Abstract
Background
Considerable interest has focused on the development of therapies that target the functionality of high-density lipoproteins (HDL). Upregulation of endogenous synthesis of the major protein on HDL particles, apolipoprotein A-I (apoA-I), represents a novel approach to generation of new HDL particles. The Study of Quantitative Serial Trends in Lipids with Apolipoprotein A-I Stimulation (SUSTAIN, NCT01423188) study aims to evaluate the lipid efficacy, safety and tolerability of an apoA-I inducer (RVX-208). The ApoA-I Synthesis Stimulation and Intravascular Ultrasound for Coronary Atheroma Regression Evaluation (ASSURE, NCT01067820) study aims to evaluate the effect of RVX-208 on plaque burden.
Methods
In SUSTAIN, 172 patients with low levels of HDL-C will be randomized to receive RVX-208 100 mg bid or placebo for 24 weeks. The primary efficacy parameter will be the percentage change in HDL-C levels. In ASSURE, 310 patients with angiographic coronary artery disease and low HDL-C levels will be randomized to receive RVX-208 100 mg bid or placebo for 26 weeks. The primary efficacy parameter will be the nominal change in percent atheroma volume (PAV), determined by analysis of intravascular ultrasound (IVUS) images of matched coronary artery segments acquired at baseline and at 26-week follow-up. The effect of RVX-208 on other lipid and inflammatory markers, safety and tolerability will also be assessed in both studies.
Conclusion
ApoA-I induction represents a potential novel strategy to reduce cardiovascular risk, by generating nascent HDL particles. These studies will provide early evaluation of the effects of RVX-208 on lipids and atherosclerotic plaque.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Randomized controlled trials have demonstrated that targeting low-density lipoprotein cholesterol (LDL-C) [1] and blood pressure [2] has a beneficial effect on cardiovascular event rates. While these studies have promoted increasing utilization of medical therapies, there remains a substantial residual risk of adverse cardiovascular outcomes. Accordingly, there is an ongoing need to develop additional therapeutic strategies to complement existing medical therapies.
Potential importance of HDL
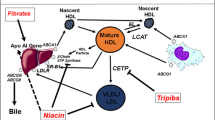
High-density lipoproteins have received considerable interest as a potential target on the basis of numerous lines of evidence that they are cardioprotective. Population studies consistently demonstrate an inverse relationship between HDL-C levels and prospective risk of cardiovascular events [3]. This relationship is observed at all levels of atherogenic lipid parameters [4]. Animal studies demonstrate that interventions promoting HDL have a favorable effect on the burden and composition of experimental atherosclerotic lesions [5–11]. These benefits are likely to result from the pivotal role played by HDL particles in the promotion of reverse cholesterol transport [12]. More recent observations that HDL are anti-inflammatory, anti-oxidant, anti-thrombotic and promote the bioavailability of nitric oxide provide additional mechanisms that are likely to exert a favorable influence on the artery wall [13].
Therapeutic strategies for HDL
The current interest in strategies that target HDL has focused primarily on their ability to raise HDL-C levels. Lifestyle measures with diet and exercise are associated with relatively modest increases in HDL-C by 5–10%, with the greatest increases observed in those who lose weight [14]. While used primarily to lower LDL-C, statins also raise HDL-C by 3–15%, with evidence that this also independently associates with their ability to slow plaque progression [15] and reduce cardiovascular event rates [16, 17]. Fibrates elevate HDL-C by 5–20% and have been demonstrated to have variable effects on cardiovascular outcomes in clinical trials [18]. A meta-analysis of all clinical trials of fibrate therapy demonstrated their greatest clinical benefit in patients with hypertriglyceridemia or low HDL-C levels at baseline [18]. Niacin is currently the most effective HDL-C raising agent in clinical practice, by up to 30%. While early formulations had a favorable effect on outcomes [19] and progression of atherosclerotic disease [20–22], difficulties with tolerance due to flushing and recent reports of no benefit with extended release formulations [23] has dampened enthusiasm for this approach. An ongoing study is currently evaluating the potential efficacy of niacin in combination with blockade of epidermal prostanoid receptors, a major pathway implicated in flushing [24].
A number of additional therapeutic strategies are currently undergoing evaluation. Early studies with various formulations of infusions of delipidated HDL demonstrated rapid regression using serial intravascular ultrasound [25–27]. The effects of these approaches on clinical events have not been evaluated. Nuclear hormone activation of factors carried on HDL particles or involved in the regulation of reverse cholesterol transport (peroxisome proliferator activated receptor [PPAR], liver X receptor [LXR] agonists) continue to be evaluated in clinical trials, despite disappointing results with earlier compounds [28]. Inhibition of cholesteryl ester transfer protein (CETP) has received considerable interest, as result of their ability to raise HDL-C to a much greater degree than currently available therapies. The first CETP inhibitor to reach an advanced stage of clinical development, torcetrapib, did not slow disease progression [29–31] and was associated with an excess rate of mortality and cardiovascular events [32]. The finding of no adverse effect on HDL functionality [33], plaque regression at very high HDL-C levels [34] and off-target toxicity with torcetrapib [35] has renewed interest to develop other CETP inhibitors, which are currently undergoing evaluation in clinical trials [36–38].
ApoA-I induction and RVX-208
An alternative strategy to promote HDL involves upregulation of endogenous synthesis of apolipoprotein A-I (apoA-I), the major protein carried on HDL particles. While the concept of turning on the system, via generation of nascent HDL particles, is attractive, intensive searches to develop effective apoA-I induction therapy has been disappointing. Recently, a small molecule compound (RVX-208) was discovered to selectively upregulate apoA-I synthesis in a liver cell system. This process involves derepression of the apoA-I gene and occurs independently of the PPAR and LXR pathways [39]. Early preclinical studies with RVX-208 demonstrated increases in levels of apoA-I and HDL-C, in association with a rise in lipid-deplete forms of HDL (pre-β HDL) and an increase in serum cholesterol efflux capacity in cell based systems [39].
The ApoA-I Synthesis Stimulation Evaluation in Patients Requiring Treatment for Coronary Artery Disease (ASSERT) study evaluated the impact of increasing doses of RVX-208 (50–150 mg bid) and placebo on lipid biomarkers, safety and tolerability in 299 statin-treated patients with established stable coronary artery disease [40]. A modest increase in apoA-I levels by up to 5.6% was observed throughout the dosing range. Increases in HDL-C by up to 8.3% and large HDL particles by up to 21.1% were observed with administration of RVX-208. In general, RVX-208 was well tolerated. A dose-dependent increase in hepatic transaminase levels, peaking at 8 weeks, with no associated elevation in bilirubin, was observed. Transaminase elevations greater than three times the upper limit of normal were observed in 17 patients treated with RVX-208. While the mechanism underlying liver enzyme elevation is unknown, it was observed more frequently in patients treated with simvastatin, high-dose statin therapy and those with liver enzyme elevations at baseline. Accordingly, the next series of phase 2 studies of RVX-208 will be performed in patients treated with either atorvastatin or rosuvastatin, excluding their highest doses, and in patients without pre-existing evidence of liver enzyme elevations.
SUSTAIN
The Study of Quantitative Serial Trends in Lipids with Apolipoprotein A-I Stimulation (SUSTAIN) will provide further characterization of the lipid efficacy, safety and tolerability of RVX-208. The primary objective of SUSTAIN is to determine the change in HDL-C levels with RVX-208 100 mg bid compared with placebo, administered for 24 weeks, in statin-treated patients with low HDL-C levels. The primary efficacy parameter is the percentage change in HDL-C. Secondary efficacy parameters include (a) within treatment group percentage changes in HDL-C, (b) percentage change in apoA-I with RVX-208 and (c) percentage changes in LDL-C, non-HDL-C, apoB, triglycerides, HDL subclasses and CRP and (d) a safety evaluation, including analysis of adverse events and laboratory data.
Study design
172 subjects aged ≥18 years taking a statin for at least 30 days with a low HDL-C level, defined as ≤45 mg/dL (1.17 mmol/L) in females and ≤40 mg/dL (1.04 mmol/L) in males in the preceding 60 days, and meeting the entry criteria (Table 1) will be randomized in the trial. Subjects will be excluded if they are treated with atorvastatin at a dose >40 mg or rosuvastatin >20 mg, have been treated with niacin/nicotinic acid at a dose of >250 mg or any dose of fibrates in the last 90 days, triglycerides >400 mg/dL or have evidence of elevation of any liver enzyme or bilirubin. After providing informed consent, patients will be randomized to treatment with RVX-208 100 mg bid or placebo for 24 weeks, in addition to background medical therapy, which will include atorvastatin (10, 20 or 40 mg) or rosuvastatin (5, 10 or 20 mg) at the discretion of the investigator. Subjects will be seen at weeks 4, 6, 8, 10, 12, 15, 18, 21 and 24 for monitoring of adverse events, study drug compliance, concomitant medication use, dietary counselling and collection of blood samples for biochemical and safety analysis.
Sample size determination and statistical analysis
Based on the assumption of a standard deviation for percentage change in HDL-C of 15.0, it was determined that a sample size of 156 subjects would be required to provide 80% power, at a 5% significance level, to demonstrate a difference between the treatment groups of 6% in terms of change in HDL-C. Subjects will be analyzed with a modified intention-to-treat approach, defined as all randomized subjects who received at least one dose of study drug and had evaluable IVUS measurements obtained at baseline and at follow-up. Anticipating that 10% of patients would discontinue from the study, a total of 172 subjects will be enrolled in the study.
ASSURE
Intravascular ultrasound (IVUS) generates high-resolution images of the full thickness of the artery wall and precisely quantifies plaque burden within the coronary vasculature. Serial IVUS studies have demonstrated beneficial effects of lowering of LDL-C [41–43] and blood pressure [44] and HDL infusions [25–27] on plaque volume. The ApoA-I Synthesis Stimulation and Intravascular Ultrasound for Coronary Atheroma Regression Evaluation (ASSURE) study will evaluate the impact of RVX-208 on plaque burden. The primary objective of ASSURE is to compare the effects of RVX-208 100 mg bid with those of placebo on the progression of atherosclerosis in a matched segment of coronary artery following 26 weeks of treatment in patients with coronary disease and low HDL-C levels. The primary efficacy parameter is the change in percent atheroma volume (PAV) measured by IVUS imaging, performed at baseline and at the end of the 26-week treatment period. Secondary end points include the change in total atheroma volume (TAV), plaque volume in the most-diseased 10-mm segment, on-therapy lipid and inflammatory biomarkers and a safety evaluation, which will include analysis of adverse events and laboratory data. An exploratory objective of ASSURE will be to characterize changes in plaque composition, pharmacokinetic parameters and correlations between changes in measures of atheroma burden and lipid biomarkers.
Study design
310 subjects aged ≥18 years with at least one >20% lumen stenosis in a native epicardial coronary artery, on visual estimation of a clinically indicated coronary angiogram, and meeting the entry criteria (Table 2) will be randomized in the trial. After providing informed consent, patients undergoing a clinically indicated coronary angiogram entered a screening period (duration up to 4 weeks). Patients meeting the inclusion criteria will be randomized to treatment with RVX-208 100 mg bid or placebo for 26 weeks. All patients will be treated with standard medical therapy, including either atorvastatin (10, 20 or 40 mg) or rosuvastatin (5, 10 or 20 mg) at the discretion of the investigator. Use of any dose of fibrates or niacin at a dose of ≥250 mg is excluded for 90 days prior to randomization and during the study.
Patients are seen every 2 weeks for the first 8 weeks and then every 3 weeks for the remainder of the study for monitoring of adverse events, study drug compliance, concomitant medication use, dietary counselling and collection of blood and samples for biochemical and safety analysis. After 26 weeks of treatment patients return for a repeat IVUS examination in the same coronary artery imaged at baseline and complete the study. Patients who require a clinically indicated coronary angiogram after 17 weeks of treatment, are to undergo early repeat IVUS imaging and complete the study at that time. If percutaneous coronary intervention of the target artery is required then IVUS imaging should be performed first, where clinically appropriate.
Intravascular ultrasound imaging
Acquisition and analysis of IVUS imaging will be performed in a similar manner to that employed in previous studies of atherosclerosis progression [25, 29, 41, 42, 44–47]. Investigators are instructed to perform IVUS imaging in the longest and least angulated coronary artery that contains no lumen stenosis greater than 50% throughout a target segment of at least 40-mm in length, has not undergone revascularization, and was not the culprit vessel responsible for a previous myocardial infarction. The vessel selected for imaging must also be considered not likely to require revascularization during the course of the study. Following anticoagulation and administration of intracoronary nitroglycerin (100–300 μg), the imaging catheter is advanced as distally as possible within the vessel. Patients will be imaged with either a 40 MHz Atlantis SR Pro (Boston Scientific Scimed, Inc., Maple Grove, MN) or a 45 MHz Revolution (Volcano Corporation, San Diego, CA) catheter. Continuous images will be acquired while the catheter was withdrawn back to the aorta by a motor drive at a constant speed of 0.5 mm per second. Images will be sent to the core laboratory at the Cleveland Clinic for analysis, by technicians who are blinded to the treatment status of the subjects.
An anatomically matched segment of artery will be selected by the core laboratory to undergo analysis, on the basis of location of proximal and distal side branches. The leading edges of the lumen and the external elastic membrane (EEM) will be defined by manual planimetry in cross-sectional images spaced 1-mm apart. Plaque area is determined as the area between the leading edges in each individual image. The volumetric extent of atherosclerotic plaque in each segment will be calculated by two approaches. Percent atheroma volume (PAV) is the proportion of the EEM volume occupied by atherosclerotic plaque.
Total atheroma volume (TAV) is the summation of plaque areas in each measured image, normalized by the median number of images analyzed in the entire cohort to account for heterogeneity in segment length between subjects, and permits each subject to be equally represented in the statistical analysis.
Changes in the 10-mm segment containing the greatest plaque burden at baseline and percentage of patients undergoing regression, defined as any reduction in PAV from baseline, will also be determined.
Sample size determination and statistical analysis
Based on the assumption of a standard deviation for mean nominal change in PAV of 2.7, it was determined that a sample size of 186 subjects would be required to provide 85% power, at a 5% significance level, to demonstrate a reduction in PAV of 0.6% in RVX-208 treated subjects compared with baseline. Given the 3:1 randomization rate, an additional 62 subjects will be enrolled to the placebo arm. Subjects will be analyzed with a modified intention-to-treat approach, defined as all randomized subjects who received at least one dose of study drug and had evaluable IVUS measurements obtained at baseline and at follow-up. Anticipating that 20% of patients would discontinue from the study or have non-evaluable IVUS imaging at follow-up, approximately 310 patients were required to achieve a total of 248 patients completing the study.
Conclusion
There continues to be interest in the development of agents that upregulate endogenous expression of apoA-I, by virtue of their potential to generate nascent HDL particles that can carry out their protective functional activities. The SUSTAIN and ASSURE studies will provide further characterization of the effects of RVX-208 in statin-treated patients. Further elucidation of the effects of RVX-208 on lipid parameters and atherosclerotic plaque will be required prior to proceeding to a large clinical outcomes trial.
References
Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81.
Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–35.
Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–14.
Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–10.
Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–41.
Badimon JJ, Badimon L, Galvez A, Dische R, Fuster V. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab Invest. 1989;60:455–61.
Nicholls SJ, Cutri B, Worthley SG, Kee P, Rye KA, Bao S, Barter PJ. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 2005;25:2416–21.
Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Nat Acad Sci (USA). 1994;91:9607–11.
Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–7.
Rong JX, Li J, Reis ED, Choudhury RP, Dansky HM, Elmalem VI, Fallon JT, Breslow JL, Fisher EA. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–52.
Shah PK, Yano J, Reyes O, Chyu K-Y, Kaul S, Bisgaier CL, Drake S, Cercek B. High-dose recombinant apolipoprotein A-IMilano mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein E-deficient mice. Circulation. 2001;103:3047–50.
Brewer Jr HB. HDL metabolism and the role of HDL in the treatment of high-risk patients with cardiovascular disease. Curr Cardiol Rep. 2007;9:486–92.
Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–72.
Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–92.
Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SL, Kapadia SR, Nissen SE. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007;297:499–508.
Cui Y, Watson DJ, Girman CJ, Shapiro DR, Gotto AM, Hiserote P, Clearfield MB. Effects of increasing high-density lipoprotein cholesterol and decreasing low-density lipoprotein cholesterol on the incidence of first acute coronary events (from the Air Force/Texas Coronary Atherosclerosis Prevention Study). Am J Cardiol. 2009;104:829–34.
Athyros VG, Mikhailidis DP, Papageorgiou AA, Symeonidis AN, Mercouris BR, Pehlivanidis A, Bouloukos VI, Elisaf M. Effect of atorvastatin on high density lipoprotein cholesterol and its relationship with coronary events: a subgroup analysis of the GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) Study. Curr Med Res Opin. 2004;20:627–37.
Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Grobbee DE, Cass A, Chalmers J, Perkovic V. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet;375:1875–1884.
Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–55.
Brown BG, Zhao X-Q, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–92.
Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial biology for the investigation of the treatment effects of reducing cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–7.
Taylor AJ, Villines TC, Stanek EJ, Devine PJ, Griffen L, Miller M, Weissman NJ, Turco M. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361:2113–22.
Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011.
Brown BG, Zhao XQ. Nicotinic acid, alone and in combinations, for reduction of cardiovascular risk. Am J Cardiol. 2008;101:58B–62B.
Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–300.
Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–82.
Waksman R, Torguson R, Kent KM, Pichard AD, Suddath WO, Satler LF, Martin BD, Perlman TJ, Maltais JA, Weissman NJ, Fitzgerald PJ, Brewer Jr HB. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol. 2010;55:2727–35.
Nicholls SJ. HDL: still a target for new therapies? Curr Opin Investig Drugs. 2008;9:950–6.
Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, Ruzyllo W, Bachinsky WB, Lasala GP, Tuzcu EM. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–16.
Bots ML, Visseren FL, Evans GW, Riley WA, Revkin JH, Tegeler CH, Shear CL, Duggan WT, Vicari RM, Grobbee DE, Kastelein JJ. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 2007;370:153–60.
Kastelein JJ, van Leuven SI, Burgess L, Evans GW, Kuivenhoven JA, Barter PJ, Revkin JH, Grobbee DE, Riley WA, Shear CL, Duggan WT, Bots ML. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356:1620–30.
Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22.
Tall AR, Yvan-Charvet L, Wang N. The failure of torcetrapib: was it the molecule or the mechanism? Arterioscler Thromb Vasc Biol. 2007;27:257–60.
Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation. 2008;118:2506–14.
Vergeer M, Stroes ES. The pharmacology and off-target effects of some cholesterol ester transfer protein inhibitors. Am J Cardiol. 2009;104:32E–8E.
Schwartz GG, Olsson AG, Ballantyne CM, Barter PJ, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Shah PK, Tardif JC, Chaitman BR, Duttlinger-Maddux R, Mathieson J. Rationale and design of the dal-OUTCOMES trial: efficacy and safety of dalcetrapib in patients with recent acute coronary syndrome. Am Heart J. 2009;158:896–901 e893.
Nicholls SJ, Brewer HB, Kastelein JJ, Krueger KA, Wang MD, Shao M, Hu B, McErlean E, Nissen SE. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA. 2011;306:2099–109.
Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, Gotto AM, Stepanavage M, Liu SX, Gibbons P, Ashraf TB, Zafarino J, Mitchel Y, Barter P. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363:2406–15.
Bailey D, Jahagirdar R, Gordon A, Hafiane A, Campbell S, Chatur S, Wagner GS, Hansen HC, Chiacchia FS, Johansson J, Krimbou L, Wong NC, Genest J. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol. 2010;55:2580–9.
Nicholls SJ, Gordon A, Johansson J, Wolski K, Ballantyne CM, Kastelein JJ, Taylor A, Borgman M, Nissen SE. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J Am Coll Cardiol. 2011;57:1111–9.
Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–65.
Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, Grines CL, DeMaria AN. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–80.
Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, Raichlen JS, Uno K, Borgman M, Wolski K, Nissen SE. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–87.
Nissen SE, Tuzcu EM, Libby P, Thompson PD, Ghali M, Garza D, Berman L, Shi H, Buebendorf E, Topol EJ. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA. 2004;292:2217–25.
Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, De Larochelliere R, Staniloae CS, Mavromatis K, Saw J, Hu B, Lincoff AM, Tuzcu EM. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–73.
Nissen SE, Nicholls SJ, Wolski K, Rodes-Cabau J, Cannon CP, Deanfield JE, Despres JP, Kastelein JJ, Steinhubl SR, Kapadia S, Yasin M, Ruzyllo W, Gaudin C, Job B, Hu B, Bhatt DL, Lincoff AM, Tuzcu EM. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA. 2008;299:1547–60.
Nissen SE, Tuzcu EM, Brewer HB, Sipahi I, Nicholls SJ, Ganz P, Schoenhagen P, Waters DD, Pepine CJ, Crowe TD, Davidson MH, Deanfield JE, Wisniewski LM, Hanyok JJ, Kassalow LM. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med. 2006;354:1253–63.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nicholls, S.J., Gordon, A., Johannson, J. et al. ApoA-I Induction as a Potential Cardioprotective Strategy: Rationale for the SUSTAIN and ASSURE Studies. Cardiovasc Drugs Ther 26, 181–187 (2012). https://doi.org/10.1007/s10557-012-6373-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-012-6373-5