Abstract
Cardiovascular risk is closely associated with insulin resistance and type 2 diabetes. Therapy based on the actions of GLP-1 is currently seen as a novel approach to treat this disease. The aims of this study was therefore to use an animal model to determine whether (i) pre-treatment of obese, insulin resistant but pre-diabetic rats with a DPP4 inhibitor, PFK275-055, could protect the heart from ischaemia/reperfusion injury and (ii) the possible mechanisms involved in such protection. Obese, pre-diabetic rats (DIO) were treated for 4 weeks with 10 mg/kg/day of the DPP4 inhibitor PFK275-055. Ex vivo perfusion was used to subject hearts to ischaemia/reperfusion to determine infarct size, functional recovery and post-ischaemic activation of proteins associated with cardiac protection. Adult ventricular cardiomyocytes were isolated to determine insulin sensitivity. Other assessments included body weight, intra-peritoneal fat weight, insulin and GLP-1 levels as well as histological evaluation of the pancreata. Results showed that DIO animals had higher body mass and intra-peritoneal fat mass than chow-fed animals. They presented with elevated plasma insulin levels and lower GLP-1 levels. Treatment with the DPP4 inhibitor resulted in smaller infarct size development in hearts from DIO rats after ischaemia/reperfusion accompanied by activation of cardioprotective kinases. GLP-1 levels were elevated and plasma insulin levels lower after treatment. In addition, the beta-cell to alpha-cell ratio of the pancreas was improved. We conclude that treatment with PFK275-055 for 4 weeks protected the heart against ischaemia/reperfusion injury, elevated GLP-1 levels and improved metabolic control in obese, pre-diabetic rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abdominal obesity as well as insulin resistance and impaired glucose tolerance are currently recognized as part of the metabolic syndrome and therefore risk factors for the development of cardiovascular disease [1, 2]. All these risk factors are strongly linked to the development of type 2 diabetes mellitus [3]. It is recognized that there is an impairment in the secretion of the incretin hormone GLP-1, in type 2 diabetic patients [4, 5]. Treatment with GLP-1 has already been proven to effectively lower blood glucose levels, [6] to be cardioprotective in type 2 diabetic patients [7] and to possibly reduce cardiovascular risk when used as treatment of such patients [8]. GLP-1 can also directly protect the heart in animal models [9–11].
GLP-1 regulates plasma glucose levels not only by pancreatic effects [12] but also by extra-pancreatic effects [13, 14]. GLP-1 levels however, are tightly regulated by the enzyme dipeptidyl peptidase IV (DPP4), situated also on the endothelial cells lining the vasculature [15]. Because of the short half-life of GLP-1 and its protein nature, long-acting formulations of GLP-1-mimetics as well as DPP4 inhibitors are currently under scrutiny for their potential as treatment in type 2 diabetes [6, 16, 17]. As reviewed by Barnett [16], DPP4 inhibitors not only increase circulating levels of GLP-1 in animal models but may also have beneficial effects on pancreatic β-cell re-generation [18, 19]. As summarized by Mafong and Henry [20] it is postulated that both GLP-1 mimetic drugs on the one hand and DPP4 inhibitors on the other hand may mimic the cardio-protective effects of GLP-1.
We addressed this question in an animal model and asked whether (i) pre-treatment of obese, insulin resistant but pre-diabetic rats with a DPP4 inhibitor, PFK275-055, could protect the heart from ischaemia/reperfusion injury and (ii) the possible mechanisms involved in such protection.
Animals
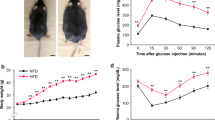
Obesity was induced in male Wistar rats (180–200 g) by feeding them a hyperphagia-inducing diet [21], previously also characterized in our laboratory [22]. Animals were randomly divided into control fed (normal rat chow) and DIO groups (normal rat chow supplemented with sucrose and condensed milk for a period of 16 weeks). They had free access to food and water and were kept on a 12 h day/night cycle in an AAALAC (Association for the assessment and accreditation of laboratory animal care international) accredited facility. After 12 weeks of diet, half of each group of animals was treated with 10 mg/kg/day of the DPP4 inhibitor PFK275-055 [23] for the next 4 weeks. The substance was weighed off for each animal individually and set in a 1 ml volume of gelatine flavoured with a small amount of jelly. This block was fed to each animal, ensuring full compliance and control of ingested dose. Control fed animals received a similar jelly block without medication.
Rats were anaesthetized by lethal intraperitoneal injection of sodium pentobarbital (160 mg/kg) for sacrifice. The study had ethical clearance from the committee for the ethical use of animals in research of the Faculty of Health Sciences, University of Stellenbosch and conformed to the Guide for the Care and Use of Laboratory Animals of the NIH (Publication No. 85-23, revised 1996).
Materials
The DPP4 inhibitor PFK275-055, a Vildagliptin analogue, was kindly supplied by Novartis. According to Villhauer et al. [23], PFK275-055 is a potent inhibitor of DPP4 activity in an in vitro assay using CaCo-2 cells. It has an IC50 of 13 ± 0.6 nM, in vitro protease selectivity and in vivo pharmacodynamic profiles that are similar to vildagliptin. Vildagliptin (10 μmol/kg) significantly improved an oral glucose tolerance test in Zucker fa/fa rats over a 90 min period and demonstrated a ∼8 fold increase in DPP4 activity over this period.
Collagenase type 2 was from Worthington, BSA (fraction V, fatty acid free) from Boehringer Mannheim and all primary antibodies from Cell Signaling. HRP-coupled secondary antibody and the ECL-detection system were from Amersham. The GLP-1(7-36) Active ELISA kit and Glucagon RIA kit were both from Linco Research, Inc., and the insulin coat-a-count RIA kit from Diagnostic Products Corporation, LA. 2-deoxy-D-[3H]glucose was bought from Perkin–Elmer. All other chemicals were of the highest grade commercially available.
Methods
Determination of plasma levels of insulin, GLP-1 and glucagon
Blood was collected at time of sacrifice of the animals. For determination of GLP-1 levels, blood samples were immediately treated with valine pyrrolidide (20 μM) as DPP4 inhibitor. Samples were allowed to clot on ice and then centrifuged for 15 min (14,000 rpm) at 4°C and stored at −20°C. Kits were used according to the manufacturer’s instructions.
Perfusion protocol of isolated hearts
-
(i)
To determine functional recovery on reperfusion after ischaemia:
After anaesthesia, hearts were quickly excised, arrested in ice cold Krebs–Henseleit solution (KR) (in mM: NaCl 119; NaHCO3 25; KCl 4.75; KH2PO4 1.2; MgSO4.7H2O 0.6; Na2SO4 0.6; CaCl2.2H2O 1.25; Glucose 11) and perfused retrogradely [24]. Hearts were fitted with an intraventricular balloon to monitor left ventricular pressure.
The myocardial temperature and left ventricular developed pressure (LVDevP) were monitored throughout the experiment as reported previously [24]. Global low-flow ischaemia was induced by reducing the coronary flow rate from 11.4 ± 0.4 mL/min to 0.2 mL/min, by means of a Gilson Minipuls 2 peristaltic pump. Hearts were allowed to stabilize for 30 min, subjected to 45 min low-flow ischaemia and reperfused for 30 min where after they were freeze-clamped and stored in liquid nitrogen for biochemical analyses. Functional parameters were continuously recorded throughout the experiment.
-
(ii)
Determination of infarct size after regional ischaemia:
Infarct size was determined after coronary artery ligation for 35 min, followed by reperfusion for 30 min. Conventional Evans Blue and TTC staining procedures were followed as described previously [24, 25] and infarct size expressed as a percentage of the area at risk.
Isolation of adult ventricular myocytes
Calcium-resistant adult ventricular myocytes in an unstimulated state were prepared as described previously [26]. After isolation, myocytes were suspended in a buffer containing in mmol/L HEPES 10, KCL 6, NaH2PO4 0.2, Na2HPO4 1, MgSO4 1.4, NaCl 128, pyruvate 2, glucose 5.5, 2% BSA (fraction V, fatty acid free) plus 1.25 mmol/L calcium, pH 7.4. The cells were left for 1–2 h under an oxygen atmosphere on a gently shaking platform to recover from the trauma of isolation. This procedure routinely rendered in excess of 80% viable cells as measured by trypan blue exclusion.
After recovery, the cells were allowed to settle into a loose pellet and the supernatant removed. They were washed twice with and suspended in a suitable volume of the abovementioned medium from which glucose and pyruvate were omitted.
Determination of 2-deoxy-D-glucose uptake by myocytes
2-deoxy-D-glucose uptake was measured essentially as described previously [26] in a final assay volume of 750 μL. Cells prepared from the four groups of animals were incubated with or without 1, 10 or 100 nM Insulin under the same conditions for 30 min. Here after glucose uptake was initiated by addition of 2-deoxy-D-[3H]glucose (1.5 μCi/mL; final 2-deoxy-D-glucose concentration 1.8 μM). Glucose uptake was stopped after 30 min by addition of phloretin (final concentration 400 μM). The cells were spun down and the pellet washed twice with HEPES buffer and dissolved in 0.5 N NaOH. The protein concentration [27] and radioactivity of each sample was determined.
Immunoblotting for analysis of the kinases
Frozen tissues were pulverized with a liquid nitrogen pre-cooled mortar and pestle and then extracted in lysis buffer containing in mM: Tris–HCl 20 (pH 7.5), EGTA 1, EDTA 1, NaCl 150, Na2VO3 1, beta-glycerophosphate 1, sodium-pyrophosphate 2.5, PMSF 0.3, Triton X-100 1% (v/v) plus 10 μg/mL leupeptin and aprotinin respectively using a Polytron PT10 homogenizer, 2 × 4 s, setting 4. Lysates were cleared from particulate matter by centrifuging for 15 min at 14,000 rpm in a microfuge. Equal amounts of cytosolic proteins (30 μg per lane) were separated on a 10% SDS poly-acrylamide gel and electro-transferred to Immobilon™-P membranes. Transfer and equal loading of proteins were determined with Ponceau Red reversible stain. The membranes were blocked for 2 h in Tris-buffered saline (TBS) containing 0.1% Tween-20 and 5% non-fat milk powder. Membranes were probed with primary antibodies directed against: total PKB/Akt (protein kinase B) protein and Ser473 phosphorylated PKB/Akt, total and phosphorylated ERK42/44 (extracellular regulated kinase), total and phosphorylated p38MAPK (mitogen activated protein kinase), total and phosphorylated JNK (jun n-terminal kinase) followed by a horse-radish peroxidase coupled anti-rabbit secondary antibody. Lysates of 3 or 4 individual hearts were always probed with all the different antibodies. Blots were stripped and reprobed with a beta-tubulin antibody to confirm equal loading. Bands were visualized using the ECL detection system and quantified by laser scanning densitometry and suitable software (Silk Scientific Inc, USA).
Histology of the pancreas
Pancreata were harvested at the time of sacrifice of the animals and fixed in 4% phosphate buffered formaldehyde until processing (within 24 h of harvesting). Fixed tissue was processed in an automatic tissue processor through ascending grades of ethanol, xylene and embedded in paraffin wax. Sections, 4–6 μm thick, were cut on a rotary microtome, mounted onto glass slides and placed in an oven for 30 min at 60°C. Each section was immunolabeled for alpha cells with a polyclonal glucagon (Dako, Carpinteria, CA) antibody for 30 min at room temperature. Thereafter, a secondary biotinylated anti-rabbit link antibody was applied and positive labelling was visualised using the ABC peroxidase diaminobenzidine tetrachloride method. This was followed by immunolabeling for beta cells with a monoclonal insulin antibody (Sigma ImmunoChemicals, St. Louis, MO) overnight at 4°C. Subsequently, a secondary biotinylated anti-mouse link antibody was applied and positive labelling was visualised using the APAAP alkaline phosphatase fuchsin method. Primary antibody was omitted in the method controls. All sections were counterstained with Mayers haematoxylin for 2 min, air dried and mounted in Entallen®.
The image analysis system comprised a Leica DC290 digital camera mounted on an Olympus BX60 light microscope which interfaced with a personal computer via Leica Qwin® Professional Software. For each pancreas the entire section was viewed with a ×10 objective and each alternate field of view captured and digitized to 768 × 1,024 pixels. The system was calibrated for the ×10 objective in the X and Y direction using a micrometric square with dimensions of 50 μm. All tissue parameters were measured using a Leica Qwin® routine. Positive staining was discriminated by colour segmentation using RGB thresholding. Firstly, the whole section area was identified and measured. Then islets were visually identified, interactively demarcated, and their total areas determined. Subsequently, alpha cell area (brown positive staining) and beta cell area (red positive staining) were identified and the areas measured. All data was exported to Microsoft Excel for analysis. Beta cell to alpha cell ratio was calculated by dividing the total beta cell area by the total alpha cell area measured. All histological evaluation of the pancreata was done by an external department blinded to the origin of the samples.
Statistical analyses
Data are presented as mean ± SEM. Comparisons between multiple groups were performed by either a one-way or a two-way ANOVA followed by the Bonferroni post-hoc test (Graph-Pad Prism 5) and between 2 groups using a Student’s t-test. A value of p < 0.05 was considered statistically significant.
Results
Biometric data
Animals on the obesity-inducing diet (DIO) had increased body weight as well as increased intraperitoneal fat weight when compared to their age-matched controls (Table 1). Blood was collected from animals in a non-fasting state. Under these conditions, plasma GLP-1 levels was reduced in the DIO animals and increased after PFK275-055 treatment. Glucagon levels were low in treated and untreated DIO animals. Insulin levels were significantly elevated in DIO animals vs controls (Table 1) while treatment with the DPP4 inhibitor lowered plasma insulin levels in both control and DIO animals (P < 0.05; 2-way ANOVA). Blood glucose levels, determined with a standard glucometer, did not differ between control and DIO animals (6.3 ± 0.32 vs 6.6 ± 0.31) nor did treatment with PFK275-055 change this (6.3 ± 0.34 vs 6.1 ± 0.31).
Histological data
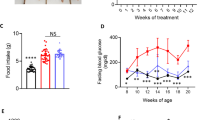
Histological evaluation of the pancreas showed 2 important results. Firstly, as indicated in Fig. 1, DIO significantly suppressed the ratio of beta cells vs alpha in the pancreatic tissue and secondly, that treatment of DIO animals with PFK275-055 partially corrected this.
Functional parameters
Before the induction of low-flow ischaemia, the function of hearts, as indicated by the aortic output, was similar (Table 2). Control animals recovered function after the ischaemic period while hearts from DIO could not recover function. PFK275-055 treatment could not improve this functional recovery of the DIO hearts. Treatment of control animals with PFK275-055 resulted in a higher coronary flow during baseline perfusion of the hearts but did not have a further effect on hearts from the DIO animals. The coronary flow of DIO hearts was already high without treatment.
Infarct size
As shown in Fig. 2, DIO resulted in significantly larger infarct development, expressed as a percentage of the area at risk, after a period of 35 min coronary artery ligation followed by 30 min of reperfusion, when compared to control fed animals (47.66 ± 4.63% vs 30.08 ± 3.73%). The area at risk was similar between all groups. Treatment with PFK275-055 did not significantly reduce infarct size in control fed animals (26.51 ± 3.33 vs 30.08 ± 3.73) but significantly reduced the infarct size in DIO animals to 29.84 ± 3.17%, indicating cardioprotection. However, a 2-way ANOVA indicated that the effects of both DIO and PFK275-055 was significant with P < 0.05, n = 6 hearts per group.
Infarct development was induced by ligation of the left anterior coronary artery for a period of 35 min followed by 30 min of reperfusion and conventional staining methods to demarcate viable tissue, infarcted tissue and the area at risk of infarction. Data is expressed as a ratio of infarct to area at risk. *P < 0.05 vs control minus inhibitor, n = 6. The effect of the inhibitor on infarct size was significant according to a 2-way ANOVA with P < 0.05
Myocyte glucose uptake
2-deoxy-D-[3H]glucose uptake by cardiomyocytes, stimulated with a concentration range of insulin were used as an indication of insulin sensitivity of the cells to document any changes in glucose handling by the cells. Cells prepared from DIO animals had a significantly impaired response to insulin notably with 10 nM and 100 nM of insulin (Fig. 3). Treatment of the DIO animals with PFK275-055 did not improve this. Basal glucose uptake in control fed animals treated with PFK275-055 was lower than untreated controls (6.3 ± 0.9 vs 13.19 ± 2.23 pmol/mgprot/30 min, P < 0.05, n = 3 individual cell preparations per group analysed in duplicate and in tandem), however stimulation with all concentrations of insulin rendered similar increases.
Insulin-stimulated 2-deoxy[3H]glucose accumulation by isolated cardiomyocytes prepared from treated vs untreated animals was measured as described in Materials and Methods. A concentration range from 1 to 100 nM insulin was used. *P < 0.05 DIO vs Control; #P < 0.05 Basal Control vs Control + PFK275-055 treatment. N = 3 individual myocyte preparations per group
Kinase profile
The profile of phosphorylation and expression of kinases associated with myocardial protection or injury was determined in the reperfusion phase after low-flow ischaemia. No differences in the expression or activation of either p38 MAPK or JNK were found (Fig. 4a & b). However, the ratio of phosphorylated to total protein of PKB/Akt was significantly lower in hearts from the DIO animals and significantly improved by PFK275-055 treatment, underscoring protection of these hearts by PFK275-055 treatment (Fig. 4c) Similarly, the ratio of phospho- to total ERK 42 and 44 were both lower in hearts from the DIO animals. Only phosphorylation of the 42 isoform of the protein was significantly higher during reperfusion after PFK275-055 treatment (Fig. 4d). Figure 5 is a compilation of the Western blot data including beta-tubulin expression to show equal loading of proteins.
Hearts subjected to global low-flow ischaemia for 35 min followed by 30 min reperfusion were freeze-clamped. This material was lysed as described in Materials and Methods and subjected to Western blotting using 10% PAGE and commercially available antibodies. In all instances, the phosphorylated and total proteins were determined from the same blot and expressed as a ratio. a represents this ratio for p38 MAPKinase, b for JNK54 and 46, c for PKB/Akt with*P < 0.05 vs control and DIO+PFK275-055 and d for ERK42 and 44 with *P < 0.05 vs control **P < 0.01 vs control and #P < 0.05 vs DIO. N = 3–4 individual hearts
Discussion
The role of GLP-1 in the aetiology of insulin resistance and type 2 diabetes and as potential treatment of this debilitating disease has been extensively researched over the past 15 years. Research has lead to the formulation of both long-acting GLP-1 analogues as well as DPP4 inhibitors for clinical use [reviews 6,16]. Direct protective effects of GLP-1 on the cardiovascular system have been demonstrated in animal models [9–11] as well as in patients [7]. It is therefore speculated that the DPP4 inhibitors will have similar effects [20] also because Holst and Deacon have shown that the therapeutic actions of the DPP4 inhibitors can be ascribed to their potential to elevate GLP-1 levels [28].
Using an animal model of pre-diabetes, we have demonstrated that indeed, treating insulin resistant, obese rats with a DPP4 inhibitor, PFK275-055, for a period of 4 weeks, not only restored GLP-1 levels back to control levels (Table 1) but was cardioprotective, as evidenced by smaller infarct development in the hearts of the DIO rats after a period of regional ischaemia followed by reperfusion. In addition, we evaluated the expression and phosphorylation of different proteins known to be associated with either myocardial injury or protection, in the reperfusion phase after low-flow ischaemia,. We found no difference in the two pro-apoptotic kinases (JNK and p38 MAPK) [25] but showed enhanced phosphorylation vs expression of the kinases involved in the RISK (reperfusion-induced salvage kinase) pathway, both known to be associated with cardioprotection [29].
DPP4 treatment resulted in enhanced coronary flow in hearts from control animals. Although not directly measured in this study, we can speculate that this can be ascribed to enhanced activation of the cGMP-nitric oxide pathway [30] via the elevated GLP-1 levels. As hearts from the DIO animals are bigger, they have a higher baseline coronary flow than hearts from control animals. The model that we used, has previously been demonstrated to suffer from endothelial dysfunction, specifically an impairment in acetylcholine-induced vasorelaxation [31]. As coronary flow was not enhanced in hearts from the DIO animals it can be concluded that treatment with the DPP4 inhibitor could not alleviate this impairment.
We furthermore evaluated the insulin sensitivity of isolated cardiomycytes after DPP4 treatment. As shown in Fig. 3, cells prepared from the hearts of DIO animals accumulated significantly less 2-deoxy-D-glucose than cells from control animals. We could not show that DPP4 treatment improved this. The improved metabolic control in the form of lower HbA1C levels reported in patients treated with either long-acting GLP-1 analogues or DPP4 inhibitors [reviews 6,16] may therefore reflect the actions of GLP-1 per se and not an insulin sensitizing effect. We have demonstrated that GLP-1 can directly elicit glucose uptake by cardiomyocytes (unpublished data). According to Vilhauer et al. [23], Vildagliptin was able to maintain DPP4 inhibition for 6–7 h after a single administration. This would strongly argue that the negative results obtained on the insulin sensitivity of isolated cardiomyocytes are not because of lack of adequate inhibition of the DPP4 enzyme. We confirmed previous reports of lower GLP-1 levels in animal models of insulin resistance [16] as well as in type 2 diabetic patients [4] and showed that DPP4 therapy will elevate these levels, as also shown by others and summarized extensively [16, 32].
Similar to what Augstein et al. (2007) has described in Zucker fatty rats [33], we found a significant decline in fasting plasma insulin levels after DPP4 treatment (Table 1). In contrast to their study, on histological examination of the pancreata, we also found an increase in the beta-cell to alpha-cell ratio of treated animals (Fig. 1), confirming the findings of Pospisilik et al. [34] using a model of streptozotocin-induced type 1 diabetic rats. An improved beta-cell mass may therefore account for the improved plasma insulin levels after treatment, indicating improved metabolic control. This result would have been strengthened by an evaluation of pancreatic transcription factors known to be associated with beta-cell neogenesis, e.g. PDX-1, as previously shown for GLP-1 [19].
In summary therefore, we showed that treatment of obese, pre-diabetic rats with a DPP4 inhibitor normalized GLP-1 levels, improved the beta-cell to alpha cell ratio of the pancreata, lowered plasma insulin levels over time and protected the heart against ischaemia/reperfusion injury, resulting in smaller infarct size development in conjunction with activation of the RISK pathway of cardioprotection.
References
Alexander CM, Landsman PB, Teusch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes and prevalence of coronary hart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–3.
Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen D, Pyorala K, et al. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164:1066–76.
Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120:S12–8.
Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52.
Pratley RE. Overview of glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors for type 2 diabetes. Medscape J Med. 2008;10:171.
Tahrani AA, Piya MK, Kennedy A, Barnett AH. Glycaemic control in type 2 diabetes: targets and new therapies. Pharmacol Ther. 2009.
Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-s in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5.
Mannucci E, Rotella CM. Future perspectives on glucagon-like peptide-1, diabetes and cardiovascular risk. Nutr Metab Cardiovasc Dis. 2008;18:639–45.
Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen Y-T, et al. The direct effects of Glucagon-like peptide-1 (GLP-1) on myocardial contractility and glucose uptake in normal and post-ischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–13.
Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–50.
Huisamen B, Genade S, Webster I, Lochner A. Signalling pathways activated by glucagon-like peptide-1 (7-36) amide in the heart and their role in protection against ischemia. Cardiovasc J Afr. 2008;19:77–83.
Montrose-Rafizadeh C, Egan JM, Roth J. Incretin hormones regulate glucose-dependent insulin secretion in RIN 1046-38 cells: mechanism of action. Enocrinology. 1994;135:589–94.
Egan JM, Montrose-Rafizadeh C, Wang Y, Bernier M, Roth J. Glucagon-like peptide-1 (7-36) amide (GLP-1) enhances insulin-stimulated glucose metabolism in 3T3-L1 adipocytes: one of several potential extrapancreatic sites of GLP-1 action. Endocrinology. 1994;135:2070–5.
Valverde I, Morales M, Clementi F, Lopez-Delgado MI, Delgado E, Perea A, et al. Glucagon-like peptide-I: a potent glycogenic hormone. FEBS Lett. 1994;349:313–6.
Deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept. 2005;128:117–24.
Barnett A. DPP4 inhibitors and their potential role in the manegement of type 2 diabetes. Int J Clin Pract. 2006;60:1454–70.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705.
Ahrén B, Foley JE. The islet inhancer cildagliptin: mechanisms of improved glucose metabolism. Int J Clin Pract Suppl. 2008;159:8–14.
Vilsbøll T. The effects of glucagon-like peptide-1 on the beta cell. Diab Obes Metab. 2009;11:11–8.
Mafong DD, Henry RR. The role of incretins in cardiovascular control. Curr Hypertens Rep. 2009;11:18–22.
Pickavance LC, Tadayyon M, Widdowson PS, Buckinham RE, Wilding JPH. Therapeutic index for rosiglitazone in dietary obese rats: separation of efficacy and haemodilution. Br J Pharmacol. 1999;128:1570–6.
Du Toit EF, Nabben M, Lochner A. A potential role for angiotensin II in obesity induced cardiac hypertrophy and ischaemic/reperfusion injury. Basic Res Cardiol. 2005;100:346–54.
Villhauer EB, Brinkman JA, Naderi GA, et al. 1-[[(3-Hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J Med Chem. 2005;46:2774–89.
Lochner A, Genade S, Moolman JA. Ischemic preconditioning: infarct size is a more reliable endpoint than functional recovery. Basic Res Cardiol. 2003;98:337–46.
Marais E, Genade S, Salie R, Huisamen B, Maritz S, Moolman JA, et al. The temporal relationship between p38 MAPK and HSP27 activation in ischaemic and pharmacological preconditioning. Basic Res Cardiol. 2005;100:35–47.
Donthi R, Huisamen B, Lochner A. The effect of vanadate and insulin on glucose transport in isolated adult rat cardiomyocytes. Cardiovasc Drugs Ther. 2000;14:463–70.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPPIV inhibitors. Diabetologia. 2005;48:612–5.
Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res. 2004;15:448–60.
Ban K, Noyan-Ashraf H, Hoefer J, Bolz S-B, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor dependent and independent pathways. Circulation. 2008;117:2340–50.
Naderali EK, Pickavance LC, Wilding JPH, Williams G. Diet-induced endothelial dysfunction in the rat is independent of the degree of increase in total body weight. Clin Sci. 2001;100:635–41.
Ahrén B. Inhibition of depeptidyl peptidase-4 (DPP-4)—a novel approach to treat type 2 diabetes. Curr Enzyme Inhib. 2005;1:65–73.
Augstein P, Berg S, Heinke P, et al. Diab Obes Metab. 2008;10:850–61.
Pospisilik JA, Martin J, Dory T, et al. Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes. 2003;52:741–50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huisamen, B., Genis, A., Marais, E. et al. Pre-treatment with a DPP-4 Inhibitor is Infarct Sparing in Hearts from Obese, Pre-diabetic Rats. Cardiovasc Drugs Ther 25, 13–20 (2011). https://doi.org/10.1007/s10557-010-6271-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-010-6271-7