Abstract
Purpose
The antianginal and anti-ischemic efficacy of the selective I f inhibitor ivabradine is established in patients with stable angina in monotherapy and in combination with other antianginals, including beta-blocker. This pilot study compared the antianginal and anti-ischemic efficacy and hemodynamic profile of ivabradine plus 5 mg bisoprolol versus those of 10 mg bisoprolol in patients with stable angina.
Patients and methods
Twenty-nine patients with stable angina and moderate left ventricular systolic dysfunction already on bisoprolol 5 mg od were randomized into 2 groups. Group 1 (n = 17) received ivabradine (5–7.5 mg bid) in addition to bisoprolol 5 mg od, while in group 2 (n = 12) bisoprolol was uptitrated first to 7.5 mg and then 10 mg od. Patients underwent a treadmill test, 6-minute walking test, and echocardiography at baseline and after 2 months.
Results
Mean resting heart rate decreased in both groups, from 76.6 ± 4.6 bpm to 59.3 ± 2.5 bpm (P < 0.001) in group 1 and from 75.9 ± 3.0 bpm to 60.5 ± 2.3 bpm (P = 0.002) in group 2. The effect on resting heart rate did not differ significantly between the two groups. However, more patients became asymptomatic in group 1 than in group 2. Addition of ivabradine also improved exercise capacity, as shown by the results of the 6-minute walking and exercise tolerance tests, whereas in group 2 neither parameter was significantly affected. Chronotropic reserve significantly improved with ivabradine, but not with bisoprolol 10 mg.
Conclusions
These results suggest that combining ivabradine with low dose bisoprolol in stable angina patients produces additional antianginal and anti-ischemic benefits and improves chronotropic reserve.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart rate (HR) reduction is an integral part of optimal pharmacological antianginal therapy. The importance of HR reduction is reinforced by the association between elevated resting HR and outcomes in patients with cardiovascular disease [1].
Ivabradine belongs to a new therapeutic class of antianginal agents that specifically inhibits the pacemaker I f current [2], resulting in selective HR reduction. Its clinical efficacy and safety in stable angina either alone or on top of beta-blockers, and in comparison with established antianginal treatments like atenolol or amlodipine, is well documented [3–6]. Although the BEAUTIFUL (morBidity-mortality EvAlUaTion of the I f inhibitor ivabradine in patients with coronary disease and left ventricULar dysfunction) and the SHIFT (Systolic Heart failure treatment with the I f inhibitor ivabradine Trial) trials [7, 8] targeted different populations, they showed that when ivabradine is added to standard evidence-based therapy, it is beneficial for prevention of cardiovascular events and improvement of prognosis when HR is above 70 bpm. Interestingly, a post hoc analysis of the BEAUTIFUL trial in patients with left ventricular (LV) dysfunction whose symptom at entry was angina showed that the addition of ivabradine to conventional therapy, including beta-blockers, further reduced HR with a benefit in terms of major cardiovascular events [9]. Of course, these data are the result of a post hoc analysis and therefore need to be adequately validated. They do however indicate that in real practice, on the one hand, target HR with beta-blockers is not always reached whilst, on the other hand, it is important to reduce HR below 70 bpm. Therefore, it is a relevant clinical question to ask whether it is better to reduce HR by increasing the dose of beta-blocker further or by adding ivabradine instead, in patients with stable angina. The aim of the present pilot study was to compare the efficacy of different therapeutic strategies, the combination of ivabradine with bisoprolol versus the uptitration of bisoprolol to target dose, in patients with stable angina and reduced LV function, such as those in the BEAUTIFUL study [9].
Patients and methods
Objectives
The present pilot study addresses two objectives. Firstly, it compares the antianginal efficacy of ivabradine (7.5 mg twice daily) when added to bisoprolol 5 mg/day (group 1) versus the uptitration of bisoprolol to a target dose of 10 mg/day (group 2) in patients with coronary artery disease, who had stable angina and LV dysfunction. Secondly, it compares the hemodynamic parameters (HR, blood pressure [BP]) at rest and during exercise of the two groups.
Study population
Twenty-nine patients with a history of MI and moderate left ventricular systolic dysfunction (LVSD) were included. Only patients with Canadian Cardiovascular Society (CCS) class I or II angina, ie, those with no or few episodes of angina on exertion, took part. Furthermore, the patients were: (1) hemodynamically stable for ≥1 month; (2) in sinus rhythm with a resting HR above 60 bpm; and (3) had a left ventricular ejection fraction (LVEF) < 45%, despite constant doses of evidence-based recommended therapy, including bisoprolol 5 mg od for at least 3 months. Patients with sick sinus node syndrome, atrial fibrillation and flutter, frequent and multifocal premature ventricular contractions, severe kidney and liver dysfunction (serum creatinine >200 μmol/l or more than threefold elevation of levels of alanine transaminase or aspartate transaminase), and decompensated diabetes were excluded.
The study was performed in accordance with the ethical principles stated in the Declaration of Helsinki, 1964, as revised in Washington, 2002. The study was approved by the institutional review board.
Study design and measurements
This was a single-blind, randomized pilot study. All patients received bisoprolol 5 mg od. In addition to bisoprolol 5 mg od, patients in group 1 received ivabradine 5 mg uptitrated to 7.5 mg after 2 weeks. In group 2, the dose of bisoprolol was first uptitrated to 7.5 mg od and then to the target dose of 10 mg od.
Patient evaluation included physical examination, office HR measurement by 12-lead electrocardiography (ECG), Doppler echocardiography, two-dimensional echocardiography using an Aloka 5000 Pro Sound ultrasonic scanner (Japan), a 6-minute walking test, and a treadmill test. LV systolic function parameters, LV end-systolic and end-diastolic volumes in relation to body surface area (end-diastolic and end-systolic indices [EDI and ESI]), and LVEF were assessed according to Simpson’s method (as suggested by the recommendations of the American Society of Echocardiography and European Association of Echocardiography) [10]. The anteroposterior dimensions of the left atrium and the thickness of the interventricular septum and posterior wall of the left ventricle were estimated using standard methods. The 6-minute walking test was used to determine the distance a patient could walk on a horizontal surface. Reasons for stopping were onset of dyspnea, angina, fatigue, or vertigo.
The treadmill test was performed according to the Bruce symptom-limited procedure in the morning and in the fasting state using Welch Allyn Cardio Perfect (USA) to conduct stress tests at the trough of drug activity (12 h after last intake of ivabradine and 24 h after last intake of bisoprolol). Twelve-lead ECG monitoring was performed throughout the procedure. HR and BP were measured at the beginning of the test and monitored throughout. The test was symptom-limited and interrupted in cases of angina, acute fatigue, prominent dyspnea, ventricular arrhythmias, ST-segment depression (more than 1 mm at a distance of 60–80 ms from the J point of the QRS complex), or decrease in systolic BP of 10 mm Hg or more. The rate of exercise, which was measured in metabolic equivalents (1 metabolic equivalent = 3.6 ml/kg/min), and the duration of exercise were assessed as well. The double product (DP) was calculated during the last stage of exercise performed by multiplying systolic BP, measured according to Korotkoff’s method, by HR, and dividing by 100.
Peak exercise chronotropic reserve was estimated using the formula: 100 × (peak HR–basal HR) / (220–age–basal HR) [11]. Short-acting nitrates were allowed if needed up to 3 h before the exercise tolerance test (ETT). Drugs with possible interactions with ivabradine such as non-dihydropyridine calcium channel blockers, class I antiarrhythmics, and strong inhibitors of cytochrome P450 3A4 were not allowed.
Statistical analysis
Nonparametric criteria were used to measure the significance of the difference between mean values: the Wilcoxon criteria for dependent variables and Mann-Whitney criteria for independent variables. Correlation analysis was performed using Pearson’s scale (for data, expressed on an interval scale) and Spearman’s rank correlation test (for data, expressed on a non-interval scale).
Results
Table 1 shows baseline patient characteristics and treatment. The patients were mostly men (89.7%) and were aged from 48 to 71 (mean 59 ± 5.4 years). All patients received treatment according to guidelines of the European Society of Cardiology [12]. This included aspirin, statins, and angiotensin-converting enzyme (ACE) inhibitors. At the end of the study, 10 patients (58.8%) were receiving 5 mg of ivabradine bid and 7 patients (41.2%) 7.5 mg bid. The mean daily dose of ivabradine was 6.02 ± 1.27 mg bid.
Antianginal and anti-ischemic efficacy
At the end of the investigation period, there was a decrease in mean weekly number of angina attacks requiring sublingual nitrate consumption (from 3.3 ± 1.1 to 1.7 ± 0.6 in group 1 and from 3.2 ± 1.0 to 2.5 ± 0.9 in group 2; P between groups after the treatment was 0.041). Thus, after treatment there were more patients with CCS classification I stable angina in group 1 (82% vs 53% at baseline) than in group 2 (67% vs 58% at baseline), P = 0.037.
The addition of ivabradine (group 1) resulted in an improvement in exercise capacity: 6-minute walking test distance increased from 388 ± 76 to 446 ± 55 m (P < 0.001) and workload increased from 5.9 ± 1.6 to 7.0 ± 1.4 metabolic equivalents (P = 0.004), whereas in group 2 neither parameter changed significantly (from 386 ± 69 to 400 ± 84 m, P = 0.216; and from 5.7 ± 1.4 to 6.2 ± 1.4 metabolic equivalents, P = 0.141) (Fig. 1, Table 2). The reasons for test termination are shown in Table 3.
Changes in hemodynamic parameters and left ventricular systolic function
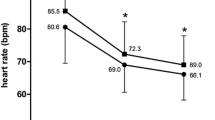
Resting HR decreased similarly in both groups during the treatment period: from 76.6 ± 4.6 to 59.3 ± 2.5 bpm in group 1 (18.9%, P < 0.001); and from 75.9 ± 2.97 to 60.5 ± 2.3 bpm in group 2 (by 18.9%, P = 0.002) (Table 4). There was a statistically significant relation between HR prior to treatment and relative decrease in HR after treatment in both groups, but the correlation was stronger in group 1 (r s = 0.760 [P < 0.01] versus r s = 0.588 [P < 0.05] in group 2).
Significant decreases in systolic BP and diastolic BP were observed in group 2: from 129.2 ± 22.6 to 120.4 ± 20.5 mm Hg (P = 0.016) for systolic BP and from 81.7 ± 9.1 to 77.1 ± 11.8 mm Hg (P = 0.026) for diastolic BP, whereas ivabradine did not significantly change systolic or diastolic pressures (Table 4).
Ivabradine allowed a larger increase in mean HR during exercise than bisoprolol: HR at peak of exercise was 132.2 ± 15.8 bpm with ivabradine (P = 0.006) versus 110.4 ± 9.0 bpm with bisoprolol (Table 2). Chronotropic reserve significantly improved with ivabradine (from 46.9 ± 2.1 to 67.1 ± 4.1%, P < 0.01), but not with bisoprolol 10 mg od (from 48.4 ± 3.5 to 48.7 ± 3.1%; P > 0.05).
There were no significant changes in EDI or ESI during treatment. The ejection fraction (EF) of patients in group 1 increased from 39.1 ± 5.5% to 42.7 ± 4.7% (P = 0.03) after 2 months, whereas no significant change was observed in group 2 (Table 4).
Safety
Ivabradine was well tolerated. In group 2, the increase in bisoprolol dosage was associated with side effects including asymptomatic arterial hypotension (BP <100/60 mm Hg), which necessitated a temporary decrease of the dosage (2 patients), and transient worsening of bronchospasm (in one patient with chronic obstructive lung disease). One patient had signs of heart failure (HF) one month after the increase in bisoprolol dosage requiring hospitalization, reduction in beta-blocker dosage, and the use of diuretic therapy. However, concomitant spontaneous unauthorized discontinuation of ACE inhibitor therapy by the patient may have contributed to triggering the decompensation of HF.
Discussion
A post hoc analysis of the BEAUTIFUL trial in 1507 patients with angina at entry showed that further reduction of HR when ivabradine is added to standard antianginal therapy is beneficial [9]. Ivabradine improved the primary outcome (composite of cardiovascular death, hospitalization for MI or HF) by 24% and hospitalization for MI alone by 42%, compared with placebo. Importantly, this benefit was observed across the entire spectrum of HR and in patients receiving background therapy with beta-blockers (90% of patients) at doses considered optimal by their physicians [9].
The recent ASSOCIATE (evaluation of the Antianginal efficacy and Safety of the aSsociation Of the I f Current Inhibitor ivAbradine with a beTa-blockEr) study demonstrated that the addition of ivabradine 7.5 mg bid to patients with chronic stable angina pectoris receiving a commonly used dosage of atenolol (50 mg) produced additional efficacy with no untoward effect on safety or tolerability [6].
A relevant and unresolved clinical question is whether it is more convenient (for HR control in angina patients) to further increase beta-blocker dosage or to add ivabradine. Our preliminary data suggest that the second option, ie, addition of ivabradine to beta-blockade, is preferable as it results in improved chronotropic reserve and exercise tolerance (both in the 6-minute walking test and the treadmill test) and in an additional antianginal effect.
Interestingly, in the present study, a similar decrease in resting HR was obtained in both groups (≤60 bpm). This excludes the influence of HR on the differences in clinical efficacy between the 2 groups and suggests that other mechanisms are involved.
Ivabradine preserved the adaptation of HR to physical exercise and significantly improved chronotropic reserve, which was not the case when the dose of bisoprolol was doubled. It has been suggested that a low increase in HR during exercise may contribute to reduced exercise tolerance [13]. The GISSI 2 (Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto miocardico 2) investigators showed that a DP increase during stress testing using a stationary bicycle or treadmill in survivors of MI has positive predictive significance [14]. This suggests that the increase of chronotropic reserve and DP during exercise is related to improved myocardial function, and may have a favorable predictive value.
The increase in HR and DP during exercise observed in group 1 also reflects an increase in myocardial oxygen consumption. However, the improvement of exercise capacity observed in group 1 can be explained by the maintenance of exercise-induced increase in coronary blood flow, as shown experimentally [15]. This in turn will match the increase in oxygen consumption. Beta-blockers are expected to oppose the physiological increase in coronary blood flow during exercise by affecting the vasomotion of coronary circulation. Beta-blockade in fact unmasked alpha-adrenergic vasoconstriction at the level of epicardial coronary arteries, and this effect may be even more prominent in the coronary microcirculation, resulting in an increase in coronary artery resistance [16, 17].
The decrease in HR achieved with ivabradine was not associated with BP changes, whereas doubling the dose of bisoprolol resulted in a significant reduction in systolic and diastolic BP (by 7.5% and 5.2%, respectively; P < 0.05), which might affect coronary artery perfusion. The absence of an effect of ivabradine on BP is related to it selectively affecting sinus node automaticity without affecting cardiac contractility or vascular tone.
In this pilot study, we deliberately enrolled patients with LV dysfunction as this was the population involved in the BEAUTIFUL trial [7, 9]. Interestingly, echocardiography after 2 months showed that that there was a small increase in EF in patients receiving ivabradine (P < 0.05), but not in those receiving a double dose of bisoprolol. The ability of ivabradine to improve systolic and diastolic function of the myocardium has been demonstrated experimentally, in clinical trials in patients with stable angina with LVSD [18–20] and recently in the Echo substudy of the BEAUTIFUL trial [21]. Our failure to observe improvement of EF with beta-blocker may be explained by the short follow-up period (2 months). It is known that in patients with LV dysfunction, improvement of EF by beta-blockade requires several months.
Obviously, this pilot study had several limitations. The design was not double-blind, the number of patients was very limited, and the population was restricted to patients with angina and LV dysfunction. The question examined is however clinically relevant, and the results are encouraging and deserve to be confirmed by further studies including a larger patient population and proper randomization.
References
Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–30.
DiFrancesco D. Funny channels in the control of cardiac rhythm and mode of action of selective blockers. Pharmacol Res. 2006;53:399–406.
Borer JS, Fox K, Jaillon P, Lerebours G. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003;107:817–23.
Ruzyllo W, Tendera M, Ford I, Fox KM. Antianginal efficacy and safety of ivabradine compared with amlodipine in patients with stable effort angina pectoris: a 3-month randomised, double-blind, multicentre, noninferiority trial. Drugs. 2007;67:393–405.
Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26:2529–36.
Tardif JC, Ponikowski P, Kahan T. Efficacy of the If current inhibitor ivabradine in patients with chronic stable angina receiving beta-blocker therapy: a 4 month, randomized, placebo-controlled trial. Eur Heart J. 2009;30:540–8.
Fox K, Ford I, Steg PG, Tendera M, Ferrari R. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–16.
Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled trial. Lancet. 2010;376:875–85.
Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Relationship between ivabradine treatment and cardiovascular outcomes in patients with stable coronary artery disease and left ventricular systolic dysfunction with limiting angina: a subgroup analysis of the randomized, controlled BEAUTIFUL trial. Eur Heart J. 2009;30:2337–45.
Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67.
Elhendy A, Mahoney DW, Khandheria BK, Burger K, Pellikka PA. Prognostic significance of impairment of heart rate response to exercise: impact of left ventricular function and myocardial ischemia. J Am Coll Cardiol. 2003;42:823–30.
Fox K, Garcia MA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341–81.
Witte KK, Cleland JG, Clark AL. Chronic heart failure, chronotropic incompetence, and the effects of beta blockade. Heart. 2006;92:481–6.
Villella M, Villella A, Barlera S, Franzosi MG, Maggioni AP. Prognostic significance of double product and inadequate double product response to maximal symptom-limited exercise stress testing after myocardial infarction in 6296 patients treated with thrombolytic agents. GISSI-2 Investigators. Grupo Italiano per lo Studio della Sopravvivenza nell-Infarto Miocardico. Am Heart J. 1999;137:443–52.
Simon L, Ghaleh B, Puybasset L, Giudicelli JF, Berdeaux A. Coronary and hemodynamic effects of S 16257, a new bradycardic agent, in resting and exercising conscious dogs. J Pharmacol Exp Ther. 1995;275:659–66.
Baumgart D, Haude M, Gorge G, et al. Augmented alpha-adrenergic constriction of atherosclerotic human coronary arteries. Circulation. 1999;99:2090–7.
Heusch G, Baumgart D, Camici P, et al. Alpha-adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation. 2000;101:689–94.
Ceconi C, Comini L, Suffredini S, et al. Heart rate reduction with ivabradine prevents the global phenotype of left ventricular remodeling. Am J Physiol Heart Circ Physiol. 2011;300:H366–73.
Manz M, Reuter M, Lauck G, Omran H, Jung W. A single intravenous dose of ivabradine, a novel I(f) inhibitor, lowers heart rate but does not depress left ventricular function in patients with left ventricular dysfunction. Cardiology. 2003;100:149–55.
Mulder P, Barbier S, Chagraoui A, et al. Long-term heart rate reduction induced by the selective I(f) current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674–9.
Ceconi C, Freedman SB, Tardif JC, et al. Effect of heart rate redution by ivabradine on left ventricular remodeling in the echocardiographic substudy of BEAUTIFUL. Int J Cardiol. 2011;146:408–14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amosova, E., Andrejev, E., Zaderey, I. et al. Efficacy of Ivabradine in Combination with Beta-Blocker Versus Uptitration of Beta-Blocker in Patients with Stable Angina. Cardiovasc Drugs Ther 25, 531–537 (2011). https://doi.org/10.1007/s10557-011-6327-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-011-6327-3