow-up at the department of nephrology) [87]. Patients who developed CI-AKI after coronary angiography were excluded from this study. Although all included individuals had not developed CI-AKI, the composite outcome occurred more frequently in the CM group compared to the control group. Patients receiving CM had a significantly higher decrease in eGFR values compared to the aforementioned control group. Since CM exposure, but not comorbidities such as diabetes mellitus and hypertension, was found to be an independent predictor of long-term adverse events, one could argue in favor of the importance of CM exposure for the occurrence of the composite outcome. Noteworthy, not even baseline eGFR was an independent predictor of the outcome. In accordance to these findings creatinine levels at 30 days have been associated with increased mortality in the study of Holscher et al., whereas no such association was found for CI-AKI [88].
The results mentioned above seem to lead to the conclusion that CI-AKI is associated with an increased risk for development of CKD. However, it is obviously not essential that the course leading from CM administration to the establishment of CKD passes through the intermediate step of CI-AKI. Undoubtedly, more studies investigating the effects of CM administration on renal function in the long-term are needed, in order to clarify whether a mid-term examination of kidney function after CM administration should become a routine, at least for patients in high-risk for developing CKD.
Contrast-Induced Acute Kidney Injury Prediction
Risk Scores
Various groups have developed and validated risk scores for CI-AKI, in an effort to create a tool useful for the clinicians [44, 45, 89] (Supplementary Table 3). Such a tool would help identify patients at high risk and accordingly affect clinical decision making. Mehran’s score [45] and Bartholomew’s score [89] are the two scores most often used in the literature. It should be noted that the clinical setting of a study, from which a score derives may affect the general applicability of the score in different clinical settings. For example, Marenzi’s score was derived from patients undergoing primary PCI for STEMI [44] and may therefore not apply in an elective PCI setting. It is therefore important that such scores are validated in different settings, before they can be generally applied in clinical practice. Other important disadvantages of risk scores are summarized in Table 5.
Our group has also developed a risk score for CI-AKI after enrolling an unselected cohort of 488 patients undergoing PCI, on an elective or an emergency basis [90]. This score was internally and externally validated by bootstrapping and in a validation cohort respectively [90]. We also prospectively validated its performance in a large multicenter international cohort of 2,689 patients undergoing PCI [91], in which our score achieved good accuracy (c-statistic 0.741; 95 % CI, 1.98-2.90) and discrimination (area under the curve ≥ 0.700) across all predefined subgroups of the study population.
The performance and clinical usefulness of various published risk scores for CI-AKI were assessed in a recent systematic review published in the British Medical Journal [92]. A total of 12 risk scores published from 2004 to 2015 were included in this review. The authors state that, although the majority of these risk scores achieve an adequate accuracy, their usability in clinical practice is extremely limited, mainly due to three factors. First, the lack of external validation in multicenter studies. Second, an unclear association between the stratification to a risk category and clinical decision making, and third, the lack of easy-to-use electronic risk calculators.
Conclusively, despite the development of a number of risk scores, we are probably still far from a risk score that can be widely used in everyday clinical practice and actually affect clinical decisions regarding the risk/benefit ratio of a coronary diagnostic/interventional procedure or the peri- or post-procedural prophylactic strategies. Apart from its simplicity and accuracy, such a score would definitely need to be extensively validated on an external basis and in various clinical settings, before it can find its way in cardiologists’ everyday clinical practice.
Risk Biomarkers
As already discussed in a previous section, quantification of sCr levels for the diagnosis and prognosis of AKI has a number of disadvantages. Among other reasons, this inadequacy of sCr as a biomarker for AKI has led to a continuous search for the “ideal AKI biomarker”, which, of course, would be of major value also for the diagnosis and management of CI-AKI. The latter would ideally also be specific to AKI due to administration of CM. There is a number of novel biomarkers that have been proposed to be able to diagnose CI-AKI and also to contribute to patient risk stratification and improvement of clinical outcomes (Table 6). Neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18) and Cystatin C (CyC) are the most known relevant biomarkers studied from various groups, including ours [93], with NGAL being the most investigated one in the clinical setting of CI-AKI [56]. However, these novel biomarkers have not yet been established in everyday clinical management of AKI and particularly CI-AKI. In this section, we refer to published meta-analyses regarding the three aforementioned biomarkers, in order to avoid presenting the results of numerous studies available in the literature, which would make this section confusing and inconclusive.
The clinical value of NGAL was assessed by a meta-analysis published some years ago which included 19 studies with a total of 2,538 patients [94]. NGAL level measurement was found to be a clinically useful early predictor of AKI, with urine and serum/plasma levels both performing well in this regard. Measured NGAL levels had a good association with clinical outcomes, such as mortality and need for dialysis. NGAL performed well in all clinical settings of AKI investigated, including CI-AKI. Particularly for the diagnosis of CI-AKI, NGAL levels had a pooled sensitivity of 77.8 % (95 % CI, 62.8 %-88.0 %) and a pooled specificity of 96.3 % (95 % CI, 74.4 %-99.6 %) with a median cut-off NGAL value of 100 ng/ml (95 % CI, 80-100).
Another meta-analysis published in the New England Journal of Medicine in 2013 aimed at investigating the usefulness of serum levels of CyC as a biomarker for AKI [95]. It included 11 general-population studies (with 90,750 individuals) and also 5 studies enrolling patients with CKD (with 2,969 patients). The authors investigated the association of eGFR calculated by measurement of CyC compared to the traditional eGFR (calculated by measurement of creatinine) and found a net reclassification improvement of 0.23 (95 % CI, 0.18-0.28) for death and 0.10 (95 % CI, 0.00-0.21) for progression to ESRD.
Finally, the value of urine levels of IL-18 as a biomarker of AKI was investigated in a meta-analysis published in 2013 [96], which included 23 studies enrolling a total of 4,512 patients. IL-18 was found to have a relatively suboptimal sensitivity of 58 % (95 % CI, 52 %-64 %) and specificity of 75 % (95 % CI, 70 %-80 %). The predictive value of urinary IL-18 did not differ significantly across the various time points of measurement. Similar were the results about IL-18 presented in another meta-analysis published this year [97].
Obviously, there is no “perfect” biomarker for AKI and CI-AKI. Given the nature of AKI itself, it would be rational to support the view that research should not be orientated towards one ideal biomarker, able to produce a simple binary result (positive or negative). Maybe it would be more meaningful to follow a synergistic approach and combine a biomarker with clinical history plus other useful traditional laboratory examinations, such as sCr, conventional urine analysis and fractional excretion of sodium [98]. Taking into consideration the particular epidemiological and other characteristics of AKI due to CM administration is undoubtedly of major significance
Contrast-Induced Acute Kidney Injury And Guidelines
In contrast to other medical issues with lots of different guidelines and statements by relevant medical societies, there is a relatively small number of guidelines specifically on CI-AKI. There is of course a number of guidelines addressing the matter of AKI in general (see Supplementary Table 4 for a summary of available guidelines). In 1999, the European Society of Urogenital Radiology produced guidelines for the use of contrast media, which had, however, the character of a consensus report [99]. These guidelines were updated many times during the years that followed, in form of online or booklet versions. One of these updates was also published in European Radiology in 2011 [58]. At present, version 9.0 represents the most up-to-date printed version, whereas the electronic form of version 8.1 is currently available online [100]. The Kidney Disease: Improving Global Outcomes (KDIGO) workgroup, a panel consisting of experts in the field, also published the 2011 KDIGO Clinical Practice Guideline for Acute Kidney Injury, with section 4 focusing on CI-AKI [101]. These guidelines were endorsed and updated by the European Renal Best Practice (ERBP) position statement, which was published in two parts in 2012 and 2013 [40, 102]. Finally, the Canadian Association of Radiologists also published relevant guidelines in 2011, which were updated in 2012 [61, 103].
These guidelines were produced after reviewing the available literature on CI-AKI, a large part of which consists of studies examining clinical settings of CM administration other than the ones met in a cardiac catheterization laboratory, such as contrast-enhanced CT scans. Although these guidelines are definitely useful, one could rationally argue that the wide utilization of cardiac catheterization nowadays creates a need for guidelines specially focusing on CI-AKI after cardiac catheterization procedures. Such guidelines would help to better understand the risks of CI-AKI development after a patient’s visit in a catheterization laboratory and allow physicians to better organize their pre- and post-procedural clinical strategy.
Conclusions
CI-AKI remains an important cause of morbidity and mortality after CM exposure, especially in the clinical setting of percutaneous coronary interventions. Various issues regarding clinical co-morbidities (age, DM, renal function), clinical setting (elective or urgent), periprocedural logistics (CM type and volume) or biochemical aspects (poor sCr performance) raise some ambiguity as far as definition, incidence and true prognostic effect is concerned. A general consensus is warranted for a strict definition of CI-AKI to be adapted so as the incidence of this pathology to be robustly associated with poor outcome. Furthermore, specific risk factors should universally be acknowledged in order appropriate clinical vigilance to be applied in these sub-group of patients. Emerging acute kidney injury biomarkers could serve as an alternative mean to diagnose CI-AKI earlier and more accurately. Finally, risk stratification with validated risk scores is of paramount importance to identify high-risk patients who will benefit from optimal renal prophylaxis, staged procedures or careful and prolonged monitoring of kidney function.
References
Laville M, Juillard L. Contrast-induced acute kidney injury: how should at-risk patients be identified and managed? Am J Nephrol. 2010;23(4):387–98.
Solomon R. Preventing contrast-induced nephropathy: problems, challenges and future directions. BMC Med. 2009;7:24.
Blackman DJ, Pinto R, Ross JR, Seidelin PH, Ing D, Jackevicius C, et al. Impact of renal insufficiency on outcome after contemporary percutaneous coronary intervention. Am Heart J. 2006;151(1):146–52.
Lindsay J, Apple S, Pinnow EE, Gevorkian N, Gruberg L, Satler LF, et al. Percutaneous coronary intervention-associated nephropathy foreshadows increased risk of late adverse events in patients with normal baseline serum creatinine. Catheter Cardiovasc Interv. 2003;59(3):338–43.
McCullough PA, Adam A, Becker CR, Davidson C, Lameire N, Stacul F, et al. Epidemiology and prognostic implications of contrast-induced nephropathy. Am J Cardiol. 2006;98(6a):5k–13k.
Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105(19):2259–64.
Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74(2):243–8.
McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103(5):368–75.
Chong E, Poh KK, Liang S, Soon CY, Tan HC. Comparison of risks and clinical predictors of contrast-induced nephropathy in patients undergoing emergency versus nonemergency percutaneous coronary interventions. J Interv Cardiol. 2010;23(5):451–9.
Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–6.
Gruberg L, Mehran R, Dangas G, Mintz GS, Waksman R, Kent KM, et al. Acute renal failure requiring dialysis after percutaneous coronary interventions. Catheter Cardiovasc Interv. 2001;52(4):409–16.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292.
Bartorelli AL, Marenzi G. Contrast-induced nephropathy. J Interv Cardiol. 2008;21(1):74–85.
Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354(4):379–86.
Guitterez NV, Diaz A, Timmis GC, O'Neill WW, Stevens MA, Sandberg KR, et al. Determinants of serum creatinine trajectory in acute contrast nephropathy. J Interv Cardiol. 2002;15(5):349–54.
Finn WF. The clinical and renal consequences of contrast-induced nephropathy. Nephrol Dial Transplant. 2006;21(6):i2–10.
Solomon R, Dauerman HL. Contrast-induced acute kidney injury. Circulation. 2010;122(23):2451–5.
McCullough PA. Radiocontrast-induced acute kidney injury. Nephron Physiol. 2008;109(4):61–72.
Reinecke H, Fobker M, Wellmann J, Becke B, Fleiter J, Heitmeyer C, et al. A randomized controlled trial comparing hydration therapy to additional hemodialysis or N-acetylcysteine for the prevention of contrast medium-induced nephropathy: the dialysis-versus-diuresis (DVD) trial. Clin Res Cardiol. 2007;96(3):130–9.
Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33(16):2007–15.
Persson PB, Tepel M. Contrast medium-induced nephropathy: the pathophysiology. Kidney Int Suppl. 2006;100:S8–10.
Hardiek K, Katholi RE, Ramkumar V, Deitrick C. Proximal tubule cell response to radiographic contrast media. Am J Physiol Renal Physiol. 2001;280(1):F61–70.
Brezis M, Rosen S, Silva P, Epstein FH. Selective vulnerability of the medullary thick ascending limb to anoxia in the isolated perfused rat kidney. J Clin Invest. 1984;73(1):182–90.
Katzberg RW, Morris TW, Burgener FA, Kamm DE, Fischer HW. Renal renin and hemodynamic responses to selective renal artery catheterization and angiography. Investig Radiol. 1977;12(5):381–8.
Katzberg RW, Schulman G, Meggs LG, Caldicott WJ, Damiano MM, Hollenberg NK. Mechanism of the renal response to contrast medium in dogs. Decrease in renal function due to hypertonicity. Investig Radiol. 1983;18(1):74–80.
Talner LB, Davidson AJ. Renal hemodynamic effects of contrast media. Investig Radiol. 1968;3(5):310–7.
Katholi RE, Taylor GJ, McCann WP, Woods Jr WT, Womack KA, McCoy CD, et al. Nephrotoxicity from contrast media: attenuation with theophylline. Radiology. 1995;195(1):17–22.
Erley CM, Duda SH, Schlepckow S, Koehler J, Huppert PE, Strohmaier WL, et al. Adenosine antagonist theophylline prevents the reduction of glomerular filtration rate after contrast media application. Kidney Int. 1994;45(5):1425–31.
Arend LJ, Bakris GL, Burnett Jr JC, Megerian C, Spielman WS. Role for intrarenal adenosine in the renal hemodynamic response to contrast media. J Lab Clin Med. 1987;110(4):406–11.
Schnackenberg CG. Physiological and pathophysiological roles of oxygen radicals in the renal microvasculature. Am J Physiol Regul Integr Comp Physiol. 2002;282(2):R335–42.
Liss P, Carlsson PO, Nygren A, Palm F, Hansell P. Et-A receptor antagonist BQ123 prevents radiocontrast media-induced renal medullary hypoxia. Acta Radiol. 2003;44(1):111–7.
Wang A, Holcslaw T, Bashore TM, Freed MI, Miller D, Rudnick MR, et al. Exacerbation of radiocontrast nephrotoxicity by endothelin receptor antagonism. Kidney Int. 2000;57(4):1675–80.
Bakris GL, Lass N, Gaber AO, Jones JD, Burnett Jr JC. Radiocontrast medium-induced declines in renal function: a role for oxygen free radicals. Am J Physiol. 1990;258(1 Pt 2):F115–20.
Katholi RE, Woods Jr WT, Taylor GJ, Deitrick CL, Womack KA, Katholi CR, et al. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis. 1998;32(1):64–71.
Beeri R, Symon Z, Brezis M, Ben-Sasson SA, Baehr PH, Rosen S, et al. Rapid DNA fragmentation from hypoxia along the thick ascending limb of rat kidneys. Kidney Int. 1995;47(6):1806–10.
McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51(15):1419–28.
Hizoh I, Strater J, Schick CS, Kubler W, Haller C. Radiocontrast-induced DNA fragmentation of renal tubular cells in vitro: role of hypertonicity. Nephrol Dial Transplant. 1998;13(4):911–8.
Ueda J, Nygren A, Hansell P, Erikson U. Influence of contrast media on single nephron glomerular filtration rate in rat kidney. A comparison between diatrizoate, iohexol, ioxaglate, and iotrolan. Acta Radiol. 1992;33(6):596–9.
Thomsen HS. Guidelines for contrast media from the European Society of Urogenital Radiology. AJR Am J Roentgenol. 2003;181(6):1463–71.
Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27(12):4263–72.
Solomon R, Deray G. How to prevent contrast-induced nephropathy and manage risk patients: practical recommendations. Kidney Int Suppl. 2006;100:S51–3.
Lakhal K, Ehrmann S, Chaari A, Laissy JP, Regnier B, Wolff M, et al. Acute kidney injury network definition of contrast-induced nephropathy in the critically ill: incidence and outcome. J Crit Care. 2011;26(6):593–9.
Slocum NK, Grossman PM, Moscucci M, Smith DE, Aronow HD, Dixon SR, et al. The changing definition of contrast-induced nephropathy and its clinical implications: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Am Heart J. 2012;163(5):829–34.
Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44(9):1780–5.
Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–9.
Iakovou I, Dangas G, Mehran R, Lansky AJ, Ashby DT, Fahy M, et al. Impact of gender on the incidence and outcome of contrast-induced nephropathy after percutaneous coronary intervention. J Invasive Cardiol. 2003;15(1):18–22.
Budano C, Levis M, D'Amico M, Usmiani T, Fava A, Sbarra P, et al. Impact of contrast-induced acute kidney injury definition on clinical outcomes. Am Heart J. 2011;161(5):963–71.
Caruso M, Balasus F, Incalcaterra E, Ruggieri A, Evola S, Fattouch K, et al. Contrast-induced nephropathy after percutaneous coronary intervention in simple lesions: risk factors and incidence are affected by the definition utilized. Intern Med. 2011;50(9):983–9.
Chousterman BG, Bouadma L, Moutereau S, Loric S, Alvarez-Gonzalez A, Mekontso-Dessap A, et al. Prevention of contrast-induced nephropathy by N-acetylcysteine in critically ill patients: different definitions, different results. J Crit Care. 2013;28(5):701–9.
Sinert R, Brandler E, Subramanian RA, Miller AC. Does the current definition of contrast-induced acute kidney injury reflect a true clinical entity? Acad Emerg Med. 2012;19(11):1261–7.
Caixeta A, Mehran R. Evidence-based management of patients undergoing PCI: contrast-induced acute kidney injury. Catheter Cardiovasc Interv. 2010;75 Suppl 1:S15–20.
Chiou WL, Hsu FH. Pharmacokinetics of creatinine in man and its implications in the monitoring of renal function and in dosage regimen modifications in patients with renal insufficiency. J Clin Pharmacol. 1975;15(5-6):427–34.
Moran SM, Myers BD. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985;27(6):928–37.
Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20(3):672–9.
Lin J, Fernandez H, Shashaty MG, Negoianu D, Testani JM, Berns JS et al. False-Positive Rate of AKI Using Consensus Creatinine-Based Criteria. Clin J Am Soc Nephrol. 2015
Briguori C, Quintavalle C, Donnarumma E, Condorelli G. Novel biomarkers for contrast-induced acute kidney injury. Biomed Res Int. 2014;2014:568738.
Toprak O. Risk markers for contrast-induced nephropathy. Am J Med Sci. 2007;334(4):283–90.
Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, et al. Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol. 2011;21(12):2527–41.
Marenzi G, Assanelli E, Campodonico J, Lauri G, Marana I, De Metrio M, et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann Intern Med. 2009;150(3):170–7.
Kane GC, Doyle BJ, Lerman A, Barsness GW, Best PJ, Rihal CS. Ultra-low contrast volumes reduce rates of contrast-induced nephropathy in patients with chronic kidney disease undergoing coronary angiography. J Am Coll Cardiol. 2008;51(1):89–90.
Owen RJ, Hiremath S, Myers A, Fraser-Hill M, Barrett BJ. Canadian association of radiologists consensus guidelines for the prevention of contrast-induced nephropathy: update 2012. Can Assoc Radiol J. 2014;65(2):96–105.
Keaney JJ, Hannon CM, Murray PT. Contrast-induced acute kidney injury: how much contrast is safe? Nephrol Dial Transplant. 2013;28(6):1376–83.
Cigarroa RG, Lange RA, Williams RH, Hillis LD. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med. 1989;86(6 Pt 1):649–52.
Freeman RV, O'Donnell M, Share D, Meengs WL, Kline-Rogers E, Clark VL, et al. Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. Am J Cardiol. 2002;90(10):1068–73.
Mager A, Vaknin Assa H, Lev EI, Bental T, Assali A, Kornowski R. The ratio of contrast volume to glomerular filtration rate predicts outcomes after percutaneous coronary intervention for ST-segment elevation acute myocardial infarction. Catheter Cardiovasc Interv. 2011;78(2):198–201.
Gurm HS, Dixon SR, Smith DE, Share D, Lalonde T, Greenbaum A, et al. Renal function-based contrast dosing to define safe limits of radiographic contrast media in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2011;58(9):907–14.
Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541–619.
Park HS, Kim CJ, Yi JE, Hwang BH, Kim TH, Koh YS, et al. Contrast volume/Raw eGFR ratio for predicting contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention for myocardial infarction. Cardiorenal Med. 2015;5(1):61–8.
Yoon HJ, Hur SH. Determination of safe contrast media dosage to estimated glomerular filtration rate ratios to avoid contrast-induced nephropathy after elective percutaneous coronary intervention. Korean Circ J. 2011;41(5):265–71.
Nyman U, Bjork J, Aspelin P, Marenzi G. Contrast medium dose-to-GFR ratio: a measure of systemic exposure to predict contrast-induced nephropathy after percutaneous coronary intervention. Acta Radiol. 2008;49(6):658–67.
Azzalini L, Spagnoli V, Ly HQ. Contrast-Induced Nephropathy: From Pathophysiology to Preventive Strategies. Can J Cardiol. 2015
Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation. 2006;113(14):1799–806.
Katsiki N, Athyros VG, Karagiannis A, Mikhailidis DP. Contrast-induced nephropathy: an "All or none" phenomenon? Angiology. 2015;66(6):508–13.
Ohno Y, Maekawa Y, Miyata H, Inoue S, Ishikawa S, Sueyoshi K, et al. Impact of periprocedural bleeding on incidence of contrast-induced acute kidney injury in patients treated with percutaneous coronary intervention. J Am Coll Cardiol. 2013;62(14):1260–6.
Wi J, Ko YG, Kim JS, Kim BK, Choi D, Ha JW, et al. Impact of contrast-induced acute kidney injury with transient or persistent renal dysfunction on long-term outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Heart. 2011;97(21):1753–7.
Mitchell AM, Kline JA, Jones AE, Tumlin JA. Major Adverse Events One Year After Acute Kidney Injury After Contrast-Enhanced Computed Tomography. Ann Emerg Med. 2015.
James MT, Samuel SM, Manning MA, Tonelli M, Ghali WA, Faris P, et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv. 2013;6(1):37–43.
Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95(1):13–9.
Kini AS, Sarkar K, Rafael OC, Jakkula M, Kaplish D, Lee P, et al. Serum creatinine ratio: a novel predictor of mortality after percutaneous coronary intervention in patients with normal and abnormal renal function. Catheter Cardiovasc Interv. 2009;74(1):49–55.
Weisbord SD, Chen H, Stone RA, Kip KE, Fine MJ, Saul MI, et al. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol. 2006;17(10):2871–7.
Abe M, Morimoto T, Akao M, Furukawa Y, Nakagawa Y, Shizuta S, et al. Relation of contrast-induced nephropathy to long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2014;114(3):362–8.
Chen SL, Zhang J, Yei F, Zhu Z, Liu Z, Lin S, et al. Clinical outcomes of contrast-induced nephropathy in patients undergoing percutaneous coronary intervention: a prospective, multicenter, randomized study to analyze the effect of hydration and acetylcysteine. Int J Cardiol. 2008;126(3):407–13.
Wickenbrock I, Perings C, Maagh P, Quack I, van Bracht M, Prull MW, et al. Contrast medium induced nephropathy in patients undergoing percutaneous coronary intervention for acute coronary syndrome: differences in STEMI and NSTEMI. Clin Res Cardiol. 2009;98(12):765–72.
Weisbord SD, Hartwig KC, Sonel AF, Fine MJ, Palevsky P. The incidence of clinically significant contrast-induced nephropathy following non-emergent coronary angiography. Catheter Cardiovasc Interv. 2008;71(7):879–85.
Gruberg L, Mintz GS, Mehran R, Gangas G, Lansky AJ, Kent KM, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36(5):1542–8.
Nemoto N, Iwasaki M, Nakanishi M, Araki T, Utsunomiya M, Hori M, et al. Impact of continuous deterioration of kidney function 6 to 8 months after percutaneous coronary intervention for acute coronary syndrome. Am J Cardiol. 2014;113(10):1647–51.
Abaci O, Harmankaya O, Kocas B, Kocas C, Bostan C, Coskun U, et al. Long-term follow-Up of patients at high risk for nephropathy after contrast exposure. Angiology. 2015;66(6):514–8.
Holscher B, Heitmeyer C, Fobker M, Breithardt G, Schaefer RM, Reinecke H. Predictors for contrast media-induced nephropathy and long-term survival: prospectively assessed data from the randomized controlled Dialysis-Versus-Diuresis (DVD) trial. Can J Cardiol. 2008;24(11):845–50.
Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, et al. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93(12):1515–9.
Tziakas D, Chalikias G, Stakos D, Apostolakis S, Adina T, Kikas P, et al. Development of an easily applicable risk score model for contrast-induced nephropathy prediction after percutaneous coronary intervention: a novel approach tailored to current practice. Int J Cardiol. 2013;163(1):46–55.
Tziakas D, Chalikias G, Stakos D, Altun A, Sivri N, Yetkin E, et al. Validation of a new risk score to predict contrast-induced nephropathy after percutaneous coronary intervention. Am J Cardiol. 2014;113(9):1487–93.
Silver SA, Shah PM, Chertow GM, Harel S, Wald R, Harel Z. Risk prediction models for contrast induced nephropathy: systematic review. BMJ. 2015;351:h4395.
Tziakas D, Chalikias G, Kareli D, Tsigalou C, Risgits A, Kikas P, et al. Spot urine albumin to creatinine ratio outperforms novel acute kidney injury biomarkers in patients with acute myocardial infarction. Int J Cardiol. 2015;197:48–55.
Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–24.
Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932–43.
Liu Y, Guo W, Zhang J, Xu C, Yu S, Mao Z, et al. Urinary interleukin 18 for detection of acute kidney injury: a meta-analysis. Am J Kidney Dis. 2013;62(6):1058–67.
Lin X, Yuan J, Zhao Y, Zha Y. Urine interleukin-18 in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Nephrol. 2015;28(1):7–16.
Molitoris BA. Urinary biomarkers: alone Are they enough? J Am Soc Nephrol. 2015;26(7):1485–8.
Morcos SK, Thomsen HS, Webb JA. Contrast-media-induced nephrotoxicity: a consensus report. Contrast media safety committee, european society of urogenital radiology (ESUR). Eur Radiol. 1999;9(8):1602–13.
ESUR Guidelines on Contrast Media, version 8.1. European Society of Urogenital Radiology. http://www.esur.org/guidelines/. Accessed September 2015.
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney inter. 2012;2(1):1.
Jorres A, John S, Lewington A, ter Wee PM, Vanholder R, Van Biesen W, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guidelines on Acute Kidney Injury: part 2: renal replacement therapy. Nephrol Dial Transplant. 2013;28(12):2940–5.
Consensus Guidelines for the Prevention of Contrast Induced Nephropathy. Canadian Association of Radiologists. http://www.car.ca/en/standards-guidelines/standards.aspx. Accessed October 2015.
Gupta R, Gurm HS, Bhatt DL, Chew DP, Ellis SG. Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv. 2005;64(4):442–8.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–12.
Harjai KJ, Raizada A, Shenoy C, Sattur S, Orshaw P, Yaeger K, et al. A comparison of contemporary definitions of contrast nephropathy in patients undergoing percutaneous coronary intervention and a proposal for a novel nephropathy grading system. Am J Cardiol. 2008;101(6):812–9.
Watabe H, Sato A, Hoshi T, Takeyasu N, Abe D, Akiyama D, et al. Association of contrast-induced acute kidney injury with long-term cardiovascular events in acute coronary syndrome patients with chronic kidney disease undergoing emergent percutaneous coronary intervention. Int J Cardiol. 2014;174(1):57–63.
Jabara R, Gadesam RR, Pendyala LK, Knopf WD, Chronos N, Chen JP, et al. Impact of the definition utilized on the rate of contrast-induced nephropathy in percutaneous coronary intervention. Am J Cardiol. 2009;103(12):1657–62.
Goldenberg I, Chonchol M, Guetta V. Reversible acute kidney injury following contrast exposure and the risk of long-term mortality. Am J Nephrol. 2009;29(2):136–44.
Roy P, Raya V, Okabe T, Pinto Slottow TL, Steinberg DH, Smith K, et al. Incidence, predictors, and outcomes of post-percutaneous coronary intervention nephropathy in patients with diabetes mellitus and normal baseline serum creatinine levels. Am J Cardiol. 2008;101(11):1544–9.
Cho JY, Jeong MH, Hwan Park S, Kim IS, Park KH, Sim DS, et al. Effect of contrast-induced nephropathy on cardiac outcomes after use of nonionic isosmolar contrast media during coronary procedure. J Cardiol. 2010;56(3):300–6.
Kume K, Yasuoka Y, Adachi H, Noda Y, Hattori S, Araki R, et al. Impact of contrast-induced acute kidney injury on outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Cardiovasc Revasc Med. 2013;14(5):253–7.
Narula A, Mehran R, Weisz G, Dangas GD, Yu J, Genereux P, et al. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur Heart J. 2014;35(23):1533–40.
Patti G, Nusca A, Chello M, Pasceri V, D'Ambrosio A, Vetrovec GW, et al. Usefulness of statin pretreatment to prevent contrast-induced nephropathy and to improve long-term outcome in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2008;101(3):279–85.
Kim JH, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH, et al. Predictors of outcomes of contrast-induced acute kidney injury after percutaneous coronary intervention in patients with chronic kidney disease. Am J Cardiol. 2014;114(12):1830–5.
Crimi G, Leonardi S, Costa F, Ariotti S, Tebaldi M, Biscaglia S, et al. Incidence, prognostic impact, and optimal definition of contrast-induced acute kidney injury in consecutive patients with stable or unstable coronary artery disease undergoing percutaneous coronary intervention. insights from the all-comer PRODIGY trial. Catheter Cardiovasc Interv. 2015;86(1):E19–27.
Senoo T, Motohiro M, Kamihata H, Yamamoto S, Isono T, Manabe K, et al. Contrast-induced nephropathy in patients undergoing emergency percutaneous coronary intervention for acute coronary syndrome. Am J Cardiol. 2010;105(5):624–8.
Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73(9):1008–16.
McCullough PA, Tumlin J, Szerlip H, Krishnaswami V, Jyothinagaram S, Rausch JF et al. Cardiorenal Syndromes: Advances in Determining Diagnosis, Prognosis and Therapy. J Cardiovasc Dis Diagn. 2015;3(221)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 309 kb)
Rights and permissions
About this article
Cite this article
Chalikias, G., Drosos, I. & Tziakas, D.N. Contrast-Induced Acute Kidney Injury: An Update. Cardiovasc Drugs Ther 30, 215–228 (2016). https://doi.org/10.1007/s10557-015-6635-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-015-6635-0