Abstract
Purpose
Volume overload is a common complication associated with heart failure (HF) and is recommended to be treated with loop or thiazide diuretics. However, use of diuretics can cause serum electrolyte imbalances and diuretic resistance. Tolvaptan, a selective, oral, non-peptide vasopressin V2-receptor antagonist, offers a new option for treating volume overload in HF patients. The aim of this study was to investigate the efficacy and safety of tolvaptan in Japanese HF patients with volume overload.
Methods
Fifty-one HF patients with volume overload, despite using conventional diuretics, were treated with 15 mg/day tolvaptan for 7 days. If the response was insufficient at Day 7, tolvaptan was continued for a further 7 days at either 15 mg/day or 30 mg/day. Outcomes included changes in body weight, symptoms and safety parameters.
Results
Thirty-six patients discontinued treatment within 7 days, therefore 15 patients entered the second phase of treatment. In two patients, tolvaptan was increased to 30 mg/day after 7 days. Body weight was reduced on Day 7 (−1.95 ± 1.98 kg; n = 41) and Day 14 (−2.35 ± 1.44 kg; n = 11, 15 mg/day). Symptoms of volume overload, including lower limb edema, pulmonary congestion, jugular venous distention and hepatomegaly, were improved by tolvaptan treatment for 7 or 14 days. Neither tolvaptan increased the incidence of severe or serious adverse events when administered for 7–14 days.
Conclusions
This study confirms the efficacy and safety of 15 mg/day tolvaptan for 7–14 days in Japanese HF patients with volume overload despite conventional diuretics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF), the end-stage of all heart diseases, has a very poor outcome [1]. Patients with HF have edema, pulmonary congestion, dyspnea, jugular venous distention, hepatomegaly, third heart sounds, pulmonary rales, and other symptoms associated with fluid retention. HF patients also suffer from fatigue, malaise, cold extremities, cyanosis, oliguria and other symptoms associated with decreased cardiac output. Together, these symptoms markedly reduce the quality of life [1, 2]. Therefore, there are two goals in treating HF: (1) to treat the disease to prolong life, and (2) to alleviate symptoms caused by fluid retention and/or decreased cardiac output to improve and regain quality of life.
Loop and thiazide diuretics are widely prescribed [2–5] and are recommended as standard therapy to reduce excess body fluid [1, 6]. However, these diuretics may pose problems when administered in HF patients with low serum electrolyte concentrations. In particular, if these diuretics are used in patients with serum electrolyte imbalances, they are usually administered at low doses, with limited efficacy for volume overload [3, 5].
Tolvaptan competitively blocks the vasopressin V2-receptor and inhibits vasopressin-mediated water reabsorption in the renal collecting ducts, causing an increase in free water clearance (water diuresis) [7]. Tolvaptan, when administered in combination with conventional diuretics to patients who exhibit an insufficient response to conventional diuretics or who are susceptible to serum electrolyte imbalance, has been shown to improve volume overload without adversely affecting serum electrolyte levels or renal function [8, 9].
Tolvaptan was developed for the treatment of edematous diseases because its aquaretic effect was thought to improve volume overload in patients with HF [8] and cirrhosis [10]. In a dose-ranging study, Matsuzaki et al. [11] administered tolvaptan at 15, 30 or 45 mg to Japanese HF patients with volume overload, despite ≥40 mg furosemide. Tolvaptan decreased body weight and improved lower limb edema. Furthermore, urine output increased dose-dependently [11]. No difference in body weight loss was observed among the three groups and the adverse events related to the pharmacological effects of tolvaptan increased dose-dependently, prompting their recommendations for the use of tolvaptan at a dose of 15 mg. In their study, tolvaptan was administered for 7 days; however, in clinical practice, ≥8 days of treatment may be required before the volume overload state reaches a level at which it can be controlled by conventional diuretics. For example, a dose of 30 mg/day may be required in patients who do not respond adequately to 15 mg of tolvaptan in terms of water diuresis or weight loss. Thus, the present study sought to examine the safety and efficacy of administering tolvaptan for longer than 7 consecutive days at a dose of 15 or 30 mg/day, in patients with an insufficient response to initial treatment with 15 mg/day tolvaptan for 7 days.
Methods
The present study was conducted in a multicenter, uncontrolled setting and consisted of a run-in period (3 days), a treatment period (14 days) and a follow-up observation. This study was approved by the institutional review board at each participating site (see Appendix) before initiation. Written informed consent to participate in the study was obtained from all patients before entering the study.
Patients
Patients aged 20–85 years with HF, who exhibited sustained lower limb edema, pulmonary congestion or jugular venous distention due to excess body fluid despite the use of conventional diuretics, were considered candidates for this study and were screened. Of these candidates, only those who had received diuretic therapy to treat volume overload for ≥7 days and who had received any of the following diuretic therapies for ≥3 days before this study were eligible: a loop diuretic equivalent to ≥40 mg furosemide; combination therapy with a loop diuretic and a thiazide diuretic (irrespective of dose); or combination therapy with a loop diuretic and an aldosterone antagonist (irrespective of dose).
Patients with any of the following were excluded from the study: (1) marked fluctuations in signs and symptoms of HF; (2) use of a ventricular assist device; (3) suspected hypovolemia; (4) hypertrophic cardiomyopathy (excluding the condition in the dilated phase), valvular diseases with dominant stenosis, or hepatic coma; (5) onset of acute myocardial infarction within 30 days before screening; (6) active myocarditis or amyloid cardiomyopathy; (7) poorly controlled diabetes mellitus, anuria or dysuria; (8) history of persistent ventricular tachycardia or ventricular fibrillation within 30 days before screening, a history of cerebrovascular disorder within 6 months before screening; (9) history of hypersensitivity or specific reactions to benzazepines; (10) BMI >35 kg/m2 or systolic blood pressure <90 mmHg; or (11) any of the following laboratory abnormalities: total bilirubin >3.0 mg/dL, serum creatinine >3.0 mg/dL, serum Na+ >147 mEq/L, or serum potassium (K+) >5.5 mEq/L.
Prohibited concomitant medications
Concomitant use of the following injectable drugs for the treatment of HF was prohibited between the run-in period and the end of the examination period (1 day after the last dose was administered): human atrial natriuretic peptides, phosphodiesterase III inhibitors, catecholamines, colforsin and diuretics. During this time, the doses of oral diuretics, infused solutions, drugs for the treatment of HF other than the prohibited concomitant medications, and antihypertensive drugs were not changed. In patients on salt-restricted diets, the prescribed salt intake was not changed.
Study protocol
All patients underwent screening tests after giving written consent to participate in the study, and eligible patients were enrolled. The dose of diuretics administered was fixed from Day 1 of the run-in period (i.e., Day −3). Subsequently, tolvaptan (15 mg) was administered in an open-label manner, once daily after breakfast for 7 days (treatment period I), after which drug efficacy was evaluated. At the end of treatment period I, tolvaptan therapy was ceased in patients who exhibited significant improvements or eradication of lower limb edema, jugular venous distention and pulmonary congestion. Patients who continued to show any of these symptoms at the end of treatment period I entered treatment period II. In this period, tolvaptan was increased to 30 mg once daily for 7 days in patients who met both of the following criteria: (1) ≤500-mL increase in 24-hour urine output from Day 1 of treatment period I, and (2) ≤1.0-kg difference in body weight between Days 6 and 7 during treatment period I (a decrease in body weight ≤1.0 kg at the last 2 days in period I was considered stable with 15 mg tolvaptan administration). Patients who did not meet one or either of these criteria continued to receive 15 mg of tolvaptan for a further 7 days. All patients were hospitalized from the run-in period to the follow-up observation. Fluid intake was not restricted during the study period.
Drug efficacy was evaluated after each 7-day treatment period (i.e., after 7 and 14 days of treatment), relative to the baseline values measured during the run-in period. Examination on completion or discontinuation of treatment was performed the day after the last dose was administered. Follow-up observation was performed 2–3 or 7–10 days after the last dose was given, and a post-study examination (final observation) was performed 14–20 days after the final dose. Body weight, urine output and fluid intake were measured daily during hospitalization.
Endpoints
The primary efficacy endpoint was the change in body weight from baseline. The secondary endpoints were physical symptoms associated with volume overload (lower limb edema, pulmonary congestion, jugular venous distention, and hepatomegaly). Lower limb edema was assessed at the tibial border or at the dorsum of the foot. The severity of lower limb edema was classified into the four grades: None (no indentation at all), Mild (slight indentation), Moderate (clear indentation) and Severe (apparent edema). Pulmonary congestion was assessed with chest X-ray. The degree of pulmonary congestion was classified into four categories: None (no congestion), Mild (pulmonary venous congestion), Moderate (interstitial pulmonary edema) and Severe (alveolar edema). To assess jugular venous distension, the vertical distance from the sternal angle to the internal jugular venous pulsation was measured in a sitting or half-sitting position. To assess hepatomegaly, the width of palpable liver (distance from the costal arch over the right mammary gland) was measured.
To investigate the pharmacological action of tolvaptan, serum Na+ , serum K+ concentrations, and daily urine output were measured.
To evaluate the safety of tolvaptan, physical signs and symptoms, laboratory data, vital signs, 12-lead electrocardiography, Holter monitoring, and New York Heart Association functional classification were assessed.
Statistical analysis
The present study was conducted in an uncontrolled manner. Tolvaptan was administered in an open-label manner for 7 days, after which the drug was administered at the same or an elevated dose dependent on the patient’s response. Therefore, intergroup comparisons were not performed.
Changes from baseline values to each evaluation point and the last observation were calculated (with last-observation carried forward where necessary). Results are presented as the mean ± standard deviation.
To assess lower limb edema and pulmonary congestion, symptoms were evaluated at specific times, and the rate of improvement was calculated as a percentage relative to the total number of patients (excluding those who had not exhibited such symptoms throughout the treatment period until the last observation).
Results
Patient disposition
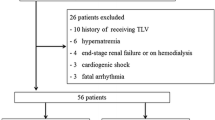
A total of 52 patients were enrolled across 27 centers between October 12, 2007 and June 19, 2008. One patient was excluded from the analysis because of a Good Clinical Practice deviation (Fig. 1). Of the remaining 51 patients, 15 proceeded to treatment period II. The other 36 patients did not proceed to treatment period II because 24 patients met the criteria to cease treatment, and 12 patients discontinued use of the drug. Tolvaptan was discontinued because of adverse events in six patients, at the physician’s discretion in one patient, elimination of congestive symptoms in three patients, an increase in the serum Na+ concentration of ≥155 mEq/L in one patient and a poor response in one patient.
Of the 15 patients who entered treatment period II, 13 continued to receive 15 mg tolvaptan, and the remaining two patients received 30 mg tolvaptan. Of the patients who continued to receive 15 mg tolvaptan, two discontinued its use because of adverse events and a poor response, respectively. Both patients who received 30 mg tolvaptan completed the 7-day treatment period.
Patient characteristics at baseline
Table 1 summarizes the baseline characteristics of all patients in each treatment group included in the analysis. Males accounted for 72.5% of all patients, and 78.4% were aged ≥65 years. More than half of the patients had both right and left ventricular failure, and at least 90% of patients also had an arrhythmia. 60.8% (31 patients) had an atrial fibrillation and 23 patients of them were treated with warfarin (data not shown). The mean cardiothoracic ratio was 61.2%, and the left-ventricular ejection fraction was 43.9%. According to the New York Heart Association (NYHA) functional classification, five patients were class I, 31 were class II, 14 were class III and one was class IV. Assessment of congestive symptoms revealed lower limb edema in 36 patients (70.6%), pulmonary congestion in 48 patients (94.1%), jugular venous distention in 22 patients (43.1%), and hepatomegaly in 15 patients (29.4%).
The previously used conventional diuretics included loop diuretics alone in 17 patients (33.3%), a combination of loop and thiazide diuretics in four patients (7.8%), a combination of loop diuretics and aldosterone antagonists in 28 patients (54.9%), and a combination of all three types of diuretics in two patients (3.9%).
Evaluation of drug efficacy in treatment period I
Figure 2 shows the changes in body weight (primary endpoint) over the 7-day treatment period. Body weight began to decrease on Day 1, and continued to decrease gradually throughout the 7 days, showing a mean decrease of 1.95 ± 1.98 kg on Day 7 as compared with baseline.
The rates of improvement and changes in congestive symptoms were analyzed in patients exhibiting these symptoms, and were compared with baseline values. At the last observation point in treatment period I, 75.0% of patients (27/36) showed improvements in lower limb edema, and 61.7% (29/47) showed improvements in pulmonary congestion. The change from baseline was −3.87 ± 3.78 cm (n = 23) for jugular venous distention, and −1.42 ± 1.26 cm (n = 15) for hepatomegaly (Table 2).
Daily urine output increased after starting tolvaptan therapy and was notably elevated on Day 1. An increase of approximately 500 mL from baseline was maintained between Days 3 and 7 (Fig. 3).
Evaluation of drug efficacy in treatment period II
Of the 15 patients who entered treatment period II, 13 continued to receive 15 mg tolvaptan, and two received 30 mg tolvaptan. Therefore, descriptive statistics were calculated for patients who continued to receive 15 mg of tolvaptan.
The time course of body weight changes in patients who continued to receive 15 mg tolvaptan is shown in Fig. 4. Body weight remained below the baseline level throughout treatment period II, with mean change from baseline of −1.55 ± 2.13 kg on Day 7, –2.35 ± 1.44 kg on Day 14, and −1.31 ± 2.95 kg at the last observation.
The mean weight change on Day 1 and Day 2 of the follow-up observation were similar between patients who completed or discontinued in treatment period I and patients who proceeded to treatment period II. Although body weight on Day7-10 of the follow-up observation in both patient groups was tended to return to baseline levels, it was still less than baseline (Fig. 4). Assessment of congestive symptoms revealed that lower limb edema improved in 44.4% of patients (4/9) on Day 7, and this increased to 75.0% (6/8) on Day 14. Lower limb edema improved at the last observation in 66.7% of patients (6/9) (Table 3). Pulmonary congestion improved in 30.8% of patients (4/13) on Day 7, and in 46.2% (6/13) at the last observation. The change in jugular venous distention was −1.74 ± 1.34 cm on Day 7 and continued to decrease throughout treatment period II, reaching −3.14 ± 1.80 cm on Day 14, and −2.50 ± 2.02 cm at the last observation. Hepatomegaly decreased by 1.50 ± 1.55 cm on Day7, by 2.00 ± 1.71 cm on Day 14, and by −2.00 ± 1.71 cm at the last observation.
Urine output remained ~500 mL/day higher than baseline after Day 7, and was ~300 mL/day higher than baseline at the last observation (Fig. 5).
Of the two patients who received an increased dose of 30 mg, one experienced marked weight loss, while the other did not show any weight changes (data not shown).
Evaluation of drug efficacy at the last observation
The mean weight change of all patients was −1.90 ± 2.19 kg at the last observation—the day after the last dose. Although the patients’ body weight began to increase after the last dose, it remained lower than the baseline values. At the last observation in all patients, the rates of improvement were 80.6% (29/36) for lower limb edema and 68.1% (32/47) for pulmonary congestion. The mean change in jugular venous distention was −4.33 ± 3.82 cm, and that for hepatomegaly was -1.72 ± 1.47 cm.
Safety
During the study period, 163 adverse events occurred in 45 patients (88.2%). The most common adverse events included thirst and increases in blood urea nitrogen (BUN), serum uric acid, serum creatinine, blood sugar and serum K + levels (Table 4). Of these adverse events, four events in four patients (7.8%) were serious, and ten events in nine patients (17.6%) led to drug withdrawal. Three events in three patients (5.9%) were severe. Three of the patients who experienced serious adverse events (cerebral artery embolism, renal failure chronic and cardiac failure) died, while the remaining patient suffered an intracardiac thrombus, but recovered. Novel adverse events, which occurred in patients who continued to receive 15 mg tolvaptan were mild except for cardiac failure (severe) and urinary tract infection (moderate). This indicates that continued administration of tolvaptan did not significantly influence tolerability.
During treatment period I, serum Na+ (Fig. 6a), BUN (Fig. 6c) and serum creatinine (Fig. 6d) concentrations increased slightly at the start of therapy, while the serum K+ concentration did not change (Fig. 6b). Interestingly, during treatment period II, none of these parameters showed a notable increase. After treatment period II, the average BUN and serum creatinine levels were increased in 2 of 13 patients.
a Time course in the concentrations of serum Na+ and (b) K+, in patients who proceeded to treatment period II and received 15 mg tolvaptan. c Time course of changes in the concentrations of blood urea nitrogen (BUN) and (d) creatinine in patients who proceeded to treatment period II and received 15 mg tolvaptan. Values are means ± standard deviation
Nineteen patients improved in NYHA functional classification at the last observation, 10 patients from II to I and 9 patients from III to II. One patient was worsened from II to III.
Discussion
The present study was conducted in an open-label manner without a placebo group. Therefore, compared with a double-blind study that uses a placebo group, this study could evaluate the efficacy and safety of tolvaptan in patients in actual clinical settings. In this study, after 15 mg of tolvaptan was administered for 7 days, body weight, congestive symptoms, and urine output were assessed. Based on the assessment at this time, tolvaptan was continued at the same or an elevated dose of 30 mg for a further 7 days, mimicking daily clinical practice. Congestive symptoms were assessed after tolvaptan had been administered for 7 or 14 days to provide clinically relevant information on the optimal duration of treatment for such symptoms.
The present study included HF patients with sustained lower limb edema, pulmonary congestion or jugular venous distention because of excessive body fluid retention, despite treatment with conventional diuretics. A comparison of the rate of patients with congestive symptoms and their severity between this study and the QUEST study [12] (a Japanese phase III double-blind, placebo-controlled study) revealed that this study included a higher percentage of patients with lower limb edema or pulmonary congestion, and that the severity of these symptoms was greater in patients enrolled in the present study. From a comparison of baseline characteristics, the severity of heart failure in patients who continued to receive 15 mg was greater than that in patients completed or discontinued in treatment period I.
Twelve patients discontinued tolvaptan during treatment period I. Of these, three discontinued its use because of sufficient treatment of congestive symptoms. Considering these three patients and the 24 patients who displayed sufficient amelioration of congestive symptoms after taking 15 mg tolvaptan for 7 days, 7 days seems to be an optimal treatment duration, as previously reported in a dose-response [11] and a phase III trial [12] of tolvaptan conducted in Japanese patients. We found that the changes in body weight and urine output were greatest on Day 1 of treatment, and these changes were maintained throughout the treatment period. The clinical and pharmacological effects of tolvaptan are consistent with those demonstrated in a previous study [11, 12].
Of the 15 patients who entered treatment period II, 13 continued to receive 15 mg tolvaptan. The body weight of these 13 patients was lower than their baseline weight, with a mean decrease in weight of 1.55 ± 2.13 kg on Day 7 and 1.31 ± 2.95 kg at the last observation. Congestive symptoms (lower limb edema, pulmonary congestion, jugular venous distention and hepatomegaly) remained improved between Day 8 and the end of treatment period II. This clinically significant finding indicates that the continued administration of tolvaptan longer than 7 days in patients with sustained congestive symptoms is highly beneficial.
In the present study, 7 days of treatment with tolvaptan resolved congestive symptoms in more than half of the patients. Furthermore, even in those patients who still exhibited congestive symptoms on Day 7, tolvaptan reduced body weight and increased urine output. Accordingly, only two patients received the higher dose of 30 mg. Therefore, we could not achieve the original objective of evaluating the efficacy and safety of 30 mg tolvaptan. Nevertheless, the present study demonstrated that 7–14 days of treatment with 15 mg tolvaptan improved congestive symptoms, reduced body weight, and increased urine output. The body weight of one of the two patients who received 30 mg tolvaptan was markedly reduced, which supports the rationale of increasing the dose of this drug to 30 mg when necessary. Clearly, further studies in a larger number of patients are warranted.
The most common adverse events in this study included thirst and increased levels of BUN, serum uric acid, serum creatinine, blood sugar and serum K+ concentrations. Severe or serious adverse events did not increase in frequency by increasing the treatment period from 7 to 14 days. Assessment of laboratory data did not indicate any significant changes in serum Na+ or K+ concentrations after 14 days of tolvaptan treatment. BUN and serum creatinine concentrations increased slightly during the first 7 days, but no further increase was observed after 8 days of treatment. Taken together, these findings suggest that safety profile of tolvaptan is acceptable for clinical use and that tolvaptan can be safely administered for up to 14 consecutive days. However, as BUN and serum creatinine were increased in two patients after treatment period II, and the causality of the adverse events to the study drug in one patient could not be ruled out, careful observation is required after tolvaptan treatment in severe patients. Dosage of warfarin was increased or introduced in 7 patients, decreased in 3 patients and increased followed by a decrease in one patient in the treatment periods. Adjusting the dosage of warfarin in patients with atrial fibrillation will be required. There were no serious or severe adverse events among patients given 30 mg tolvaptan. However, caution must be given to interpreting this finding as only two patients received this higher dose.
Limitations
Although the present study was conducted in an open-label manner with a single group, our data demonstrate the continued efficacy of tolvaptan; however, a definitive conclusion could not be drawn. To evaluate the efficacy of tolvaptan over ≥2 weeks in patients who do not respond to 7 days of tolvaptan, a comparative study is required in which the non-responsive patients are randomized to receive either tolvaptan or placebo. However, such a study design will likely impose ethical issues and may not be feasible considering the beneficial effects of tolvaptan on treating congestion and volume overload as described above. It is also necessary to investigate the clinical usefulness of long-term treatment with tolvaptan to prevent relapse of fluid retention.
Conclusions
The present study demonstrated the efficacy and safety of 15 mg/day of tolvaptan administered for 7–14 days in Japanese patients with heart failure suffered from sustained excessive body fluid despite the treatment with conventional diuretics.
References
Japanese Circulation Society. Guidelines for the diagnosis and treatment of cardiovascular disease (Joint Study Group Report 2009): guidelines for treatment of chronic heart failure (revised 2010).http://www.j-circ.or.jp/guideline/pdf/JCS2010_matsuzaki_h.pdf . Accessed 13 April 2011.
Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–804.
Raftery EB. Haemodynamic effects of diuretics in heart failure. Br Heart J. 1994;72(suppl):S44–7.
Anand I, Florea VG. Diuretics in chronic heart failure—benefits and hazards. Eur Heart J Suppl. 2001;3(Suppl G):G8–G18.
Gupta S, Neyses L. Diuretic usage in heart failure: a continuing conundrum in 2005. Eur Heart J. 2005;26:644–9.
Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235.
Yamamura Y, Nakamura S, Itoh S, et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther. 1998;287:860–7.
Gheorghiade M, Niazi I, Ouyang J, et al. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation. 2003;107:2690–6.
Costello-Boerrigter LC, Smith WB, Boerrigter G, et al. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol. 2006;290:F273–8.
Okita K, Sakaida I, Okada M, et al. A multicenter, open-label, dose-ranging study to exploratively evaluate the efficacy, safety, and dose–response of tolvaptan in patients with decompensated liver cirrhosis. J Gastroenterol. 2010;45:979–87.
Matsuzaki M, Masatsugu H, Izumi T, Asanoi H, Tsutamoto T, Tolvaptan Investigators. Effects of tolvaptan on volume overload in Japanese patients with heart failure: results of a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Cardiovasc Drugs Ther. 2011; e-pub ahead of print.
Matsuzaki M, Fukunami M, Hori M, Izumi T, Tolvaptan Investigators. Efficacy and safety of tolvaptan in heart failure patients with excessive fluid retention following treatment with other diuretics: a phase iii, randomized, double-blinded and placebo-controlled study (The QUEST study). Cardiovasc Drugs Ther. 2011; e-pub ahead of print.
Disclosures
Editorial support for this manuscript was provided by Elsevier Japan K.K. This support was funded by Otsuka Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendix
Appendix
The present study was conducted at the following institutions in Japan: National Hospital Organization Hakodate National Hospital, Hokkaido Prefectural Welfare Federation of Agricultural Cooperatives Sapporo Kosei General Hospital, National Hospital Organization Nishi Sapporo National Hospital, National Hospital Organization Tokyo Medical Center, Tokyo Medical University Hospital, Nihon University Itabashi Hospital, National Hospital Organization Takasaki General Medical Center, National Hospital Organization Saitama National Hospital, University of Yamanashi Hospital, Nagano Prefectural Federation of Agricultural Cooperatives for Health and Welfare Saku Central Hospital, Seirei Hamamatsu General Hospital, Mie University Hospital, Mie Prefectural General Medical Center, National Cerebral and Cardiovascular Center, Osaka Police Hospital, Osaka City General Hospital, Kansai Rosai Hospital, Nara Medical University Hospital, Hospital of Hyogo College of Medicine, Hyogo Prefectural Amagasaki Hospital, Tokushima University Hospital, National Hospital Organization Ureshino Medical Center, and Saiseikai Kumamoto Hospital.
Rights and permissions
About this article
Cite this article
Fukunami, M., Matsuzaki, M., Hori, M. et al. Efficacy and Safety of Tolvaptan in Heart Failure Patients with Sustained Volume Overload despite the Use of Conventional Diuretics: A Phase III Open-Label Study. Cardiovasc Drugs Ther 25 (Suppl 1), 47–56 (2011). https://doi.org/10.1007/s10557-011-6348-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-011-6348-y