Abstract
Purpose
Recent reports showed a significant association between vitamin D levels and cardiovascular disease events and mortality. In the current study, we investigated the effect of the vitamin D receptor activator maxacalcitol (OCT) on cardiac damage in a rat model of type 2 diabetes.
Methods
At 20 weeks of age, the rats were divided into three groups: vehicle-treated (DM), insulin-treated (INS) and OCT-treated (OCT). At 30 weeks, the rats were sacrificed and urinary and blood biochemical analyses and cardiac histological and immunohistochemical analyses were performed. To evaluate the effect of OCT on the renin-angiotensin system, we performed a further study using aliskiren (ALS). At 20 weeks, the diabetic rats were divided into two groups: the ALS-treated group (ALS) and the ALS plus OCT-treated group (ALS + OCT), and we evaluated the renin-angiotensin system (RAS) and cardiac lesions at 30 weeks.
Results
At 30 weeks, despite comparable blood pressure and renal function, heart volume, intracardiac oxidative stress by immunohistological analysis, cardiac and perivascular fibrosis and urinary excretion of 8-hydroxydeoxyguanosine and serum N-terminal pro-brain natriuretic peptide levels were significantly decreased in the OCT group compared to the DM group. mRNA expressions of dihydronicotinamide adenine dinucleotide phosphate (NADPH) p47 subunit and cardiac injury-related markers in the heart were also significantly decreased in the OCT group compared to the DM group. The cardioprotective effect of OCT was preserved even in the context of RAS inhibition.
Conclusion
Our results suggest that OCT prevents the development of cardiac damage in DM, independent of RAS inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a major risk factor associated with cardiovascular events and mortality [1, 2]. Recently, the close association between CKD and cardiovascular disease (CVD) has become the focus of investigation. However, the pathophysiological mechanisms underlying this link are very complex and remain unclear. Renin-angiotensin system (RAS) inhibitors including angiotensin converting enzyme inhibitors, angiotensin II receptor blockers and aldosterone blockers, not only delay the decline of kidney function and reduce urinary protein excretion but also prevent the progression of cardiac damage [3–5]. Furthermore, many basic and clinical reports demonstrated that β-blockers could have cardioprotective effects [6–8].
Recently, several studies have reported the organ-protective effects of vitamin D [9]. Meta-analyses and large cohort studies have demonstrated a significant association between vitamin D deficiency and negative CKD and CVD outcomes [10, 11]. Therefore, vitamin D may have an important role in preventing CKD and CVD complications. Furthermore, clinical and experimental studies have shown that vitamin D treatment reduces proteinuria, improves endothelial dysfunction, and ameliorates cardiac hypertrophy [12–15]. Among the causes of CKD, diabetes mellitus (DM) is also a crucial risk factor for CVD. It has been demonstrated that vitamin D deficiency is associated with DM [16, 17]. Considering these facts, we supposed that vitamin D therapy may improve the prognosis of patients with diabetes.
Vitamin D receptor activator (VDRA) is routinely used for dialysis patients to prevent the progression of secondary hyperparathyroidism in the clinical setting. In general, VDRA has lower affinity for vitamin D binding protein than vitamin D (25-hydroxyvitamin D and 1,25-dehydroxyvitamin D). Therefore, VDRA is promptly excreted into urine from the kidney and its half-life is shorter than that of vitamin D. Furthermore, VDRA is easy to move to every tissue and exert its physiological action for a relatively short time. Because of its properties, VDRA does not so often manifest adverse effects such as vascular calcification and hypercalcemia compared to active vitamin D.
Although there are several studies demonstrating the effect of vitamin D and VDRA on cardiac structure and function in CKD, to our knowledge, there is little data detailing the role of vitamin D and VDRA in diabetes. In the present study, we examined the effects of VDRA on the heart and the pathophysiological mechanisms underlying these effects on oxidative stress and RAS in a model rat of diabetes.
Materials and Methods
Animals
Male spontaneously diabetic Torii (SDT) rats were obtained from CLEA Japan Inc., Tokyo, Japan. The rats were housed with food and water available ad libitum in light- and temperature-controlled environments. At 20 weeks of age, these rats were divided into three groups: vehicle-treated (DM, n = 8), insulin-treated (INS, n = 8), and maxacalcitol-treated (OCT, n = 8). Insulin pellets (LinShin Canada Inc., Scarborough, Ontario, Canada), which release a controlled amount of insulin, were subcutaneously implanted every 2 weeks. Maxacalcitol (Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) was dissolved in phosphate-buffered saline (pH 8.0) containing 0.2% ethanol and 0.01% Tween 20, and administered intraperitoneally three times a week at a dosage of 0.2 μg/kg body weight/day. A 24-h urine sample was collected from each rat using a metabolic cage before the rat was sacrificed. At 30 weeks, the rats were sacrificed under ether anesthesia. Blood samples for serum measurements were collected from the left ventricle, and the hearts were removed for RNA extraction and histomorphological analysis.
Furthermore, to perform additional analyses, we used aliskiren (ALS, Novartis, Switzerland), a direct renin inhibitor. Twenty weeks-old male SDT rats were divided into two groups: an ALS-treated group (ALS, n = 6) and an ALS plus OCT-treated group (ALS + OCT, n = 6). ALS was orally administered at a dosage of 100 mg/kg bodyweight/day through a feeding tube. At 30 weeks, these rats were also sacrificed under ether anesthesia and evaluated.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee guidelines of Kobe University Graduate School of Medicine (Permit Number: P081214R). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Serum and Urine Measurements
After centrifugation for 5 min at 3000 rpm, serum samples obtained were stored at −80 °C until analysis. Urine samples were also stored at −80 °C for later analysis. Serum creatinine, total protein (TP), albumin (Alb), calcium (Ca) and phosphate (P) levels were measured using a FUJI DRI-CHEM 3500 (FUJIFILM Japan, Tokyo, Japan). Urinary creatinine levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Cayman Chemical Company, Ann Arbor, MI, USA). Urinary excretion of Alb (U-Alb) and 8-hydroxydeoxyguanosine (U-8-OHdG) were determined using an ELISA kit (Alb: Exocell Inc., Philadelphia, PA; 8-OHdG: Japan Institute for Control of Aging, Shizuoka, Japan). Serum N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were also determined (Biomedica, Wien, Austria). Serum parathyroid hormone (PTH) levels were measured using a rat PTH-ELISA kit (Immutopics, San Clemente, CA, USA). Serum fibroblast growth factor 23 (FGF23) levels were measured using an intact FGF23 ELISA kit (Kainos Laboratories, Inc., Tokyo, Japan). Serum 25-hydroxyvitamin D (25D) and 1,25-dihydroxyvitamin D (1,25D) levels were measured using a 25-Hydroxyvitamin D 125I radioimmunoassay kit (DiaSorin Inc., Stillwater, USA) and a TFB 1,25-dihydroxyvitamin D radioimmunoassay kit (Immunodiagnostic Systems Ltd., Boldon, UK), respectively.
Blood Pressure Measurements
Systolic blood pressure measurements were performed by tail-cuff plethysmography (Model MK-2000; Muromachi Kikai Co. Ltd., Japan). To avoid the possibility of stress artefacts, the rats were allowed to acclimatize sufficiently. Blood pressure was measured at the basal time point, and then, every 2 weeks for the rest of the study period and determined using ten readings for each rats.
Histological and Immunohistochemical Analyses
To evaluate cardiac abnormalities, the hearts were removed, weighed and fixed with 10% formaldehyde, dehydrated at room temperature through an ethanol series, and embedded in paraffin. The resulting paraffin blocks were cut into in 3-μm-thick sections. The sections were stained with haematoxylin–eosin for routine histology and azan for morphometric studies. Myocardial fibrosis was evaluated in 20 randomly selected microscopic fields per sample using image analysis software - Lumina Vision version 3.7.4.2 (Mitani Corp., Tokyo, Japan). As previously described [18], perivascular fibrosis was graded semiquantitatively on a scale of 1 to 4 (fibrosis score; normal to severe) in 20 random microscopic fields.
The formation of 8-OHdG in the cardiac tissue was determined using anti-8-OHdG monoclonal antibodies (NOF Corporation, Tokyo, Japan). For the analysis, the heart slices were pre-incubated with blocking agents (Simple Stain Rat; Nichirei, Tokyo, Japan), followed by incubation with the primary antibodies mentioned above for 60 min at room temperature. Immune staining was performed using a universal immunoperoxidase polymer (Histofine Simple Stain MAX PO, anti-rat and anti-rabbit; Nichirei, Tokyo, Japan). The number of 8-OHdG-positive cells was counted in 20 random microscopic fields per cardiac tissue section to calculate the 8-OHdG-positive cells scores.
RNA Extraction and Real-Time RT-PCR Analysis
Total RNA was extracted from the heart using the ISOGEN kit (Wako Pure Chemicals Industries, Ltd, Osaka, Japan) as described previously [19]. Real-time PCR was performed using a LightCycler 350s Real-Time PCR System (Roche, Mannheim, Germany) with the LightCycler FastStart DNA Master SYBR Green I Kit (Roche). We analyzed all the data with the second derivative maximum method of the LightCycler software (version. 4.0; Roche). The relative mRNA expression levels of target genes were normalized to β-actin mRNA. Primer sequences used in this study are listed in Table 1.
Statistical Analysis
We performed all statistical analyses using the computer software application StatView 5.0 (SAS Institute, Cary, NC, USA). Values are presented as means ± SEM. To evaluate the significance of differences between groups, Student’s t-test or one-way ANOVA followed by the Tukey–Kramer test was used. A p value of <0.05 was considered statistically significant.
Results
Animal Characteristics and Biochemical Data
At 20 weeks of age, there were no significant differences in animal characteristics among the three groups (data not shown). As shown in Table 2, at 30 weeks of age, body weight, blood pressure, renal function and glycemic control were comparable between the DM and OCT groups. U-Alb was significantly less in the OCT group than in the DM group. In the INS group, body weight and serum TP and Alb levels were significantly greater, and HbA1c and U-Alb were significantly lower compared to the DM and OCT groups. As for the renal function, creatinine clearance (Ccr) tended to be lower in the INS group compared to that in the other two groups although it was not statistically significant. As for CKD-mineral bone disorder parameters, serum Ca, P, and 25D levels were comparable among the three groups. By contrast, serum 1,25D and PTH levels were significantly suppressed by OCT administration and these levels were lower in the DM group compared to the INS group. Serum FGF23 levels significantly increased following OCT administration and were the lowest in the DM group.
Change of Cardiac Findings by the OCT Treatment
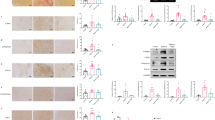
As shown in Fig. 1a, at 30 weeks, the relative heart volume was significantly lower in the OCT group than in the DM group despite similar blood pressures and serum glucose levels in the two groups. Serum NT-proBNP levels were also significantly lower in the OCT group than in the DM group (Fig. 1b). The degree of myocardial and perivascular fibrosis was more severe in the DM group than in the INS and OCT groups (Fig. 2a–d). The OCT treatment prevented the progression of cardiac fibrosis.
Histological examination of the heart sections in the DM, INS and OCT groups (a) Myocardial fibrosis (magnification ×400) (b) Perivascular fibrosis (magnification ×400) (c) Degree of myocardial fibrosis (d) Perivascular fibrosis score *; DM group v.s INS group, p < 0.05, ✝; DM group v.s OCT group, p < 0.05
Assessment of Oxidative Stress
We examined systemic and intracardiac oxidative stress among the three groups. Twenty-four-hour U-8-OHdG was greater in the DM and OCT groups than in the INS group. U-8-OHdG tended to be lower in the OCT group than in the DM group although there was no statistically significant difference (Fig. 3b). The 8-OHdG-positive cell score in the heart was significantly lower in the OCT group than in the DM group (Fig. 3c).
Immunohistochemical evaluation of oxidative stress in the cardiac tissue in the DM, INS and OCT groups. (a) Left panel, DM group; middle panel, INS group; right panel, OCT group. (b) Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) excretion in the DM, INS and OCT groups. (c) 8-OHdG-positive cell score in the heart (×400). *; DM group v.s INS group, p < 0.05, ✝; DM group v.s OCT group, p < 0.05, #; DM group v.s OCT group, p < 0.05
mRNA Expression of Markers of Cardiac Hypertrophy
To assess the expressions of cardiac hypertrophy-related genes, the mRNA expressions of atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), myosin heavy chain beta (β-MHC), myosin heavy chain alfa (α-MHC), connective tissue growth factor (CTGF) and dihydronicotinamide adenine dinucleotide phosphate (NADPH) p47-phox subunit were determined by real time RT-PCR. At 30 weeks, the mRNA expressions of ANP, BNP, β-MHC/α-MHC, CTGF and NADPH p47 phox were significantly higher in the DM group than in the INS group, and these increased expressions were reduced by OCT treatment (Fig. 4).
mRNA expressions of cardiac hypertrophy-related genes in the DM, INS and OCT groups. (a) mRNA expression of atrial natriuretic peptide (ANP) (b) mRNA expression of brain natriuretic peptide (BNP) (c) mRNA expression of beta-myosin heavy chain/alfa-myosin heavy chain (β-MHC/α-MHC) (d) mRNA expression of connective tissue growth factor (CTGF) (e) mRNA expression of NADPH p47 *; DM group v.s INS group, p < 0.05, ✝; DM group v.s OCT group, p < 0.05, #; DM group v.s OCT group, p < 0.05
Assessment of RAS
To evaluate RAS status, we measured the urinary excretion of angiotensinogen (AGT) and the mRNA expression of renin and angiotensin II type 1 receptor (AT1R). Urinary excretion of AGT was markedly elevated in the DM group, and significantly lower in the INS group than in the DM group. It did not significantly differ between the OCT group and the DM group (Fig. 5a). As for the mRNA expression of renin and AT1-R, although they were significantly lower in the INS group than in the DM and OCT groups, there was no significant difference between the DM and OCT groups (Fig. 5b, c).
mRNA expressions of renin-angiotensin system-related genes and urinary excretion of angiotensinogen (AGT) in the DM, INS and OCT groups. (a) mRNA expression of renin (b) mRNA expression of angiotensin II type 1 receptor (AT1R) (c) 24hr-urinary excretion of AGT *; DM group v.s INS group, p < 0.05, ✝; DM group v.s OCT group, p < 0.05, #; DM group v.s OCT group, p < 0.05
Effect of OCT on Heart Under RAS Inhibition
We performed a further study to ascertain whether OCT could prevent the progression of diabetic cardiomyopathy under RAS inhibition. As shown in Fig. 6, OCT could attenuate cardiac hypertrophy in the context of RAS inhibition. mRNA expressions of BNP and β-MHC/α-MHC were significantly decreased in the ALS + OCT group compared to the ALS group (Fig. 7). However, the mRNA expression of RAS genes and the urinary excretion of AGT were comparable between the ALS group and the ALS + OCT group (Fig. 8).
mRNA expressions of cardiac hypertrophy-related genes between the ALS and ALS + OCT groups. (a) mRNA expression of atrial natriuretic peptide (ANP) (b) mRNA expression of brain natriuretic peptide (BNP) (c) mRNA expression of beta-myosin heavy chain/alfa-myosin heavy chain (β-MHC/α-MHC) (d) mRNA expression of connective tissue growth factor (CTGF) (e) mRNA expression of NADPH p47 ##; ALS group v.s ALS + OCT group, p < 0.05
Discussion
Our study demonstrated that 1) at 30 weeks, despite comparable blood pressure serum glucose levels and renal function, U-Alb and U-8-OHdG were significantly lower in the OCT group than in the DM group; 2) heart volume and serum NT-proBNP levels were significantly lower in the OCT group compared to the DM group; 3) the number of 8-OHdG positive cardiomyocytes was reduced and cardiac and perivascular fibrosis was ameliorated by OCT administration; 4) the mRNA expressions of cardiac injury-related markers were significantly decreased in the hearts of OCT-treated rats compared to the DM group; and 5) the cardioprotective effect of OCT was preserved in the context of RAS inhibition.
Numerous reports have indicated that vitamin D and VDRA have various beneficial effects on physiological functions [9, 12, 13]; moreover, previous experimental and clinical studies have also demonstrated the favourable effects of vitamin D and VDRA on cardiac lesions, including left ventricular hypertrophy (LVH) [14, 15, 20]. On the other hand, randomized controlled trials in patients with CKD could not demonstrate the beneficial effects of VDRA on LVH [21, 22]. However, these studies also showed that the number of patients with cardiovascular-related hospitalizations was significantly lower in the VDRA treated group compared to the placebo group. Based on these results, we speculated that vitamin D and VDRA therapy may be effective for appropriate patients. It has been reported that vitamin D deficiency is prevalent among patients with type 2 diabetes and is associated with cardiac abnormalities [23]. Thus, in the present study we assessed the effect of VDRA therapy on cardiac abnormalities in diabetes.
CVD is the most important complication that is closely linked with mortality in diabetes. Although the rapid progression of coronary artery disease (CAD) is well known in diabetes, some patients with diabetes have prominent cardiac dysfunction and LVH despite having no significant CAD. This cardiac abnormality, now termed ‘diabetic cardiomyopathy’, occurs in diabetes independently of CAD and hypertension [24]. Several mechanisms responsible for diabetic cardiomyopathy have been proposed. Oxidative stress, activation of RAS, impaired regulation of intracellular calcium, abnormal cellular metabolism and mitochondrial dysfunction etc. seem to be involved in the progression of diabetic cardiomyopathy [25, 26].
Oxidative stress is one of the most important factors for the progression of CVD and CKD. Oxidative stress is remarkably increased in diabetes and plays a key role in the progression of various diabetic complications such as osteopenia, nephropathy and cardiac and vascular lesions. It has been demonstrated that vitamin D, including OCT, has anti-oxidative effects [27, 28]. In the present study, U-8-OHdG increased and increased oxidative stress in the heart was proven by immunohistochemical staining. These results showed that systemic and local oxidative stress was increased under diabetic conditions. Although glycemic control by insulin therapy could reduce oxidative stress and prevent the progression of organ damage, OCT therapy also decreased oxidative stress and allayed organ damage compared to the DM group despite the comparably high glucose levels. Therefore, we believe that OCT exerts anti-oxidative effects independently of glycemic control. The results of our study also revealed a significant decrease in heart volume as well as a reduced the expression of cardiac hypertrophy-related genes and cardiac and intracardiac vascular fibrosis, following OCT therapy, which is in line with previous studies under non-diabetic conditions. In the light of these results, OCT is considered to have cardioprotective effects in diabetic conditions.
To explain the favourable effects of vitamin D and VDRA on the cardiovascular system, several pathophysiological mechanisms have been speculated. Active vitamin D halts the progression of atherosclerosis by enriching Th2 lymphocytes population [29]. The improvement of endothelial dysfunction by vitamin D therapy has been also reported [12, 30]. In addition to these vascular protective effects, the suppression of RAS is thought to be one of the most important mechanisms accounting for the cardioprotective effects of VDRA [14]. However, a previous study has demonstrated that OCT does not suppress renin and angiotensin II [31]. In fact, our data also showed that there were no significant differences in the expressions of RAS-related genes in the heart and urinary excretion of AGT between the DM and OCT group. Furthermore, to ascertain whether the cardioprotective effect of OCT depends on the suppression of RAS, we performed a further study using aliskiren. Although there was no significant difference in oxidative stress between the ALS and ALS + OCT group, heart weight and expression of cardiac hypertrophy-related genes were significantly lower in the ALS + OCT group compared to those in the ALS group. Taken together, we suggest that OCT has a cardioprotective effect that is independent of RAS suppression and this effect is especially associated with the cardiac hypertrophy progression pathway. The calcineurin–nuclear factor of activated T cells (calcineurin-NFAT) signaling cascade seems to be a crucial pathway of pathological hypertrophy [32–34]. It has been reported that vitamin D could prevent the progression of cardiac hypertrophy through the inhibition of this pathway [35]. Although we could not prove the mechanisms in this study, inhibition of this pathway by vitamin D may be one of the mechanisms of reducing cardiac hypertrophy.
Increased serum levels of FGF23 and PTH have been reported to be associated with cardiac hypertrophy [36–39]. In addition, impaired regulation of intracellular Ca leads to diastolic and systolic dysfunction [40, 41]. In the present study, although the details were not evaluated, the heart volume was the lowest in the INS group despite the highest serum PTH level among the three groups, and the heart volume in the OCT group was lower than in the DM group despite the highest serum FGF23 levels. These results suggest that FGF23 and PTH did not play a key role in our study. Furthermore, because an elevation of serum Ca levels following the administration of OCT was not observed, impaired Ca homeostasis did not seem to affect cardiac abnormalities.
It has been reported that serum FGF23 levels are significantly associated with CVD events and mortality in both dialysis and non-dialysis patients [42, 43]. Certainly, though active vitamin D and VDRA raise FGF23 levels, they also increase the expression of klotho which has cardiovascular protective effects. Then, the previous experimental study has reported that not a slightly and moderately but extremely high concentration of FGF administration lead to cardiac hypertrophy [36]. Wolf has speculated that a degree of FGF response to vitamin D may be important and patients who exhibit a greater increase in FGF23 may manifest poor prognosis [44]. Our data also showed that elevation of serum FGF23 levels was not associated with cardiac hypertrophy and damage. Therefore, we think that the balance between favorable effects and adverse effects is important in using vitamin D and VDRA. Taken together, in the real world, vitamin D and VDRA treatment may be beneficial for most CKD patients.
Conclusions
Our data suggest that OCT prevents the development of cardiac damage, such as cardiac hypertrophy and cardiac fibrosis in diabetes mellitus, independently of RAS inhibition. To explore the detailed mechanisms, a further study is needed.
References
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305.
Ninomiya T, Kiyohara Y, Kubo M, et al. Chronic kidney disease and cardiovascular disease in a general Japanese population: the Hisayama Study. Kidney Int. 2005;68:228–36.
Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9.
Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–17.
Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin–angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366:2026–33.
Asanuma H, Minamino T, Sanada S, et al. ß-Adrenoceptor blocker carvedilol provides cardioprotection via an adenosine-dependent mechanism in ischemic canine hearts. Circulation. 2004;109:2773–9.
Wang R, Miura T, Harada N, et al. Pleiotropic effects of the beta-adrenoceptor blocker carvedilol on calcium regulation during oxidative stress-induced apoptosis in cardiomyocytes. J Pharmacol Exp Ther. 2006;318:45–52.
Packer M, Fowler MB, Roecker EB, et al. Carvedilol prospective randomized cumulative survival (COPERNICUS) study group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194–9.
Holick MF. Vitamin D, deficiency. N Engl J Med. 2007;357:266–81.
Mehrotra R, Kermah DA, Salusky IB, et al. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009;76:977–83.
Grandi NC, Breitling LP, Brenner H. Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Prev Med. 2010;51:228–33.
Chitalia N, Ismail T, Tooth L, et al. Impact of vitamin D supplementation on arterial vasomotion, stiffness and endothelial biomarkers in chronic kidney disease patients. PLoS One. 2014;9, e91363.
de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomized controlled trial. Lancet. 2010;376:1543–51.
Kong J, Kim GH, Wei M, et al. Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. Am J Pathol. 2010;177:622–31.
Panizo S, Barrio-Vázquez S, Naves-Díaz M, et al. Vitamin D receptor activation, left ventricular hypertrophy and myocardial fibrosis. Nephrol Dial Transplant. 2013;28:2735–44.
Kirii K, Mizoue T, Iso H, et al. Japan public health center-based prospective study group. Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia. 2009;52:2542–50.
Svoren BM, Volkening LK, Wood JR, Laffel LM. Significant vitamin D deficiency in youth with type 1 diabetes mellitus. J Pediatr. 2009;154:132–4.
Fujii H, Nishijima F, Goto S, et al. Oral charcoal adsorbent (AST-120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol Dial Transplant. 2009;24:2089–95.
Fujii H, Kono K, Nakai K, et al. Oxidative and nitrosative stress and progression of diabetic nephropathy in type 2 diabetes. Am J Nephrol. 2010;31:342–52.
Lemmilä S, Saha H, Virtanen V, Ala-Houhala I, Pasternack A. Effect of intravenous calcitriol on cardiac systolic and diastolic function in patients on hemodialysis. Am J Nephrol. 1998;18:404–10.
Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307:674–84.
Wang AY, Fang F, Chan J, et al. Effect of paricalcitol on left ventricular mass and function in CKD—The OPERA trial. J Am Soc Nephrol. 2014;25:175–86.
Scragg R, Sowers M, Bell C, Third National Health and Nutrition Examination Survey. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the third national health and nutrition examination survey. Diabetes Care. 2004;27:2813–8.
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602.
Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23.
Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121:748–57.
Noyan T, Balaharoğlu R, Kömüroğlu U. The oxidant and antioxidant effects of 25-hydroxyvitamin D3 in liver, kidney and heart tissues of diabetic rats. Clin Exp Med. 2005;5:31–6.
Nakai K, Fujii H, Kono K, et al. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am J Hypertens. 2014;27:586–95.
Andress DL. Vitamin D, in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int. 2006;69:33–43.
Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–5.
Inoue K, Matsui I, Hamano T, et al. Maxacalcitol ameliorates tubulointerstitial fibrosis in obstructed kidneys by recruiting PPM1A/VDR complex to pSmad3. Lab Invest. 2012;92:1686–97.
Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–75.
Muslin AJ. MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin Sci (Lond). 2008;115:203–18.
Vega RB, Bassel-Duby R, Olson EN. Control of cardiac growth and function by calcineurin signaling. J Biol Chem. 2003;278:36981–4.
Chen S, Law CS, Grigsby CL, et al. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838–47.
Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408.
Gutiérrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–52.
Cha H, Jeong HJ, Jang SP, et al. Parathyroid hormone accelerates decompensation following left ventricular hypertrophy. Exp Mol Med. 2010;42:61–8.
Fujii H, Kim JI, Abe T, Umezu M, Fukagawa M. Relationship between parathyroid hormone and cardiac abnormalities in chronic dialysis patients. Intern Med. 2007;46:1507–12.
Choi KM, Zhong Y, Hoit BD, et al. Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in Type 1 diabetic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1398–408.
Zhao XY, Hu SJ, Li J, Mou Y, Chen BP, Xia Q. Decreased cardiac sarcoplasmic reticulum Ca2+− ATPase activity contributes to cardiac dysfunction in streptozotocin-induced diabetic rats. J Physiol Biochem. 2006;62:1–8.
Isakova T, Xie H, Yang W, et al. Chronic renal insufficiency cohort (CRIC) study group. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–9.
Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92.
Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82:737–47.
Acknowledgments
This study was partly presented at the annual meeting of American Society of Nephrology Congress, 2013.
We thank Dr. Riko Kitazawa of the division of diagnostic molecular pathology, Kobe University Graduate School of Medicine, for the pathological evaluation and Kayo Tsubota for technical assistance.
This study was partly supported by Chugai Pharmaceutical Co., Ltd and M. H. is an employee of Chugai Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Author declares that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fujii, H., Nakai, K., Yonekura, Y. et al. The Vitamin D Receptor Activator Maxacalcitol Provides Cardioprotective Effects in Diabetes Mellitus. Cardiovasc Drugs Ther 29, 499–507 (2015). https://doi.org/10.1007/s10557-015-6629-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-015-6629-y