Abstract
Introduction
Medium-chain fatty acids (MCFAs) have physical and metabolic properties that are distinct from those of long-chain fatty acids, which make them a readily available cellular energy source. These properties have been used advantageously in the clinics for more than 50 years for treating lipid absorption disorders, undernourished patients, and more recently subjects with long-chain fatty acid oxidation defects. In these latter subjects, nutritional interventions with MCFA-containing triglycerides have been shown to improve clinical symptoms, particularly cardiomyopathies.
Potential benefits of MCFA metabolism in cardiac diseases
There is, however, only a limited number of studies that have considered the potential use of MCFAs as metabolic therapy for cardiac diseases in general. Nevertheless, current experimental evidence does support the notion that the diseased heart is energy deficient and that alterations in myocardial energy substrate metabolism contribute to contractile dysfunction and cardiac disease development and progression. Hence, this article will review current literature on MCFAs with a specific emphasis on their metabolism and potential benefits for the heart. It will include practical considerations about the potential clinical application of MCFA therapy for the management of patients with cardiac diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Medium-chain fatty acids (MCFAs) have a chain length between 6 to 12 carbons and are saturated. In contrast, long-chain FAs (LCFAs) contain 14 or more carbons and often carry one or more double bonds. These structural differences confer to MCFAs distinct metabolic properties such as their unregulated β-oxidation for energy production, which have been used advantageously in various clinical conditions, in which cases MCFAs are traditionally administered as medium-chain triglycerides (MCTs). For clinical use, there are various commercial preparations of MCTs, containing either MCFAs alone in the form of oil or margarine, or in combination with carbohydrates, proteins, essential FAs, vitamins and minerals in the form of milk or enteral solutions [1, 2]. Furthermore, MCT-containing lipid emulsions, which are classically administered as a mix with LCFA-containing triglycerides (LCTs), are also available for parenteral infusion [3]. It is noteworthy that in contrast to LCTs, MCFA-containing triglycerides are minor constituents of the classical diet, except in populations consuming large amounts of coconut oil, which contains over 50% even-carbon MCFAs, as it occurs in Sri Lanka and other developing countries [4]. Milk is also another natural source of MCFAs; their proportion can reach ~50% of FAs in some mammals such as rabbits and rats [5]. Interestingly, results from a prospective cohort study including more than 80,000 US females showed that in contrast to LCFAs, intakes of MCFAs were not significantly associated with the risk of coronary heart disease [6].
Since their first introduction in the 1950s for the treatment of lipid absorption disorders [2], MCT-enriched diets have been proposed for clinical conditions associated with increased energy needs, such as undernourished patients or premature neonates [7, 8], and more recently for the management of obesity [9–12]. Over the past decades, MCTs have also been used for the nutritional management of patients with inherited LCFA β-oxidation disorders [13]. These patients often present in the clinics during their first years of life with fasting-induced hypoketotic hypoglycaemia, myopathy, cardiomyopathy or liver dysfunction. MCFAs, which bypass the enzyme defect and thereby can restore energy production, have been shown to improve clinical symptoms, particularly cardiac hypertrophy and dysfunction, in most patients [14–17]. More recently, MCFAs with an odd number of carbons were reported to be superior to even-carbon MCFAs in improving the cardiomyopathy of one patient with genetic LCFA oxidation deficiency [18]. Beyond being readily oxidized for energy production, odd-carbon MCFAs are anaplerotic for the citric acid cycle (CAC), a process that has been shown to be crucial for maintenance of gluconeogenesis in liver, neurotransmitter synthesis in the brain, insulin signalling in β-cells as well as optimal contraction in the heart [19].
In other cardiomyopathies, unrelated to inherited FA oxidation defects, MCFAs or MCTs have only been used in a restricted number of animal studies. Nevertheless, numerous evidence support the notion that the diseased heart is energy deficient and that alterations in myocardial energy substrate metabolism contribute to contractile dysfunction and cardiac disease development and progression [20–24]. Beyond the potential benefit of pharmacological interventions with “metabolic modulators,” which have been proposed as adjunctive therapies in these diseases [20–24], there appears to be also a potential for nutritional interventions with even- and/or odd-carbon MCFAs in the treatment of cardiac diseases given the reported benefits of MCTs in the management of cardiomyopathies associated with inherited LCFA oxidation defects. Furthermore, findings from recent studies in animals and humans have emphasized the importance of LCFAs as determinant of contractile function, structural remodelling, and mitochondrial energy metabolism in the failing, post-infarcted or hypertrophied heart [25–27].
This article will review current experimental evidence suggesting the potential benefits of MCFAs as metabolic therapy in cardiac diseases. Herein, metabolism of even- and odd-carbon MCFAs will be presented, followed by a description of their reported effects on myocardial substrate metabolism, function and remodelling. Finally, their effects beyond the heart as well as potential limitations in their clinical use will be discussed.
2 Metabolic effects of MCFAs
This section will describe MCT and MCFA metabolism at the whole-body and cellular levels. Thereafter, their impact on myocardial substrate metabolism, a potential determinant of contractile function, energy status, disease progression, and resistance to ischemia–reperfusion [20, 21, 27], will also be presented. For each section, we will discuss potential differences between even- and odd-carbon MCFAs, although unless specified, the text refers predominantly to even-carbon MCFAs.
2.1 MCFA metabolism at the cellular level
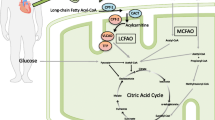
MCFAs display numerous unique metabolic properties that contrast with LCFAs and contribute to their metabolic effects [12, 28]. The β-oxidation of LCFAs is highly regulated by substrate availability, transport across plasma and mitochondrial membranes, and specific FA oxidation enzyme activities. Specifically, LCFA uptake is mediated by sarcolemmal transporters, such as FA translocase (FAT/CD36), plasmalemmal FA-binding protein and FA transport protein (Fig. 1) [29]. Subsequently, LCFAs are activated into their corresponding acyl-CoA derivatives, which are either incorporated into intracellular lipid pools or enter the mitochondria for β-oxidation. Transport of long-chain acyl-CoAs (LC-acyl-CoAs) into the mitochondria is mediated by the concerted action of three carnitine-dependent enzymes [30]. The first enzyme of this carnitine shuttle, carnitine palmitoyltransferase I (CPT-I), is the key regulatory step for LCFA transport and oxidation into mitochondria. In contrast, MCFAs do not rely on membrane transporters for their uptake into cells and mitochondria; they are directly activated in the mitochondrial matrix by medium-chain acyl-CoA (MC-acyl-CoA) synthetase prior to β-oxidation, which is not subject to regulation at the level of CPT-I [12, 28, 31]. Accordingly, MCFAs are rapidly taken up by cells, readily and preferentially β-oxidized in the mitochondria at rate that is determined by their availability, that is their blood concentrations. There appears to be little peroxisomal oxidation or incorporation into triglycerides of MCFAs [10, 32, 33].
Long-chain and medium-chain fatty acid oxidation pathways in cardiomyocytes. Compared to long-chain fatty acids, medium-chain fatty acids are directly oxidized into mitochondria. Circles represent atoms of carbon from fatty acids that enter into the citric acid cycle. Complete β-oxidation of one molecule of octanoate yields 4 molecules of acetyl-CoA, while one molecule of heptanoate yields two molecules of acetyl-CoA and one molecule of propionyl-CoA (from carbons 5, 6 and 7), which is anaplerotic in that it enters the pool of citric acid cycle intermediates at the level of succinyl-CoA. FABP, plasmalemmal fatty acid-binding protein; CD36, fatty acid translocase/CD36; CPT, carnitine palmitoyltransferase; CAT, carnitine-acylcarnitine translocase
Recently, the use of odd-carbon MCTs has been proposed as an alternative to even-carbon MCTs in the nutritional management of patients with LCFA oxidation defects [18]. The benefit of odd-carbon MCTs, specifically heptanoate-containing triglycerides or triheptanoin, is attributed to their anaplerotic properties. Anaplerosis is the re-filling of the catalytic intermediates of the CAC that carry acetyl-CoA as it is oxidized. In fact, the oxidation of one molecule of heptanoate, which have seven carbons, yields two acetyl-CoA and one propionyl-CoA molecules, while that of octanoate, a eight-carbon containing MCFA, yields four acetyl-CoA units (Fig. 1). Propionyl-CoA derived from heptanoate is further metabolised to succinyl-CoA, which is an anaplerotic reaction that feeds the pool of CAC intermediates [19]. Supporting evidence for this metabolic scheme is provided by labelling experiments conducted in perfused hearts with heptanoate differentially labelled with carbon 13 (13C) on carbon 1 or carbons 5, 6 and 7. Indeed, using [1–13C]heptanoate, there was incorporation of label into citrate and the expected fall in enrichment from citrate to succinate concurred with the notion that the carbon-1 of heptanoate enters the CAC via β-oxidation and not through anaplerosis. In contrast, the use of [5,6,7–13C3]heptanoate resulted in the formation of [13C3]succinate in a proportion that supported the notion of heptanoate metabolism to propionyl-CoA [26].
2.2 MCFA metabolism at the whole-body level
While traditionally, MCFAs are given in the form of MCTs, their route of administration, oral or parenteral, has a major impact on the formation of their derived metabolites [2, 12, 34]. When given orally, MCTs which are hydrosoluble, are readily hydrolysed to MCFAs that are absorbed into the portal vein and are rapidly taken up and oxidized by the liver. This contrasts with LCFAs that are packed into chylomicrons, which bypass the liver via the lymphatic system and are delivered to extrahepatic tissues. Accordingly, MCTs are hydrolysed faster and are better absorbed than LCTs [2, 12]. Results from animal and human studies provide also evidence that MCT diets lead to less deposition of body fat than LCT diets. Furthermore, because ingested MCFAs are directed toward oxidation rather than storage, they also influence energy expenditure [2, 12]. In the liver, MCFAs are not significantly incorporated into lipids. Rather, excess formation of acetyl-CoA through β-oxidation of MCFAs leads to ketone body synthesis [2, 12]. In fact, ketone bodies, namely acetoacetate and β-hydroxybutyrate, are the quantitatively most important MCFA-derived metabolites that become available to non-hepatic organs when MCTs are administered orally. In contrast, intravenous MCTs are hydrolysed presumably by lipoprotein lipase to MCFAs, which are rapidly taken up and oxidized by peripheral tissues, particularly heart, muscles and kidneys, but also liver [33, 35]. The oxidation rate of enterally or parenterally administered MCTs is similar, but it is faster than that of LCTs [35].
Similarly, the hepatic metabolism of odd-carbon MCFAs leads to ketone body synthesis. However, beyond the formation of the classical four carbons (C4) ketone bodies acetoacetate and β-hydroxybutyrate from acetyl-CoA metabolism, odd-carbon MCFAs lead also to the formation of the C-5 ketone body analogs β-ketopentanoate and β-hydroxypentanoate from propionyl-CoA metabolism. Recently, Kinman et al. [34] have demonstrated that heptanoate and C5-ketone bodies were the predominant circulating metabolites when triheptanoin was infused intravenously, whereas only C5-ketone bodies were detected when triheptanoin was given intraduodenally. Irrespective of the route of administration of MCFAs, their hepatic metabolism can contribute to energy production as well as favour anaplerosis and hence gluconeogenesis and thereby induce changes in the availability of other circulating substrates, such as LCFAs or glucose, for non-hepatic organs, a phenomenon that can indirectly modulate cardiac energy substrate metabolism.
2.3 Metabolic effects of MCFAs in the heart
Metabolic effects of MCFAs that have been documented in the normal and diseased hearts include modulation of substrate selection, anaplerosis, and energy status. These effects have been described in hearts from rats and pigs perfused ex vivo or in situ. It is noteworthy that a number of earlier studies that is prior to 2000, have been conducted using MCFAs, principally octanoate, as the sole source of FAs. While results from these studies provide useful metabolic informations, care should be taken in extrapolating conclusions from these studies to the in vivo situation. Indeed, as discussed below, the metabolic effects of MCFAs differ when supplied alone or together with LCFAs, a condition that is more physiologically relevant.
Modulation of substrate selection
In the heart, as in other tissues, MCFAs are more easily oxidized than LCFAs and their contribution to energy production is related to their coronary concentration [36, 37]. In rat hearts perfused with carbohydrates and FAs, replacing LCFAs by MCFAs at a concentration providing the same quantity of acetyl-CoA units (assuming complete β-oxidation) induced a two- to six-fold increase in the myocardial levels of acetyl-CoA as well in the rate of FA β-oxidation, while the rate of glycolysis and glucose oxidation was decreased [37, 38]. When supplied at concentration greater than 0.5 mM, MCFAs provided more than 80% of acetyl-CoA molecules for oxidation into the CAC [36–39]. Evidence supporting the notion that β-oxidation of MCFAs is uncontrolled when supplied as the sole FA to perfused hearts is supported by our finding of a high rate of ketone body release, representing as much as 25% of acetyl-CoA production, when hearts were perfused with carbohydrates and 0.2 mM octanoate. This ketone body release is attributed to the high activity and reversibility of cardiac 3-oxoacidtransferase [40]. However, the latter metabolic effect of MCFAs, as well as an increased myocardial malonyl-CoA levels [37, 41] are not observed when hearts are perfused with a mixture of MCFAs and LCFAs.
In hearts perfused with 1–1.2 mM LCFA (oleate or palmitate), decreasing LCFA concentration to 0.4–0.6 mM combined with addition of 0.6–1.2 mM MCFA (octanoate) led to the following metabolic changes. First, there was an increased contribution of total exogenous FAs to energy production, which was due to MCFAs since that of LCFAs was decreased [42, 43]. Second, the contribution of carbohydrates, namely glucose, pyruvate and/or lactate, to energy production remained unchanged or was slightly increased, providing about 10% of acetyl-CoA production [42, 43]. Third, glycolytic flux was unaffected, but the cytosolic redox state, as reflected by the lactate-to-pyruvate production rate, was less reduced than with LCFAs alone [42, 43]. Finally, the myocardial levels of endogenous energy stores, glycogen and triglycerides, were also unchanged [42].
Similar metabolic effects of MCFAs have been reported in animal models of left ventricular hypertrophy, which display enhanced glycolysis and decreased FA oxidation. First, we conducted a study in hearts from 15-week-old spontaneously hypertensive rats (SHR) [44], which also show hypertension, insulin resistance and dyslipidemia, thereby mimicking some of the characteristics of the metabolic syndrome [45]. These rats also exhibit a genetic defect in FAT/CD36, concurring with the reported reduced contribution of exogenous LCFAs to β-oxidation in the heart [44–47]. In our study, SHR hearts were perfused in a working mode with physiological concentrations of substrates, including 0.4 mM oleate, and hormones and subjected to an adrenergic stimulation to increase energy demand (Fig. 2). Addition of 0.2 mM octanoate increased exogenous FA contribution to energy metabolism, principally due to MCFA contribution (10.5 ± 0.6%), with an equivalent decrease in endogenous non-carbohydrate substrate contribution, probably endogenous LCTs [44]. In contrast, glycolysis and glucose oxidation were unaffected. When octanoate was replaced by 0.2 mM heptanoate, there was a two-fold lower contribution of this odd-carbon MCFA to acetyl-CoA formation for energy production (4.9 ± 0.8%; n = 4; Fig. 2, unpublished data), concurring with the notion that oxidation of one molecule of heptanoate yields two times less acetyl-CoA molecules compared to octanoate (Fig. 1). Furthermore, concurring with Okere et al., we detected the formation of M + 3 isotopomers of succinate (MPE M + 3 = 1.1 ± 0.1%; n = 4) when hearts were perfused with [5,6,7–13C3]- but not [1–13C]heptanoate, in agreement with the metabolism of the last three carbons of heptanoate to succinyl-CoA.
Relative substrate contribution to acetyl-CoA for citrate synthesis in isolated working SHR hearts perfused with different mixtures of fatty acids. SHR hearts were perfused in a working mode with either 0.49 mM oleate (C18:1), 0.4 mM oleate + 0.2 mM octanoate (+C8), or 0.4 mM oleate + 0.2 mM heptanoate (+C7) and with physiological concentrations of other substrates and hormones, and subjected to an adrenergic stimulation. Results depicted for groups C18:1 and +C8 were adapted from [44]. Bars represent the relative contribution of carbohydrates (PDC; black bars), exogenous oleate (OLE: white bars), octanoate (OCT) or heptanoate (HEPT; dotted bars); and other non-glucidic endogenous substrates (OS; grey bars) to acetyl-CoA for citrate synthesis. *p < 0.05 vs C18:1 group
More recently Allard et al. [42] have examined the effects of MCFAs in a rat model of left ventricular hypertrophy induced by aortic banding. Hearts were perfused with glucose and 1.2 mM palmitate, or 0.6 mM palmitate plus 1.2 mM octanoate. These two mixtures of FAs supply an equivalent amount of acetyl-CoA molecules. In the presence of octanoate, there was a two-fold increased contribution of exogenous FAs, attributed to MCFAs, reduced glycolysis, whereas glucose oxidation remained unchanged. Collectively, these metabolic effects concurred with our findings in SHR except that they were of greater magnitude, possibly due to the use of a greater octanoate concentration (1.2 versus 0.2 mM in our study).
Anaplerosis
Another potential beneficial effect of MCFAs is to increase myocardial levels of CAC intermediates. This anaplerotic effect was reported in different conditions of heart perfusions with the even-carbon MCFA, octanoate or hexanoate [26, 43, 44, 48–50]. This increase in CAC intermediate levels, more specifically that of isocitrate and malate, appears to be due to a preferential partitioning of pyruvate to carboxylation rather than to decarboxylation combined with an increased supply of acetyl-CoA from the MCFAs. There were no reported changes in the cardiac efflux of CAC intermediates, a process that is often referred to as cataplerosis [43, 44, 48, 49].
The magnitude of the anaplerotic effect was more marked with odd- than even-carbon MCFAs. In pig hearts perfused in situ and subjected to ischemia–reperfusion, the addition of 0.4 mM heptanoate induced a 25–50% increase in CAC intermediate tissue levels, particularly succinate, fumarate and malate [26]. We also obtained similar results in working SHR hearts perfused ex vivo and subjected to adrenergic stimulation [44] in the presence of either 0.49 mM oleate (C18:1), or a mixture of 0.4 mM oleate and 0.2 mM octanoate (+C8) or 0.2 mM heptanoate (+C7; Fig. 3).
Effects of medium-chain fatty acids on the myocardial levels of citric acid cycle intermediates. SHR hearts were perfused in a working mode with different mixtures of fatty acids, as described in Fig. 2. Tissue levels of citrate (Cit), isocitrate (Iso), α-ketoglutarate (αKG), succinate (Suc), fumarate (Fum), malate (Mal) and total citric acid cycle intermediates were quantified by gas chromatography–mass spectrometry in tissue homogenates spiked with standards. *p < 0.05, **p < 0.001 vs C18:1 group
Energy production
Much remain to be learned, however, on the mechanism by which the aforementioned unique metabolic effects of MCFAs impact on cardiac energy production. In our studies conducted in hearts from SHR and Wistar rats, despite differences in substrate selection for energy production induced by the addition of MCFAs, there was no difference in the absolute CAC flux rates, calculated from oxygen consumption and flux ratios [43, 44, 49]. However, Allard et al. [42] found that the metabolic effects of octanoate were associated with an increased myocardial concentration of adenosine triphosphate (ATP) both in control and hypertrophic rat hearts. Furthermore, the lower glycolytic rate of hypertrophied hearts perfused in the presence of octanoate was associated with a decreased activity of both isoforms of adenosine monophosphate kinase (α1 and α2), suggesting that beyond their metabolic effects, MCFAs may also impact on the activity of this signalling pathway which is considered as a cellular metabolic and energy fuel sensor [20, 42].
3 Potential benefits of MCFAs in cardiac diseases
The following section will describe the potential benefits of MCFAs on the normal and diseased heart beyond metabolism. This includes contractile function as well as the development of hypertrophy or its progression to failure. First, we will discuss findings related to cardiomyopathy development in subjects with LCFA oxidation defects, principally humans. Then, we will describe findings in studies using other models of cardiomyopathy, which were conducted exclusively in animals. As a whole, while part of the reported benefits of MCFAs may potentially be attributed to the aforementioned metabolic effects on the heart, the contribution of additional indirect effects cannot be excluded and hence will also be discussed.
3.1 LCFA oxidation defects
The clinical phenotype of patients affected with genetic FA oxidation defects is very diversified but frequently includes cardiomyopathy and/or heart beat disorders [13, 51]. Interestingly, cardiac symptoms are specifically associated with LCFA oxidation defects, either disorders of carnitine shuttle for transport across the mitochondrial membrane or defects of specific mitochondrial enzymes for LCFA oxidation. In contrast, they are exceptional in MCFA or short-chain FA oxidation disorders. Cardiac decompensation occurs under conditions where LCFAs usually become the major energy source such as fasting or during a stress challenge. The treatment regimen that has been successful in improving the outcome of many patients with LCFA oxidation defects, particularly in alleviating their cardiomyopathy [14–17], include dietary MCTs (~30% of calorie intake) in conjunction with frequent carbohydrate feedings, carnitine supplementation, and reduced consumption of LCTs. In these patients, MCTs was found to restore ketone body production and improves metabolic control following exercise [52]. Among the proposed underlying pathophysiological mechanisms in these patients are defective energy production combined with accumulation of toxic LCFA derivatives, either LC-acyl-CoAs or carnitines [51, 53]. The benefit of MCT administration is attributed to MCFAs and their derived metabolites, which bypass the defective enzyme block and thereby, can restore energy production beyond that provided by carbohydrates. This is suggested by the finding that cold tolerance of mouse with LCFA oxidation defects can be increased by feeding a MCT-enriched diet prior to cold challenge but not by glucose infusion [54]. Furthermore, MCFAs appear also to decrease the accumulation of toxic LCFA-derived metabolites, although the underlying mechanism remains to be clarified. In fact, experiments conducted in fibroblasts from patients with LCFA oxidation deficiencies demonstrated that MCFAs can be normally oxidized in these cells, inducing a decreased accumulation of toxic LCFA derivatives and correction of secondary metabolic disturbances [55–57]. Actually, MCT supplementation is generally considered to be beneficial in FA oxidation defects, even if MCFA utilization slightly differs between the different enzyme defects [13, 58].
Nevertheless, despite marked improvements in the clinical status of most FA oxidation defective patients, there remain persistent cardiac and muscle dysfunctions in some of these subjects. Recently, Roe et al. [18] reported a dramatic improvement of cardiomyopathy and muscle symptoms after substituting even-carbon (trioctanoin) by odd-carbon (triheptanoin) MCTs in a few patients with very long-chain acyl-CoA dehydrogenase deficiency. In one of these patients, cardiac hypertrophy and dysfunction was normalized after a few weeks of triheptanoin treatment. Triheptanoin ingestion induced a rapid appearance of both C4- and C5-ketone bodies in plasma. There was no evidence for any accumulation of toxicity linked to propionyl-CoA overload. It was postulated that the beneficial effect of triheptanoin results from its anaplerotic property. Presumably this would compensate for a decreased tissue CAC intermediate level attributed to excessive leakage and thereby improve acetyl-CoA metabolism in the CAC and energy production. However, controlled clinical studies appear warranted to substantiate the potential advantageous role of anaplerotic odd-carbon MCTs in patients with LCFA oxidation defects.
3.2 Cardiac hypertrophy and failure
Collectively, studies that have examined the effects of MCFAs in animal models of hypertrophy, both using the ex vivo perfused heart model or in vivo, have reported some benefits on cardiac function or disease progression. First, in their rat model of pressure-overload induced hypertrophy, Allard et al. [42] reported an improved contractile function of working hearts perfused with 0.6 mM palmitate and 1.2 mM octanoate compared to 1.2 mM palmitate alone. Similarly, we demonstrated that the capacity of SHR hearts to withstand an acute adrenergic stress, as evidenced by a rapid decline in cardiac functions and enhanced lactate dehydrogenase release (reflecting loss in cell membrane integrity), can be improved by increasing the contribution of exogenous FA oxidation to energy production by supplementation with 0.2 mM octanoate [44]. Replacing octanoate with 0.2 mM heptanoate did not result in additional benefits (unpublished data), in spite of heptanoate’s greater anaplerotic effect as illustrated in Fig. 3. Collectively, these results suggest that impaired energy production rather than defective anaplerosis contributes to cardiac dysfunction in this model of acute heart failure.
In addition to these aforementioned effects of acute supplementation with MCFAs, there have been a number of studies reporting some benefits following feeding SHR with a MCT-enriched diet [47, 59, 60]. These benefits include prevention of cardiac hypertrophy and improvement of cardiac function, despite persistent hypertension.
3.3 Ischemia and reperfusion
Earlier studies in perfused rat hearts have reported improved functional recovery following an ischemic insult with high concentrations of hexanoate or octanoate [38, 61]. However, using a less severe model of ischemia–reperfusion (i.e. 60% reduction of regional coronary flow) in the pig heart perfused in situ and lower concentrations of MCFAs (0.4 mM hexanoate or heptanoate), Okere et al. [26] did not observe any improvement in cardiac function.
3.4 Diabetic cardiomyopathy
To the best of our knowledge, there has been only one study in a rat model of diabetes with MCFAs, in which it was shown that the addition of hexanoate as sole FA source to hearts perfused with glucose alone normalized contractile function [39]. However, interestingly in mice overexpressing peroxisome proliferator-activated receptor α (PPARα) in a cardiomyocyte specific manner, which recapitulates the diabetic metabolic phenotype, administration of a diet enriched in LCFAs exacerbated the cardiomyopathy and contractile dysfunction, whereas a MCFA-enriched diet rescued these abnormalities [62]. Collectively, these results suggest that MCT-enriched diet may improve the diabetic cardiomyopathy, however further work is necessary to confirm this favourable effect, notably in human diabetic patients.
3.5 Other effects of MCFAs
Much remains to be learned about the mechanisms underlying the reported beneficial effects on cardiac function and disease progression. While these effects may result from the aforementioned unique metabolic properties and effects of MCFAs, there are additional potential indirect both cardiac and extra-cardiac effects, which ought also to be considered as potential mechanisms to explain the benefits of MCFAs. For example, replacing LCFAs by MCFAs as energy substrate may contribute to decrease potentially adverse effects of LCFAs, which has been referred to as lipotoxicity [62, 63]. This phenomenon has been proposed to occur whenever there is a mismatch between LCFA uptake and oxidation, leading to their conversion to biologically active metabolites such as ceramide and/or diacylglycerol that may impact on signalling pathways and gene expression. For example, in contrast to LCFAs, MCFAs do not activate PPARα and genes regulated thereby such as uncoupling protein 3 and pyruvate dehydrogenase kinase 4 [64]. In addition, while LC- and MC-acyl-CoAs differentially activate cardiac KATP channels, an important regulator of cellular excitability [65], only LC-acylcarnitine derivatives were found to decrease inward K+ current and elicit electrophysiological alterations [51, 66]. Finally, LC-acyl-CoAs have been shown to inhibit ex vivo the activity of enzymes involved in energy substrate metabolism and its regulation. This includes acetyl-CoA carboxylase, the mitochondrial tricarboxylate transporter, and several CAC enzymes [67, 68]. Figure 4 illustrates the inhibitory effects of increasing concentrations of LC-acyl-CoAs, but not MC-acyl-CoAs, on myocardial citrate synthase, nicotinamide adenine dinucleotide phosphate (NADP+)-isocitrate dehydrogenase and aconitase activities.
Effects of acyl-CoAs of different chain lengths on the activity of myocardial mitochondrial enzymes. Data are means ± SE of four experiments. The enzyme activity of citrate synthase, aconitase and NADP+-isocitrate dehydrogenase (NADP-ICDH) was determined spectrophotometrically as previously described [44] in frozen powered rat hearts in the absence (open bar), or in presence of 0.1 (grey bar) or 0.5 mM (black bar) of different acyl-CoA derivatives. Note that the enzyme activity was unaffected in presence of MCFA derivatives (octanoyl-CoA), in contrast to LC-acyl-CoAs, either palmitoyl-CoA or oleyl-CoA, which induce a drastic reduction in enzyme activity. *p < 0.0001 vs control condition
At the whole-body level, MCFAs also have other effects that may indirectly impact on cardiac function and metabolism as well as on risk factors for cardiac diseases. For example, MCT-enriched diets have been associated with improved insulin sensitivity and glucose tolerance [47, 69], increased thermogenesis and down-regulation of adipogenic genes, resulting in a reduced fat storage and a decreased body weight [11, 69]. In addition, they may also impact on the immune response or coagulation pathways [70, 71]. Interestingly, MCFAs have recently been reported to be ligands for G-protein coupled receptors acting as signalling molecules, for example to potentiate insulin secretion at the level of β-cells [72, 73]. The potency of MCFAs as agonist appears, however, to be chain length dependent.
3.6 Potential limitations or safety of MCFAs
Only few deleterious effects have been reported with the use of MCFAs, many of which have been reported mainly when MCTs were administered as the main dietary energy source representing >50% of the total energy intake. These include decreased absorption of LCTs, which may result in deficiency in essential polyunsaturated LCFA, steatorrhea, gastrointestinal discomfort, increased risk of ketogenesis and acidosis, and in some studies, though not all, changes in blood lipid profile such as a raise in triglycerides or non-high-density lipoprotein cholesterol [1–3, 74, 75]. However, MCTs should be well tolerated while providing an adequate source of energy when given at less than 30% of the total energy intake.
MCFAs may, however, interfere with protein metabolism, as suggested from perfused rat heart experiments [76]. In fact, octanoate being a chemical analogue of 2-ketoisocaproate, is a potent inhibitor of branched-chain alpha-keto acid dehydrogenase kinase, resulting in activation of branched-chain alpha-keto acid dehydrogenase complex and thereby leucine oxidation [77]. Further investigations appear warranted to assess the significance of this effect given that leucine modulates the mammalian target of rapamycin signaling pathway, which governs cell protein synthesis as demonstrated in skeletal muscle [78]. The impact of heptanoate on leucine metabolism should also be further considered. On additional considerations for odd-carbon MCFAs is propionyl-CoA overload, although this has not been reported in recent studies in humans and rats in which triheptanoin was supplied at <30% of the total energy intake [18, 19].
4 Conclusion
In summary, MCFAs are readily oxidized by cells, including cardiomyocytes, and provide a very efficient source of energy production. MCFAs display metabolic, biological and physical properties that are distinct from LCFAs, which may contribute to their reported benefits on the heart’s energy status and contractile function. These properties have been used advantageously for the nutritional management of cardiomyopathies in subjects with inherited LCFA β-oxidation defects [13]. Studies in animal models indicate that MCFAs may favourably modulate cardiac disease progression [42, 44, 62]. Interestingly, these beneficial effects of MCFAs have been reported in animal models for which the expression of myocardial genes coding for FA β-oxidation are decreased (pressure-overload hypertrophy [42]) or increased (diabetic cardiomyopathy [62]). This suggests that MCFAs use would not be restricted to a given condition. However, to the best of our knowledge, the effect of MCT feeding has not yet been examined in patients with coronary diseases or heart failure. While there appear to be many potential beneficial direct and indirect effects linked to substituting dietary LCTs for MCTs as an energy source, much remain to be learned about the metabolic alterations prevailing in patients with such diseases, particularly at the myocardial level. In fact, in contrast to animal models, patients received multiple medications, for which there is little information about their impact on substrate metabolism [20]. Hence, a better understanding of the prevailing metabolic alterations in these patients would help to delineate specific conditions in which MCTs are likely to be beneficial.
References
Ruiz-Sanz JI, Aldamiz-Echevarria L, Arrizabalaga J, et al. Polyunsaturated fatty acid deficiency during dietary treatment of very long-chain acyl-CoA dehydrogenase deficiency. Rescue with soybean oil. J Inherit Metab Dis. 2001;24:493–503.
Bach AC, Babayan VK. Medium-chain triglycerides: an update. Am J Clin Nutr. 1982;36:950–62.
Ulrich H, Pastores SM, Katz DP, Kvetan V. Parenteral use of medium-chain triglycerides: a reappraisal. Nutrition. 1996;12:231–8.
Kaunitz H. Medium chain triglycerides (MCT) in aging and arteriosclerosis. J Environ Pathol Toxicol Oncol. 1986;6:115–21.
Decuypere JA, Dierick NA. The combined use of triacylglycerols containing medium-chain fatty acids and exogenous lipolytic enzymes as an alternative to in-feed antibiotics in piglets: concept, possibilities and limitations. An overview. Nutr Res Rev. 2003;16:193–209.
Hu FB, Stampfer MJ, Manson JE, et al. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70:1001–8.
Graham GG, Baertl JM, Cordano A, Morales E. Lactose-free, medium-chain triglyceride formulas in severe malnutrition. Am J Dis Child. 1973;126:330–5.
Tantibhedhyangkul P, Hashim SA. Medium-chain triglyceride feeding in premature infants: effects on fat and nitrogen absorption. Pediatrics. 1975;55:359–70.
St-Onge MP, Jones PJ. Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. J Nutr. 2002;132:329–32.
Geliebter A, Torbay N, Bracco EF, Hashim SA, Van Itallie TB. Overfeeding with medium-chain triglyceride diet results in diminished deposition of fat. Am J Clin Nutr. 1983;37:1–4.
Baba N, Bracco EF, Hashim SA. Enhanced thermogenesis and diminished deposition of fat in response to overfeeding with diet containing medium chain triglyceride. Am J Clin Nutr. 1982;35:678–82.
Papamandjaris AA, MacDougall DE, Jones PJ. Medium chain fatty acid metabolism and energy expenditure: obesity treatment implications. Life Sci. 1998;62:1203–15.
Saudubray JM, Martin D, de Lonlay P, et al. Recognition and management of fatty acid oxidation defects: a series of 107 patients. J Inherit Metab Dis. 1999;22:488–502.
Duran M, Wanders RJ, de Jager JP, et al. 3-Hydroxydicarboxylic aciduria due to long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency associated with sudden neonatal death: protective effect of medium-chain triglyceride treatment. Eur J Pediatr. 1991;150:190–5.
Touma EH, Rashed MS, Vianey-Saban C, et al. A severe genotype with favourable outcome in very long chain acyl-CoA dehydrogenase deficiency. Arch Dis Child. 2001;84:58–60.
Brown-Harrison MC, Nada MA, Sprecher H, et al. Very long chain acyl-CoA dehydrogenase deficiency: successful treatment of acute cardiomyopathy. Biochem Mol Med. 1996;58:59–65.
Cox GF, Souri M, Aoyama T, et al. Reversal of severe hypertrophic cardiomyopathy and excellent neuropsychologic outcome in very-long-chain acyl-coenzyme A dehydrogenase deficiency. J Pediatr. 1998;133:247–53.
Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest. 2002;110:259–69.
Brunengraber H, Roe CR. Anaplerotic molecules: current and future. J Inherit Metab Dis. 2006;29:327–31.
Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–129.
Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–73.
Taegtmeyer H, Ballal K. No low-fat diet for the failing heart? Circulation. 2006;114:2092–3.
Lopaschuk GD, Wambolt RB, Barr RL. An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther. 1993;264:135–44.
Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–51.
Rennison JH, McElfresh TA, Okere IC, et al. High-fat diet postinfarction enhances mitochondrial function and does not exacerbate left ventricular dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1498–1506.
Okere IC, McElfresh TA, Brunengraber DZ, et al. Differential effects of heptanoate and hexanoate on myocardial citric acid cycle intermediates following ischemia-reperfusion. J Appl Physiol. 2006;100:76–82.
Tuunanen H, Engblom E, Naum A, et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–7.
Rinaldo P, Matern D, Bennett MJ. Fatty acid oxidation disorders. Annu Rev Physiol. 2002;64:477–502.
Coort SL, Bonen A, van der Vusse GJ, Glatz JF, Luiken JJ. Cardiac substrate uptake and metabolism in obesity and type-2 diabetes: role of sarcolemmal substrate transporters. Mol Cell Biochem. 2007;299:5–18.
Saggerson ED, Carpenter CA. Carnitine palmitoyltransferase and carnitine octanoyltransferase activities in liver, kidney cortex, adipocyte, lactating mammary gland, skeletal muscle and heart. FEBS Lett. 1981;129:229–32.
Schulz H. Regulation of fatty acid oxidation in heart. J Nutr. 1994;124:165–71.
Bian F, Kasumov T, Thomas KR, et al. Peroxisomal and mitochondrial oxidation of fatty acids in the heart, assessed from the 13C labeling of malonyl-CoA and the acetyl moiety of citrate. J Biol Chem. 2005;280:9265–71.
Johnson RC, Young SK, Cotter R, Lin L, Rowe WB. Medium-chain-triglyceride lipid emulsion: metabolism and tissue distribution. Am J Clin Nutr. 1990;52:502–8.
Kinman RP, Kasumov T, Jobbins KA, et al. Parenteral and enteral metabolism of anaplerotic triheptanoin in normal rats. Am J Physiol Endocrinol Metab. 2006;291:E860–866.
Metges CC, Wolfram G. Medium- and long-chain triglycerides labeled with 13C: a comparison of oxidation after oral or parenteral administration in humans. J Nutr. 1991;121:31–6.
Ala-Rami A, Ylihautala M, Ingman P, Hassinen IE. Influence of calcium-induced workload transitions and fatty acid supply on myocardial substrate selection. Metabolism. 2005;54:410–20.
Longnus SL, Wambolt RB, Barr RL, Lopaschuk GD, Allard MF. Regulation of myocardial fatty acid oxidation by substrate supply. Am J Physiol Heart Circ Physiol. 2001;281:H1561–1567.
Montessuit C, Papageorgiou I, Tardy-Cantalupi I, Rosenblatt-Velin N, Lerch R. Postischemic recovery of heart metabolism and function: role of mitochondrial fatty acid transfer. J Appl Physiol. 2000;89:111–9.
Chatham JC, Forder JR. Relationship between cardiac function and substrate oxidation in hearts of diabetic rats. Am J Physiol. 1997;273:H52–58.
Vincent G, Comte B, Poirier M, Des Rosiers C. Citrate release by perfused rat hearts: a window on mitochondrial cataplerosis. Am J Physiol Endocrinol Metab. 2000;278:E846–856.
Poirier M, Vincent G, Reszko AE, et al. Probing the link between citrate and malonyl-CoA in perfused rat hearts. Am J Physiol Heart Circ Physiol. 2002;283:H1379–1386.
Allard MF, Parsons HL, Saeedi R, Wambolt RB, Brownsey R. AMPK and metabolic adaptation by the heart to pressure overload. Am J Physiol Heart Circ Physiol. 2007;292:H140–148.
Vincent G, Bouchard B, Khairallah M, Des Rosiers C. Differential modulation of citrate synthesis and release by fatty acids in perfused working rat hearts. Am J Physiol Heart Circ Physiol. 2004;286:H257–266.
Labarthe F, Khairallah M, Bouchard B, Stanley WC, Des Rosiers C. Fatty acid oxidation and its impact on response of spontaneously hypertensive rat hearts to an adrenergic stress: benefits of a medium-chain fatty acid. Am J Physiol Heart Circ Physiol. 2005;288:H1425–1436.
Pravenec M, Kren V. Genetic analysis of complex cardiovascular traits in the spontaneously hypertensive rat. Exp Physiol. 2005;90:273–6.
Vincent G, Khairallah M, Bouchard B, Des Rosiers C. Metabolic phenotyping of the diseased rat heart using 13C-substrates and ex vivo perfusion in the working mode. Mol Cell Biochem. 2003;242:89–99.
Hajri T, Ibrahimi A, Coburn CT, et al. Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy. J Biol Chem. 2001;276:23661–6.
Panchal AR, Comte B, Huang H, et al. Partitioning of pyruvate between oxidation and anaplerosis in swine hearts. Am J Physiol Heart Circ Physiol. 2000;279:H2390–2398.
Comte B, Vincent G, Bouchard B, Benderdour M, Des Rosiers C. Reverse flux through cardiac NADP(+)-isocitrate dehydrogenase under normoxia and ischemia. Am J Physiol Heart Circ Physiol. 2002;283:H1505–1514.
Sundqvist KE, Vuorinen KH, Peuhkurinen KJ, Hassinen IE. Metabolic effects of propionate, hexanoate and propionylcarnitine in normoxia, ischaemia and reperfusion. Does an anaplerotic substrate protect the ischaemic myocardium? Eur Heart J. 1994;15:561–70.
Bonnet D, Martin D, de Lonlay P, et al. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999;100:2248–53.
Gillingham MB, Scott B, Elliott D, Harding CO. Metabolic control during exercise with and without medium-chain triglycerides (MCT) in children with long-chain 3-hydroxy acyl-CoA dehydrogenase (LCHAD) or trifunctional protein (TFP) deficiency. Mol Genet Metab. 2006;89:58–63.
Olpin SE. Implications of impaired ketogenesis in fatty acid oxidation disorders. Prostaglandins Leukot Essent Fatty Acids. 2004;70:293–308.
Schuler AM, Gower BA, Matern D, Rinaldo P, Wood PA. Influence of dietary fatty acid chain-length on metabolic tolerance in mouse models of inherited defects in mitochondrial fatty acid beta-oxidation. Mol Genet Metab. 2004;83:322–9.
Shen JJ, Matern D, Millington DS, et al. Acylcarnitines in fibroblasts of patients with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency and other fatty acid oxidation disorders. J Inherit Metab Dis. 2000;23:27–44.
Vianey-Saban C, Divry P, Brivet M, et al. Mitochondrial very-long-chain acyl-coenzyme A dehydrogenase deficiency: clinical characteristics and diagnostic considerations in 30 patients. Clin Chim Acta. 1998;269:43–62.
Jones PM, Butt Y, Bennett MJ. Accumulation of 3-hydroxy-fatty acids in the culture medium of long-chain L-3-hydroxyacyl CoA dehydrogenase (LCHAD) and mitochondrial trifunctional protein-deficient skin fibroblasts: implications for medium chain triglyceride dietary treatment of LCHAD deficiency. Pediatr Res. 2003;53:783–7.
Parini R, Invernizzi F, Menni F, et al. Medium-chain triglyceride loading test in carnitine-acylcarnitine translocase deficiency: insights on treatment. J Inherit Metab Dis. 1999;22:733–9.
Shimojo N, Miyauchi T, Iemitsu M, et al. Effects of medium-chain triglyceride (MCT) application to SHR on cardiac function, hypertrophy and expression of endothelin-1 mRNA and other genes. J Cardiovasc Pharmacol. 2004;44:S181–185.
Rupp H, Schulze W, Vetter R. Dietary medium-chain triglycerides can prevent changes in myosin and SR due to CPT-1 inhibition by etomoxir. Am J Physiol. 1995;269:R630–640.
Madden MC, Wolkowicz PE, Pohost GM, McMillin JB, Pike MM. Acylcarnitine accumulation does not correlate with reperfusion recovery in palmitate-perfused rat hearts. Am J Physiol. 1995;268:H2505–2512.
Finck BN, Han X, Courtois M, et al. A critical role for PPAR alpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–31.
Borradaile NM, Schaffer JE. Lipotoxicity in the heart. Curr Hypertens Rep. 2005;7:412–7.
Okere IC, Chandler MP, McElfresh TA, et al. Carnitine palmitoyl transferase-I inhibition is not associated with cardiac hypertrophy in rats fed a high-fat diet. Clin Exp Pharmacol Physiol. 2007;34:113–9.
Fox JE, Magga J, Giles WR, Light PE. Acyl coenzyme A esters differentially activate cardiac and beta-cell adenosine triphosphate-sensitive potassium channels in a side-chain length-specific manner. Metabolism. 2003;52:1313–9.
Yamada KA, Kanter EM, Newatia A. Long-chain acylcarnitine induces Ca2+ efflux from the sarcoplasmic reticulum. J Cardiovasc Pharmacol. 2000;36:14–21.
Paradies G, Ruggiero FM. Enhanced activity of the tricarboxylate carrier and modification of lipids in hepatic mitochondria from hyperthyroid rats. Arch Biochem Biophys. 1990;278:425–30.
Lai JC, Liang BB, Jarvi EJ, Cooper AJ, Lu DR. Differential effects of fatty acyl coenzyme A derivatives on citrate synthase and glutamate dehydrogenase. Res Commun Chem Pathol Pharmacol. 1993;82:331–8.
Han J, Hamilton JA, Kirkland JL, Corkey BE, Guo W. Medium-chain oil reduces fat mass and down-regulates expression of adipogenic genes in rats. Obes Res. 2003;11:734–44.
Wanten GJ, Calder PC. Immune modulation by parenteral lipid emulsions. Am J Clin Nutr. 2007;85:1171–84.
Miller GJ. Effects of diet composition on coagulation pathways. Am J Clin Nutr. 1998;67:542–5.
Covington DK, Briscoe CA, Brown AJ, Jayawickreme CK. The G-protein-coupled receptor 40 family (GPR40-GPR43) and its role in nutrient sensing. Biochem Soc Trans. 2006;34:770–3.
Kostenis E. A glance at G-protein-coupled receptors for lipid mediators: a growing receptor family with remarkably diverse ligands. Pharmacol Ther. 2004;102:243–57.
Hill JO, Peters JC, Swift LL, et al. Changes in blood lipids during six days of overfeeding with medium or long chain triglycerides. J Lipid Res. 1990;31:407–16.
Wollin SD, Wang Y, Kubow S, Jones PJ. Effects of a medium chain triglyceride oil mixture and alpha-lipoic acid diet on body composition, antioxidant status, and plasma lipid levels in the Golden Syrian hamster. J Nutr Biochem. 2004;15:402–10.
Buxton DB, Barron LL, Taylor MK, Olson MS. Regulatory effects of fatty acids on decarboxylation of leucine and 4-methyl-2-oxopentanoate in the perfused rat heart. Biochem J. 1984;221:593–9.
Paxton R, Harris RA. Regulation of branched-chain alpha-keto acid dehydrogenase kinase. Arch Biochem Biophys. 1984;231:48–57.
Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr. 2006;83:500S–7S.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (CIHR Grant # 9575 to C.D.R.) and by the Montreal Heart Institute Foundation. The authors are grateful to Dr Henri Brunengraber, for providing 13C-labelled heptanoate, Dr Robert A. Harris for stimulating discussions, and Bertrand Bouchard for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grants: This study was supported by the Canadian Institutes of Health Research (CIHR Grant # 9575 to C.D.R.) and by the Montreal Heart Institute Foundation.
Rights and permissions
About this article
Cite this article
Labarthe, F., Gélinas, R. & Des Rosiers, C. Medium-chain Fatty Acids as Metabolic Therapy in Cardiac Disease. Cardiovasc Drugs Ther 22, 97–106 (2008). https://doi.org/10.1007/s10557-008-6084-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-008-6084-0