Abstract
Background
Berberine exhibits numerous pharmacological effects, but the mechanism for its protective effects against ischemia-reperfusion cardiac injury is unknown.
Methods
Male Wistar rats were treated with berberine (100 mg/Kg/day, ig) for 14 days and controls treated with water. Hearts were isolated in vitro and perfused in the Langendorff mode and subjected to 30 min of global ischemia followed by 30 min of reperfusion and hemodynamic data examined. In a separate set of experiments, hearts were subjected in vivo to left anterior descending coronary artery ligation for 30 min followed by 120 min reperfusion and hemodynamic data, type and duration of arrhythmias, and myocardial infarct size determined. AMP-activated protein kinase (AMPK) level, ADP/ATP and AMP/ATP ratios were examined in non-ischemic areas and risk areas of the heart.
Results
Subsequent to ischemia-reperfusion injury, left ventricular developed pressure, left ventricular end diastolic pressure and maximum rate of intraventricular pressure contractility and relaxation were significantly improved in the berberine treatment groups compared to controls. Berberine treatment decreased infarct size and diminished the duration and incidence of arrhythmias compared to controls. Berberine treatment significantly decreased AMPK protein concentration, and the ratio of ADP/ATP and AMP/ATP in the myocardial risk areas. In contrast, berberine treatment significantly increased AMPK protein concentration, and the ratio of ADP/ATP and AMP/ATP in the non-ischemia areas compared to controls.
Conclusion
These findings suggest that berberine may exert its cardioprotective effect on ischemia-reperfusion injury via regulation of AMPK activity in both non-ischemic areas and risk areas of the heart.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) is the leading cause of death worldwide. Early and successful restoration of blood flow to an ischemic myocardium is the most effective strategy to improve clinical outcome [1–4]. However, restoration of blood flow produces serious myocardial damage, including acute myocardial ischemia-reperfusion injury. Therefore, novel therapeutic strategies are required to protect the myocardium against ischemia-reperfusion injury in patients with CAD. Despite significant advances in our understanding of the mechanisms underlying this process, current treatments for ischemia-reperfusion injury remain rudimentary.
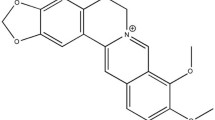
Berberine ([C20H18NO4]+) (BER) is an isoquinoline alkaloid originally isolated from the Chinese herb Coptis chinensis (Huanglian). It is an anti-microbial drug routinely prescribed for the treatment of diarrhea in many Asian countries [5–7]. Interests in its effects on metabolic disease have grown over the last decades. Several studies have focused on its effect on endothelial function and the cardiovascular system in diabetic conditions [8–12]. A previous study from our laboratory reported beneficial effects of BER on endothelial function through the eNOS pathway [13]. Other studies have indicated that BER reduced oxidative stress and vascular inflammation, and suppressed artherogenesis via stimulation of AMP-activated protein kinase (AMPK) expression [14].
AMPK is a heterogeneous protein trimer composed of three subunits, a functional subunit (α) and regulatory subunits (β γ)[15]. It plays an important role in various intracellular signaling pathways that regulate intracellular energy balance [16–18]. It is widely accepted that berberine activates AMPK to achieve its beneficial effect on diabetes and endothelial function by inhibition of mitochondrial respiratory complex I[19]. In addition, AMPK regulation may protect the heart from ischemic injury or cardiac myocyte hypertrophy [20, 21]. However several studies have demonstrated that AMPK activity might induce myocardial cell death and poorer cardiac functional recovery after ischemia reperfusion injury [22, 23]. Meanwhile, most studies have examined activation of AMPK in the risk area of the ischemic-reperfused heart but not evaluate the alteration of AMPK in both risk area and non-ischemia area [23–25]. The effect of BER on ischemia reperfusion heart injury is unknown, and whether its cardioprotective effect is exerted by mediation of AMPK activity in non-ischemia and risk areas of ischemic reperfused myocardium is unclear. In the present study, we showed that BER protects the heart in both in vitro and in vivo models of cardiac ischemia-reperfusion injury by inducing up-regulation of AMPK activity in areas of non-ischemia and down-regulation of AMPK activity in risk areas and that this is correlated with the ratio of ADP/ATP and AMP/ATP.
Materials and Methods
Materials
Antibodies against phos-AMPK, AMPK and anti-rabbit secondary antibody were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). GAPDH was obtained from Epitomics Inc. (Epitomics, CA), BER was purchased from Northeast Pharmaceutical Factory, China. Compound C was purchased from Sigma-Aldrich. Other reagents were purchased from Beijing General Chemical Reagent. BL-420 system was purchased from Chengdu Technology & Market (Co., LTD) for recording ECG and hemodynamic data.
Animals
Male Wistar rats (250–280 g) were purchased from the Experimental Animal Center, JiLin University. All rats had free access to tap water. The procedures were approved by the JiLin University Animal Care and Use Committee and followed the Principles of Laboratory Animal Care.
In Vitro Isolated Heart Perfusion
Sixty rats were randomly divided into 4 groups (n = 15) for in vitro experiments: control (CON), control plus BER group (CON+BER), ischemia reperfusion group (IR), and IR plus BER group (IR+BER). The CON+BER and IR+BER groups were pretreated with BER (100 mg/kg/d, ig) for 14 days before the experiments. CON and IR were treated with water in parallel. Rats were anesthetized with 20 % urethane, their hearts isolated and perfused in a non-recirculating Langendorff apparatus. Modified Kreb’s Henseleit buffer (KHB) was oxygenated with 95 % O2, and 5 % CO2 at 37 °C and pH 7.4. The coronary perfusion pressure was kept at 80 cm H2O and the heart connected to a stimulator to maintain the heart rate at 300/min. An incision was made down the left atrial appendage and a water-filled latex balloon was placed in the left ventricle via the left atrium. Left ventricular developed pressure (LVDP), left ventricular end diastolic pressure (LVEDP), minimum (−dp/dtmax) and maximum (+dp/dtmax) rate of pressure change in the ventricle were recorded every 10 min during perfusion after equilibration. The hearts were perfused for 20 min for stabilization prior to ischemia. The CON and CON+BER groups were perfused with KHB for 80 min. The IR and IR+BER groups were subjected to 30 min ischemia followed by 30 min of reperfusion.
In Vivo Animal Experiments
Sixty rats were randomly divided into 4 groups (n = 15) for in vivo experiments: control (CON), control plus BER group (CON+BER), ischemia reperfusion group (IR), and IR plus BER group (IR+BER). The CON+BER and IR+BER groups were pretreated with BER (100 mg/kg/d, ig) for 14 days before the experiments. CON and IR were treated with water in parallel. CON and CON+BER rats were anesthetized with 20 % urethane (0.5 ml/100 g, ip), placed face up and fixed on the operating table and connected with BL-420E biological and functional system ECG wires. After tracheal intubation and connection to a small animal respirator (tidal volume of 8–12 ml, 1:2 breathing, respiratory rate 70–80 times/min), the chest was opened between the left third and fourth rib exposing the heart. A nylon suture was placed around the left anterior descending coronary artery. IR and IR+BER groups were subjected to 30 min ischemia by ligating the artery, which was followed by 120 min reperfusion by loosening the ligature. Arrhythmias were monitored during ischemia reperfusion by ECG. ST-segment elevation and widening of R wave indicated ischemia. A 50 % decrease in ST-segment elevation indicated successful reperfusion. CON and CON+BER groups were treated identically without artery ligation. LVDP, LVEDP, ±dp/dtmax and ECG were recorded continuously. In some experiments, rats hearts subjected to left anterior descending coronary artery ligation were treated with Compound C at the beginning of ischemia or 5 min into reperfusion prior to determination of LVDP.
Determination of Tissue Injury
Evans Blue Staining
After 120 min reperfusion, the left coronary artery was injected with 0.2 ml 1 % Evans blue dye through the right common carotid artery in order to distinguish between ischemic and non-ischemic areas. The heart was isolated immediately, rinsed with phosphate buffered solution (PBS, pH 7.4) and the surface dried with filter paper.
TTC Staining
After Evans blue staining, the left ventricle was cut into 2 mm thick transverse sections, and incubated with 1 % triphenyl tetrazolium chloride (TTC) phosphate buffer (pH 7.4) at 37 °C. TTC reacted with intracellular dehydrogenases to stain viable tissue red leaving infarcted areas off-white. Digital photographs were taken of the traverse sections.
Determination of Myocardial Infarction Area
The infarct size (IS), area at risk (AAR) and percentage of infarct size to area at risk (IS/AAR) were determined by computer image analysis (Image plus 6.0 System).
Western Blot
After acute left coronary artery ligation in vivo, samples were taken from the AAR and the non-ischemia area (NIA). Quantitative analysis of AMPK (or p-AMPK, Th172) levels was performed as previously described [26]. Briefly, protein samples were prepared from hearts by homogenization with ice-cold RIPA buffer. Protein concentrations were measured using the Bradford assay (Bio-rad protein assay kit). For western blot detection of p-AMPK and AMPK protein expression, proteins (80 μg) were separated by 12 % SDS polyacrylamide gel and electro transferred onto polyvinylidene difluoride membranes (Bio-Rad). Membranes were blocked with 5 % (w/v) skim milk or 5 % BSA for 2 h at room temperature and then incubated with rabbit polyclonal antibodies (p-AMPK, 1:500, AMPK, 1:1000, Santa Cruz Biotechnology) with gentle agitation overnight at 4 °C. The membranes were washed 3 times for 10 minutes each with 15 ml of TBST (10 mM Tris–HCl, 150 mM NaCl and 0.1 % (v/v) Tween-20) and then incubated with secondary antibody (1:1000 goat anti-rabbit IgG horseradish peroxidase conjugate, Santa Cruz Biotechnology) at room temperature for 2 h. Proteins were visualized with enhanced chemiluminescence’s solution and X-ray film. An Imaging Densitometer was used to scan the protein bands and these were quantified using Image Analysis Software (Quantity One). The results were expressed as phosphorylated protein relative to total protein.
Determination of AMP, ADP and ATP Content of Ischemia and Non-ischemia Areas
ATP, ADP and AMP levels were determined using respective ELISA kits (Shanghai YanJing Biological Research Technology Co, LTD, China). Briefly, animals were killed and hearts were rapidly removed and the ischemia and non-ischemia areas isolated and frozen in liquid nitrogen. The tissues were then homogenized on ice in PBS containing protease inhibitor, pH 7.4, 4 °C and the homogenate centrifuged at 3,000 g for 20 min. AMP, ADP and ATP content was determined in the supernatant according to the manufacturer’s instructions.
Statistics
All data are expressed as mean±SE. The differences between groups were compared with one-way ANOVA followed by Tukey’s test, and the statistics of cardiac function are compared with two-way ANOVA followed by Tukey’s test, p < 0.05 was considered statistically significant.
Results
Effect of BER on IR-Induced Cardiac Dysfunction In Vitro
Hemodynamic data indicated that left ventricular function was depressed after 30 min of myocardial ischemia followed by 30 min reperfusion (Table 1). Temporal analysis indicated that systolic and diastolic functions were depressed significantly, with decline in LVDP (Fig. 1a), +dp/dtmax (Fig. 1c), −dp/dtmin (Fig. 1d) at 30 min ischemia. After 30 min reperfusion, LVDP, +dp/dtmax and −dp/dtmin slightly recovered, but to a lower level than values at the beginning of the perfusion. LVEDP increased significantly at 10 minutes reperfusion (Fig. 1b). BER treatment significantly improved cardiac dysfunction caused by ischemia. After 30 min reperfusion, BER preserved LVDP by 75 % (p < 0.01) (Fig. 1a), LVEDP by 29 % (p < 0.01) (Fig. 1b), +dp/dtmax by 75 % (p < 0.01) (Fig. 1c) and −dp/dtmin by 69 % (p < 0.01) (Fig. 1d), compared to the control group at the same time point. There were no differences in cardiac function indicated by hemodynamic data including LVDP, LVEDP, +dp/dtmax and −dp/dtmin between CON and CON+BER groups (data not shown).
Effect of BER treatment on cardiac function in ischemia reperfusion heart in vitro. a, left ventricular developed pressure (LVDP); b, left ventricular end-diastolic pressure (LVEDP); c, +dp/dtmax: maximum rate of pressure change in the ventricule; d, −dp/dtmin: minimum rate of pressure change in the ventricule. *p < 0.05, vs. the level of LVDP, LVEDP, +dp/dtmax, −dp/dtmin at 20 min stabilization, **p < 0.05, vs. IR group at the same time points, respectively. Data shown as means±SE (n = 6–8 rats/group)
Effect of BER on IR-Induced Cardiac Dysfunction In Vivo
Hemodynamic data from the in vitro study clearly indicated that BER had a beneficial effect on attenuating cardiac dysfunction induced by ischemia reperfusion. In vivo hemodynamic data indicated that left ventricular functions in both IR and IR+BER groups were depressed following 30 min myocardial infarction and partially recovered by 120 min reperfusion (Table 2). Treatment with 100 mg/kg BER for 14 days significantly reversed cardiac dysfunction in ischemic reperfused hearts compared to untreated controls. Temporal analysis indicated that BER treatment increased recovery of LVDP by 10 % at 30 min ischemia and by 9 % after 120 min of reperfusion (Fig. 2a), decreased LVEDP by 45 % at 30 min ischemia and by 40 % after 120 min reperfusion (Fig. 2b), increased +dp/dtmax 23 % at 30 min ischemia and 39 % after 120 min reperfusion (Fig. 2c), and increased −dp/dtmin 29 % at 30 min ischemia (Fig. 2d). There were no differences in cardiac LVDP, LVEDP, +dp/dtmax and −dp/dtmin between CON and CON+BER treated animals not subjected to ischemia reperfusion (data not shown).
Effect of BER treatment on cardiac function in ischemia reperfusion heart in vivo. a, left ventricular developed pressure (LVDP); b, left ventricular end-diastolic pressure (LVEDP); c, +dp/dtmax: maximum rate of pressure change in the ventricule; d, −dp/dtmin: minimum rate of pressure change in the ventricule. Data shown are means±SE (n = 6–8/group) *P < 0.05 vs. the level of LVDP, LVEDP, +dp/dtmax −dp/dtmin at 20 min stabilization, **p < 0.05, vs. IR group at the same time points, respectively
Effect of BER on the Incidence of IR-Induced Ventricular Arrhythmic Events (VAEs) In Vivo
Arrhythmia vulnerability was evaluated as the number of VAEs and the duration of these VAEs. ECG was continuously recorded during the ischemia reperfusion process in the in vivo experiment. Prior to coronary artery ligation, VAEs were negligible during stabilization period in all groups (range 0–20 events). During the first 10 min after ligation and at the beginning of reperfusion, the number of VAEs was significantly increased in both IR and IR+BER groups compared to controls (Table 3). The rate of death was markedly increased in IR rats, but significantly decreased by BER treatment. As shown in Table 3, premature beats were significantly decreased by 52 % and the duration of ventricular fibrillation and ventricular tachycardias were significantly reduced 57 % and 50 %, respectively, by BER treatment compared to controls. These results indicate that rats treated with BER are less prone to the development of arrhythmias triggered by ischemia reperfusion in vivo.
Effect of BER on the Myocardial Infarct Size After Ischemia Reperfusion
To determine whether BER could attenuate the myocardial necrosis after left coronary artery ligation and reperfusion, infarct size was determined by Evans blue and TCC staining. Ischemia reperfusion mediated infarct size was significantly decreased by BER treatment compared to the IR group (Fig. 3a–c). Significant reductions in IS/LV (Fig. 3e) and IS/AAR (Fig. 3d) were observed in BER treated animals compared to IR. There was no alteration in AAR/LV (Fig. 3f, Table 4).
Effects of BER treatment on infarct size in ischemia reperfused rat hearts. Image of heart TTC/Evens blue staining: a, non-ischemia area; b, infarcted area; c, area at risk; d, Percentage of infarcted size to left ventricular area (IS/LV%); e, Percentage of area at risk to left ventricular (AAR/LV%); f, Percentage of infarcted size to area at risk (IS/AAR%). **P < 0.05 compared to IR group
Effect of BER on the AMPK Activity in AAR and NIA of Ischemic Reperfused Hearts
To explore the mechanism of this beneficial effect of BER on ischemia reperfusion heart injury, we examined if BER attenuated cardiac dysfunction induced by ischemia reperfusion via regulating the activity of AMPK. The expression of AMPK and its phosphorylated form pThr172-AMPK in heart after ischemia reperfusion treated with or without BER are shown in Fig. 4. Data were expressed as the ratio of p-AMPK/AMPK and p-AMPK/GAPDH, and these ratios were normalized to control. P-AMPK was decreased in NIA after IR, but the expression of p-AMPK increased at AAR compared to controls. P-AMPK in NIA was increased in IR+BER compared to IR group (p < 0.01) (Fig. 4b). Interestingly, in the AAR a decreased level of p-AMPK was observed in the IR-BER group compared to the IR group (Fig. 4a). These surprising results indicated that BER may play a role in the regulation of the cardiac energy status in different sections of the heart after ischemia reperfusion injury. As expected, there was no difference between the CON and the CON+BER groups in both the AAR and the NIA. Total AMPK levels were similar between IR and IR+BER groups.
Effect of BER on the expression of AMPK in area at risk (AAR) and non-ischemia area (NIA) of ischemic reperfused hearts. a, Western blot analysis of p-AMPK and AMPK levels in AAR; b, Western blot analysis of p-AMPK and AMPK levels in NIA. AMPK activity was determined as ratio of p-AMPK to AMPK. CON, hearts from water fed rats; CON+BER, hearts from BER fed rat; IR, hearts from water fed rats subjected to ischemia reperfusion injury; IR+BER, hearts from BER fed rats subjected to ischemia reperfusion injury. *p < 0.05 compared to CON group. **p < 0.05, vs. IR group, (n = 5)
Effect of BER on the AMP, ADP and ATP Levels in Area At Risk (AAR) and Non-ischemia area (NIA) of Ischemic Reperfused Hearts
We examined if the differential regulation of AMPK activity by BER in AAR and NIA were related to the ratio of adenine nucleotides during the reperfusion after ischemia. Since the ratio of ADP/ATP is related to activation of AMPK, we examined the ratio of AMP/ATP and ADP/ATP in both the AAR and the NIA of ischemic reperfused tissues. As shown in Fig. 5 the ratio of AMP/ATP was increased in the AAR in the IR group compared to the CON group (Fig. 5a), but decreased in the NIA (Fig. 5b). Pre-treatment with BER significantly decreased the ratio of AMP/ATP in the AAR and increased the ratio of AMP/ATP in the NIA. The ratio of ADP/ATP exhibited a similar trend in both areas (Fig. 6a and b). These results were consistent with the AMPK activities in both the AAR and the NIA. These data indicate that the different AMPK activities observed in the AAR and the NIA after ischemia-reperfusion may be due to the different energy states in these distinct areas.
Effect of BER on AMP/ATP ratio in the area at risk (AAR) and non-ischemia area (NIA) of ischemic reperfused hearts. Adenine nucleotides from homogenate of myocardial tissues extracts of AAR and NIA were determined by ELISA. CON, hearts from water fed rats; CON+BER, hearts from BER fed rat; IR, hearts from water fed rats subjected to ischemia reperfusion injury; IR+BER, hearts from BER fed rats subjected to ischemia reperfusion injury. a, AMP/ATP ratio in AAR; b, AMP/ATP ratio in NIA. *p < 0.05 compared to CON group. **p < 0.05, vs. IR group (n = 5)
Effect of BER on the ADP/ATP ratio in area at risk (AAR) and non-ischemia area (NIA) of ischemic reperfused hearts. Adenine nucleotides from homogenates of myocardial tissue extracts of AAR and NIA were determined by ELISA. CON, hearts from water fed rats; CON+BER, hearts from BER fed rat; IR, hearts from water fed rats subjected to ischemia reperfusion injury; IR+BER, hearts from BER fed rats subjected to ischemia reperfusion injury. a, ADP/ATP ratio in AAR. b, ADP/ATP ratio in NIA. *p < 0.05 compared to CON group. **p < 0.05, vs. IR group (n = 5)
Effect of Compound C on the BER-mediated Recovery of LVDP of In Vivo Ischemia-reperfused Hearts
We further examined if BER would sustain its cardioprotective effect when AMPK was inhibited. Water or BER fed rats were subjected to left anterior descending coronary artery ligation and 10 mg/Kg Compound C, a potent AMPK inhibitor, was injected immediately after ligation or injected at 5 min of reperfusion and LVDP subsequently examined. As seen in Fig. 7, Compound C diminished the protective effect of BER when injected at ischemia. This indicated that down regulation of AMPK activity during ischemia may decrease the cardioprotective effect of BER in ischemia and indicate that BER mediates its cardioprotective effect, at least in part, through modulation of AMPK activity.
Effect of Compound C on BER-mediated recovery of LVDP of in vivo ischemia-reperfused hearts. Rats were fed either water or BER and then subjected to left anterior descending coronary artery ligation in vivo and Compound C added immediately after ligation or at the first 5 min of reperfusion. IR, water fed rats subjected to left anterior descending coronary artery ligation; IR+BER, rats fed BER subjected to left anterior descending coronary artery ligation; IR+BER +Compound C, rats fed BER subjected to left anterior descending coronary artery ligation and injected with 10 mg/Kg Compound C immediately after ligation of the left anterior descending coronary artery; IR+BER +Delayed Compound C, rats fed BER subjected to left anterior descending coronary artery ligation and injected with 10 mg/Kg Compound C at 5 min of reperfusion. Data shown are means±SE (n = 6–8) *p < 0.05 vs. BER group at the same time points. **p < 0.05, vs. IR group at the same time points, respectively
Discussion
In the present study, we utilized both in vitro and in vivo models to explore the beneficial effect of BER on ischemia reperfusion cardiac injury. Rats were pretreated with BER (100 mg/kg/day) for 14 days (the last dose given 2 h before the in vitro and in vivo experiments) prior to ischemia reperfusion injury in order to assess the protective effect of BER. Pretreatment is commonly used to study the effect of drugs on ischemia reperfusion injury [27, 28] and pretreatment for this period ensures a stable plasma concentration of BER. We observed that LVDP, LVEDP, ±dp/dtmax were significantly improved by BER treatment in hearts subjected to ischemia reperfusion injury in vitro in a Langendorff perfusion system. The Langendorff heart perfusion system is a classic model to estimate cardiac functional changes in the absence of a neurohumoral influence. A number of studies have utilized this system to evaluate the effect of drugs on cardiac function, heart rate and coronary arterial function [29, 30]. The limitations of this model include a lack of a truly physiological cardiac load which may not accurately estimate LV dysfunction in ischemia reperfusion injury. Thus, we further evaluated the potential beneficial effects of BER on cardiac function during ischemia reperfusion in an in vivo model, i.e. the acute left anterior descending coronary artery ligation model. This in vivo model is most representative of the clinical condition of ischemia followed by restoration of blood flow to the ischemic heart. We observed a significant improvement of LVDP, LVEDP and ±dp/dtmax by BER treatment in hearts subjected to ischemia reperfusion consistent with the results from the in vitro study. Our results are in agreement with a previous study in which BER treatment was shown to significantly improve left ventricular function, exercise capacity and dyspnea-fatigue index in patients with cardiac hypertrophy [31]. The hemodynamic data from both the in vitro and in vivo experiments demonstrated the efficacy of BER in improving cardiac function during ischemia reperfusion injury. Moreover, BER treatment significantly reduced infarct size during ischemia reperfusion injury and prevented the occurrence and duration of isolated premature beats, ventricular fibrillation (VF) and ventricular tachycardia (VT) in the in vivo model of ischemia reperfusion injury.
The energy metabolism disorder between glucose and fatty acid utilization is an important factor contributing to cardiac ischemia reperfusion injury. The majority of energy supply for normal heart tissue is provided by oxidation of fatty acid with a small amount derived from oxidation of carbohydrates [32]. Long-chain fatty acids enter into mitochondria via carnitine palmitoyl transferase-1/2 (CPT-1, CPT-2) and are converted to acetyl-CoA in order to generate ATP in the tricarboxylic acid cycle. Meanwhile, pyruvic acid produced from glycolysis is converted to acetyl-CoA by pyruvate dehydrogenase (PDH) which in turn is suppressed by acetyl-CoA [32]. Therefore excessive fatty acid oxidation can inhibit glucose oxidation by decreased PDH activity resulting in an imbalance between glycolysis and glucose oxidation [33]. During ischemia mitochondrial oxidative metabolism is inhibited and glycolysis is enhanced and becomes the predominant source of energy production. During reperfusion the rates of fatty acid oxidation are accelerated resulting in inhibition of glucose oxidation. This decreased coupling between glycolysis and glucose oxidation leads to an acceleration of the rate of myocardial H+ production and post-ischemic contractile dysfunction with subsequent myocardial cell apoptosis [34, 35].
AMPK, as a cellular fuel gauge, is an important regulator of myocardial energy metabolism during ischemia and reperfusion. AMPK is rapidly activated during myocardial ischemia and protects the heart against ischemic injury by promoting glucose uptake and glycolysis as well as fatty acid oxidation. In the hearts of transgenic mice expressing inactive AMPKα2 perfused with a low concentration of fatty acids, glucose uptake and utilization were impaired, cardiac function was decreased and cell death markers were elevated [36, 37]. Acute metformin therapy provided cardioprotection against myocardial infarction via AMPK-eNOS–mediated signaling [38]. The cardioprotective actions of metformin are consistent with that seen by the addition of the AMPK agonist AICAR and is attenuated in AMPK knockout mice [39]. These observations indicate that activation of AMPK may benefit hearts subjected to ischemia reperfusion injury. However, harmful effects of AMPK in the setting of ischemia reperfusion injury have also been observed. Recovery of cardiac function was improved during reperfusion after ischemia in AMPK-α2 dominant negative hearts perfused in the presence of a normal concentration of fatty acid [23]. In addition, AMPK-α2-specific reduction in AMPK activity did not affect baseline cardiac function but increased left ventricular end-diastolic pressure and decreased ATP levels when hearts were subjected to global ischemia [40], suggesting that AMPK inhibition is beneficial and may protect heart from ischemia. In the present study cardiac function and efficiency of the IR group were reduced and this was accompanied by an elevation of p-AMPK at the ischemia area. Activated AMPK was shown to enhance glucose uptake during early reperfusion [37]. However, persistent AMPK activation during reperfusion induced inactivation of acetyl-CoA carboxylase (ACC), decreased malonyl-CoA levels and relieved the inhibition of CPT-1 which facilitated the rates of fatty acid oxidation resulting in the accumulation of deleterious by-products of glycolysis (lactate and protons) within cardiac cells and aggravated myocardial injury at the ischemic site [41]. In our study, BER treatment decreased phosphorylation of AMPK in the AAR and improved myocardial dysfunction during ischemia reperfusion injury. In addition, p-AMPK expression was significantly decreased in the NIA in the IR group compared to the control group, while p-AMPK expression was increased in the NIA in the IR+BER group. These results suggest that BER protects the heart from IR injury by regulation of AMPK activity in both the AAR and the NIA during IR injury. Up-regulation of AMPK in the NIA mediated by BER may improve cardiac function and efficiency by promoting glucose uptake and glycolysis as well as fatty acid oxidation. Down-regulation of AMPK in the AAR mediated by BER may protect the heart from myocardial acidosis and apoptosis caused by fatty acid oxidation at the expense of glucose oxidation.
In support of the role of AMPK in mediating the protective effect of BER, we found that addition of Compound C, an inhibitor of AMPK, diminished the protective effect of BER when injected during ischemia. Interestingly, recovery of cardiac function was not attenuated when Compound C was injected at 5 min of reperfusion. Our observation is consistent with previous studies which demonstrate that phase-dependent changes of AMPK occurred during ischemia reperfusion and phase-dependent regulation of its activity might present a beneficial effect on ischemia reperfusion injury [24, 42]. Thus, BER could serve as a therapy for cardiac dysfunction in phase-dependent or area-dependent manner.
The mechanism of AMPK activation involves both an increase in AMP/ATP and activation of upstream AMPKKs, such as the tumor suppressor kinase LKB1 and a calmodulin-dependent protein kinase kinase (CamKK) [43–45]. Activation of AMPK by BER was associated with an increase in the AMP/ATP ratio due to inhibition of mitochondrial respiration complex I in L6 myotubes and this was not related to activity of either LKB1 or CamKK [19]. ADP may also protect AMPK from dephosphorylation by binding to just one of the two exchangeable AXP (AMP/ADP/ATP) binding sites on the regulatory domain of AMPK [46]. In our study, BER treatment increased AMP/ATP ratio in the NIA, but decreased AMP/ATP ratio in the AAR during IR injury. The ADP/ATP ratio exhibited the same trend. This could be a mechanism by which BER up-regulates AMPK at the NIA and down-regulates AMPK at the AAR. Alternatively, BER may differentially affect the ischemic and non-ischemic areas of the heart due to stress stimuli of AMPK activation during ischemia reperfusion cardiac injury. For example, reactive oxygen species and fatty acids accumulate during ischemia and trigger AMPK activation [47, 48]. BER has been shown to reduce triglyceride, low density lipoprotein and total cholesterol, and increase high density lipoprotein in patients with moderate dyslipidemias [49]. This might explain why BER could inhibit AMPK activity in the ischemic parts of the heart. However, the precise mechanism for BER modulation of AMPK activity requires further investigation.
In conclusion, BER remarkably improves cardiac function after ischemia reperfusion injury in both in the in vitro isolated rat heart and the in vivo heart and reduces the infarct size following an IR injury. The mechanism of this protective effect may be mediated via regulation of AMPK activity in both non-ischemic and risk areas due to modulation of the ratios of AMP/ATP and ADP/ATP.
Abbreviations
- AAR:
-
area at risk
- AICAR:
-
5-aminoimidazole-4-carboxamide -1-β-D-ribofuranoside
- AMP:
-
adenosine monophosphate
- ADP:
-
adenosine diphosphate
- AMPK:
-
adenosine-5′-monophosphate kinase
- AMPKK:
-
AMPK kinase
- ATP:
-
adenosine triphosphate
- BER:
-
berberine
- CamKK:
-
calmodulin-dependent protein kinase
- CDA:
-
coronary artery disease
- −dp/dtmin:
-
+dp/dtmax, minimum and maximum rate of pressure change in the ventricle
- ECG:
-
electrocardiogram
- GAPDH:
-
glyceraldehydes-3-phosphate dehydrogenates
- HR:
-
heart rate
- IS:
-
infract size
- KHB:
-
Krebs-Henseleit buffer
- LKB1:
-
tumor suppressor kinase
- LV:
-
left ventricular size
- LVDP:
-
left ventricular developed pressure
- LVEDP:
-
left ventricular end-diastolic pressure
- NIA:
-
non-ischemia area
- PBS:
-
phosphate buffered solution
- TTC:
-
triphenyl tetrazolium chloride phosphate buffer
- VAEs:
-
ventricular arrhythmic events
- VF:
-
ventricular fibrillation
- VT:
-
ventricular tachycardia.
References
Ferrari R. Importance of oxygen free radicals during ischemia and reperfusion in the experimental and clinical setting. Oxygen free radicals and the heart. Am J Cardiovasc Pathol. 1992;4(2):103–14.
Koch A, Bingold TM, Oberlander J, Sack FU, Otto HF, Hagl S, Schnabel PA. Capillary endothelia and cardiomyocytes differ in vulnerability to ischemia/reperfusion during clinical heart transplantation. Eur J Cardiothorac Surg. 2001;20(5):996–1001.
Okabe E. Ito H: [Biochemistry of the physiopathologic and clinical aspects of free radicals in heart ischemia: free radicals as a mediator of ischemia-reperfusion injury]. Nihon Rinsho. 1988;46(10):2190–5.
Shibata T, Yamamoto F, Kosakai Y, Kawazoe K, Komai H, Ichikawa H, Ohashi T, Shimada Y, Nakajima N, Kawashima Y. Clinical application of recombinant human SOD for the protection of ischemia reperfusion injury during open heart surgery. Nihon Kyobu Geka Gakkai Zasshi. 1993;41(3):427–32.
Desai AB, Shah KM, Shah DM. Berberine in treatment of diarrhoea. Indian Pediatr. 1971;8(9):462–5.
Khin Maung U, Myo K, Nyunt Nyunt W, Aye K, Tin U. Clinical trial of berberine in acute watery diarrhoea. Br Med J (Clin Res Ed). 1985;291(6509):1601–5.
Lahiri SC, Dutta NK. Berberine and chloramphenicol in the treatment of cholera and severe diarrhoea. J Indian Med Assoc. 1967;48(1):1–11.
Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103(3):274–84.
Bhutada P, Mundhada Y, Bansod K, Tawari S, Patil S, Dixit P, Umathe S, Mundhada D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav Brain Res. 2011;220(1):30–41.
Hsieh YS, Kuo WH, Lin TW, Chang HR, Lin TH, Chen PN, Chu SC. Protective effects of berberine against low-density lipoprotein (LDL) oxidation and oxidized LDL-induced cytotoxicity on endothelial cells. J Agric Food Chem. 2007;55(25):10437–45.
Meng S, Wang LS, Huang ZQ, Zhou Q, Sun YG, Cao JT, Li YG, Wang CQ. Berberine ameliorates inflammation in patients with acute coronary syndrome following percutaneous coronary intervention. Clin Exp Pharmacol Physiol. 2012;39(5):406–11.
Zhang H, Wei J, Xue R, Wu JD, Zhao W, Wang ZZ, Wang SK, Zhou ZX, Song DQ, Wang YM, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59(2):285–92.
Wang C, Li J, Lv X, Zhang M, Song Y, Chen L, Liu Y. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur J Pharmacol. 2009;620(1–3):131–7.
Wang Q, Zhang M, Liang B, Shirwany N, Zhu Y, Zou MH. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PLoS One. 2011;6(9):e25436.
Folmes KD, Chan AY, Koonen DP, Pulinilkunnil TC, Baczko I, Hunter BE, Thorn S, Allard MF, Roberts R, Gollob MH, et al. Distinct early signaling events resulting from the expression of the PRKAG2 R302Q mutant of AMPK contribute to increased myocardial glycogen. Circ Cardiovasc Genet. 2009;2(5):457–66.
Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol. 2006;574(Pt 1):95–112.
Xia X, Yan J, Shen Y, Tang K, Yin J, Zhang Y, Yang D, Liang H, Ye J, Weng J. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS One. 2011;6(2):e16556.
Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008;294(1):E148–56.
Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57(5):1414–8.
Hong Y, Hui SC, Chan TY, Hou JY. Effect of berberine on regression of pressure-overload induced cardiac hypertrophy in rats. Am J Chin Med. 2002;30(4):589–99.
Yang J, Zhou ZY, Xu JG. Protective effect of berberine on cardiac hypertrophy induced by L-thyroxine in rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004;35(2):223–5.
Capano M, Crompton M. Bax translocates to mitochondria of heart cells during simulated ischaemia: involvement of AMP-activated and p38 mitogen-activated protein kinases. Biochem J. 2006;395(1):57–64.
Lopaschuk GD. AMP-activated protein kinase control of energy metabolism in the ischemic heart. Int J Obes (Lond). 2008;32 Suppl 4:S29–35.
Paiva MA, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, Mocanu MM. Transitory activation of AMPK at reperfusion protects the ischaemic-reperfused rat myocardium against infarction. Cardiovasc Drugs Ther. 2010;24(1):25–32.
Paiva MA, Rutter-Locher Z, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, Mocanu MM. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300(6):H2123–34.
Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, et al. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009;119(19):2568–77.
Gonzalez-Salazar A, Molina-Jijon E, Correa F, Zarco-Marquez G, Calderon-Oliver M, Tapia E, Zazueta C, Pedraza-Chaverri J. Curcumin protects from cardiac reperfusion damage by attenuation of oxidant stress and mitochondrial dysfunction. Cardiovasc Toxicol. 2011;11(4):357–64.
Thuc LC, Teshima Y, Takahashi N, Nishio S, Fukui A, Kume O, Ezaki K, Miyazaki H, Yufu K, Hara M, et al. Cardioprotective effects of pravastatin against lethal ventricular arrhythmias induced by reperfusion in the rat heart. Circ J. 2011;75(7):1601–8.
Hermann R, Marina Prendes MG, Torresin ME, Velez D, Savino EA, Varela A. Effects of the AMP-activated protein kinase inhibitor compound C on the postconditioned rat heart. J Physiol Sci. 2012;62(4):333–41.
Mochizuki T, Yu S, Katoh T, Aoki K, Sato S. Cardioprotective effect of therapeutic hypothermia at 34 degrees C against ischaemia/reperfusion injury mediated by PI3K and nitric oxide in a rat isolated heart model. Resuscitation. 2012;83(2):238–42.
Hong Y, Hui SS, Chan BT, Hou J. Effect of berberine on catecholamine levels in rats with experimental cardiac hypertrophy. Life Sci. 2003;72(22):2499–507.
Saddik M, Gamble J, Witters LA, Lopaschuk GD. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem. 1993;268(34):25836–45.
Hopkins TA, Dyck JR, Lopaschuk GD. AMP-activated protein kinase regulation of fatty acid oxidation in the ischaemic heart. Biochem Soc Trans. 2003;31(Pt 1):207–12.
Dennis SC, Gevers W, Opie LH. Protons in ischemia: where do they come from; where do they go to? J Mol Cell Cardiol. 1991;23(9):1077–86.
Liu B, Clanachan AS, Schulz R, Lopaschuk GD. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res. 1996;79:940–8.
Russell 3rd R. The Role of AMP-activated protein kinase in fuel selection by the stressed heart. Curr Hypertens Rep. 2003;5(6):459–65.
Russell 3rd RR, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114(4):495–503.
Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57(3):696–705.
Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104(3):403–11.
Folmes CD, Wagg CS, Shen M, Clanachan AS, Tian R, Lopaschuk GD. Suppression of 5′-AMP-activated protein kinase activity does not impair recovery of contractile function during reperfusion of ischemic hearts. Am J Physiol Heart Circ Physiol. 2009;297(1):H313–21.
Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270(29):17513–20.
Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J. Differential roles of GSK-3beta during myocardial ischemia and ischemia/reperfusion. Circ Res. 2011;109(5):502–11.
Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, Vanoverschelde JL, Ashworth A, Jovanovic A, Alessi DR, et al. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1. Am J Physiol Endocrinol Metab. 2006;290(5):E780–8.
Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA, Edelman AM, Fremeau RT, Means AR. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J Biol Chem. 1998;273(48):31880–9.
Hardie DG, Carling D. The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur J Biochem. 1997;246(2):259–73.
Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472(7342):230–3.
Hwang JT, Kwon DY, Park OJ, Kim MS. Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells. Genes Nutr. 2008;2(4):323–6.
Wiczer BM, Lobo S, Machen GL, Graves LM, Bernlohr DA. FATP1 mediates fatty acid-induced activation of AMPK in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2009;387(2):234–8.
Cicero AF, Rovati LC, Setnikar I. Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol-lowering agents. A single-blind clinical investigation. Arzneimittelforschung. 2007;57(1):26–30.
Acknowledgments
This work was supported by Jilin Science & Technology Development Plan (20070728-2, 20090441), Jilin Province Administration of Traditional Chinese medicine science and technology projects No.2010-093, Opening Project of State Key Laboratory of Supramolecular Structure and Materials of Jilin University under Grant No. SKLSSM200912, and the Heart and Stroke Foundation of Manitoba. G.M.H. is a Canada Research Chair in Molecular Cardiolipin Metabolism.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wenguang Chang and Ming Zhang contributed equally to this and was considered co-first author.
Rights and permissions
About this article
Cite this article
Chang, W., Zhang, M., Li, J. et al. Berberine Attenuates Ischemia-Reperfusion Injury Via Regulation of Adenosine-5′-monophosphate Kinase Activity in Both Non-ischemic and Ischemic Areas of the Rat Heart. Cardiovasc Drugs Ther 26, 467–478 (2012). https://doi.org/10.1007/s10557-012-6422-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-012-6422-0