Abstract
Anti-cancer cancer-targeted therapies are designed to exploit a particular vulnerability in the tumor, which in most cases results from its dependence on an oncogene and/or loss of a tumor suppressor. Mutations in the phosphoinositide 3-kinase (PI3K)/AKT/mTOR pathway are freqcuently found in breast cancers and associated with cellular transformation, tumorigenesis, cancer progression, and drug resistance. Several drugs targeting PI3K/ATK/mTOR are currently in clinical trials, mainly in combination with endocrine therapy and anti-HER2 therapy. These drugs are the focus of this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The PI3K/AKT/mTOR pathway plays a central role in cell physiology by transmitting signal transduction events in response to extracellular stimuli. This pathway controls many cellular functions such as proliferation, growth, survival, motility, and metabolism [1]. Mutations in this signalling route are frequently found in cancer, being particularly common in breast cancer, where about 60% of tumors harbor genetic alterations that hyperactivate the PI3K/AKT/mTOR pathway [2]. Preclinical studies have proven these alterations to be oncogenic drivers and, as such, therapeutic targets. Several drugs against PI3K, mTOR, and AKT are in clinical development. However, so far, the results have been modest with only one drug approved, everolimus, for the treatment of metastatic estrogen receptor (ER)-positive breast cancer. Recent advances in preclinical and clinical research are shedding some light on how to best approach the therapeutic inhibition of PIK3/AKT/mTOR signaling in patients. Drugs targeting this pathway face many challenges to succeed such as the identification of a biomarker predictive of tumor response, development of rational combinations with other compounds to overcome mechanisms of adaptive resistance, and optimization of approaches to minimize toxicity while still inhibiting their molecular target(s). In this review, we will discuss the current knowledge on drug development against the pathway, its limitations, and suggestions for an effective drug development.
2 Function and genetic alterations of PI3K/AKT pathway in breast cancer
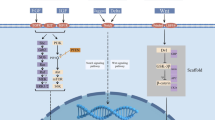
Phosphoinositide 3-kinases (PI3Ks) are a family of three different classes of lipid kinases. Class I PI3K is the most studied and clearly implicated in oncogenic transformation and tumor growth [1–3]. Class I PI3Ks are heterodimers consisting of a p85 regulatory subunit and a p110 catalytic subunit (p110α, p110β, p110γ, or p110δ). PI3K receives signals from growth factor receptor tyrosine kinases, such as ERBB receptors, FGFR and IGF-1R, and G protein-coupled receptors. Activated receptors phosphorylate adaptor proteins which, in turn, bind the amino-terminal SH2 domain of p85. This binding frees p110 from the inhibitory effect of p85 which then catalyzes the conversion of phosphatidylinositol bisphosphate, PI(4,5)P2, to phosphatidylinositol triphosphate, PI(3,4,5)P3. PIP3 recruits PDK1 and AKT, through their pleckstrin homology (PH) domain, to the plasma membrane. PDK1 phosphorylates AKT at Thr308. The mTOR/Rictor (TORC2) complex phosphorylates AKT at Ser473, resulting in full activation of this enzyme. PTEN and INPP4B dephosphorylate PIP3 in positions 3 and 5 of the inositol ring, respectively, thereby negatively regulating PI3K signaling output [4, 5]. Activated AKT phosphorylates and inhibits tuberous sclerosis complex 1 and 2 (TSC1/2) resulting in accumulation of Ras homolog enriched in brain (RHEB) which activates the complex mTOR/Raptor (TORC1). TORC1 phosphorylates ribosomal protein S6 kinase (S6K1) and eIF4E binding protein 1 (4E-BP1) promoting messenger RNA (mRNA) translation, protein synthesis, and autophagy. AKT also phosphorylates GSK3α, GSK3β, FoxO transcription factors, MDM2, BAD, and p27KIP1 to facilitate survival and cell cycle entry (Fig. 1).
The PI3K/AKT/mTOR pathway signaling as a therapeutic target. Activated growth factor receptors phosphorylate adaptor proteins like IRS1 which recruit p85/p110 (PI3K) dimers to the plasma membrane. PI3K is a heterodimer composed of a p85 regulatory subunit and a p110 catalytic subunit. p85 binding to IRS1 relieves its inhibitory effect on p110. Activated p110 catalyzes the conversion of PIP2 to PIP3. PTEN and INPP4B dephosphorylate PIP3, thus negatively regulating PI3K. PIP3 recruits PDK1 and AKT to the membrane. Full activation of AKT requires its phosphorylation by PDK1 and mTORC2. Activated AKT inhibits the complex TSC1/2, resulting in RHEB-GTP accumulation which, in turn, activates TORC1. Activated TORC1 phosphorylates ribosomal protein S6 kinase (S6K1) and eIF4E binding protein 1 (4E-BP1) promoting mRNA translation, protein synthesis, and autophagy. Small-molecule inhibitors discussed in the text are included in the figure. Dashed lines represent the inhibitory feedback loop relieved upon inhibition of the pathway
Mutations of the PIK3CA gene, which encodes p110α, are the most common genetic alteration in breast cancer occurring at frequency of 45% in luminal A, 30% in luminal B, 39% in HER2-enriched, and 9% in basal-like breast cancer subtypes. More than 80% of the mutations cluster within the helical (E542K and E545K) or the kinase (H1047R) domains of p110α [6]. Helical domain mutations increase catalytic activity by reducing the repression of p110α by p85 [7] or facilitating the interaction of p110α with IRS1 [8], whereas kinase domain mutations mainly increase the retention of p110α at the plasma membrane [9]. Preclinical data from cell-based studies and genetically engineered mice (GEMs) have clearly shown that these mutations activate PI3K/AKT/mTOR signaling and are oncogenic drivers by promoting cell transformation, tumor initiation, progression, and resistance to apoptosis [10–12]. However, data from knock-in GEMs, where the PIK3CA mutant protein is expressed at physiologic levels in the mammary gland, do not show pathway hyperactivation. In this knock-in models, mammary tumors develop after a long latency [13], suggesting that additional genetic alterations are needed to recapitulate a PI3K-induced transformed phenotype [14, 15]. This is consistent with data from primary breast cancers showing a disconnection between PIK3CA mutation and PI3K pathway activation. For example, luminal tumors, despite having the highest incidence of PIK3CA mutations, do not exhibit high levels of (activated) p-AKT, p-S6, and p-4EBP1 [16]. Further, PIK3CA mutations have been associated in early ER-positive breast cancer with good prognosis and are not a negative predictive factor for response to adjuvant endocrine therapy [17–19]. In triple-negative breast cancer, the PI3K/AKT/mTOR activation appears to be driven mainly by loss of PTEN (30%) or INPP4B (40%). Genomic loss of these tumor suppressors is associated with increased levels of p-AKT, p-S6, and p-4EBP1 [16, 20]. Around 3% of luminal tumors harbor AKT mutations in the PH domain (E17K), which result in constitutive localization at the plasma membrane and resulting activation of AKT [21]. Mutations in PIK3R1, the gene encoding the p85 regulatory subunit of PI3K, have also been reported although with a lower frequency (≈2%). Interestingly, PIK3R1 mutations cluster in the region of the protein that contacts p110, thus reducing the inhibitory effect of p85 on the isozyme [22, 23].
3 Inhibition of mTOR in cancer
mTOR is a serine/threonine kinase composed by the following two distinct protein complexes: a rapamycin- and nutrient-sensitive multiprotein complex (TORC1) and a growth factor-sensitive but nutrient- and rapamycin-insensitive complex (TORC2). TORC1 responds to amino acids, stress, oxygen, energy requirements and growth factors, and promotes cell growth and cell cycle progression. TORC2 responds to growth factors and regulates cell survival and metabolism, as well as the cytoskeleton [24]. Inhibitors of mTOR such as rapamycin (sirolimus) and the rapalogs temsirolimus (CCI-779), everolimus (RAD001), and deforolimus (AP23573) have been extensively evaluated in hematological malignancies, renal cancer, and as treatment for transplant rejection.
4 mTOR inhibitors in ER-positive breast cancer
Preclinical studies show that PI3K/AKT/mTOR activation is a mechanism of acquired resistance to long-term estrogen deprivation [25–27]. Results from two randomized trials, BOLERO-2 and TAMRAD [28, 29], suggested that the addition of the TORC1 inhibitor everolimus to anti-estrogen therapy can reverse endocrine resistance. In both studies, all patients had been previously exposed to aromatase inhibitors (AIs) and most of them developed progression after an initial response (acquired resistance). Patients were randomized to receive anti-estrogen therapy (exemestane in BOLERO-2, tamoxifen in TAMRAD) plus everolimus vs the anti-estrogen alone. The addition of everolimus increased the median progression-free survival (PFS) in both studies (BOLERO-2 PFS 7.8 vs 3.2 months, p < 0.0001; TAMRAD PFS 8.6 vs 4.5 months, p < 0.01). This led in 2012 to the approval of everolimus in combination with endocrine therapy after progression on AIs from the FDA and the EMA. The phase III trial HORIZON [30] evaluated the addition of temsirolimus to letrozole vs letrozole alone in AI-naive patients. There was no difference in outcome between the two arms of the study probably because HORIZON, different to BOLERO-2 and TAMRAD, tested the impact of TORC1 inhibition on primary endocrine resistance. In both BOLERO-2 and TAMRAD, the addition of everolimus to endocrine therapy produced higher rate of stomatitis, rash, fatigue, anorexia, anemia, and thrombocytopenia than endocrine therapy alone, which produced frequent treatment discontinuation and dose modifications. A recent study showed that stomatitis, the most common adverse event of everolimus, can be considerably reduced with the prophylactic use of dexamethasone oral solution [31]. This strategy could help to maintain the dose level and probably the drug efficacy. All this toxicity highlights one limitation of inhibitors of the PI3K/AKT/mTOR pathway and the importance of knowledge and practice on the management of adverse events associated with drugs like everolimus.
Predictive biomarkers of response to everolimus in breast cancer are not yet established. A biomarker analysis in ≈300 patients from BOLERO-2 using targeted exome sequencing, mostly of DNA from primary tumors, failed to identify any correlation between PIK3CA-activating mutations or other gene alterations in the PI3K/AKT/mTOR pathway and benefit from everolimus [32]. Also, selected PIK3CA mutations in plasma tumor at study entry did not predict for benefit from everolimus [33]. Interestingly, 10 patients with somatic mTOR kinase mutations derived clinical benefit from everolimus, which is in agreement with reports in renal and bladder cancers [34, 35].
5 mTOR inhibitors in HER2-overexpressing breast cancer
Laboratory studies have suggested that inhibition of PI3K/AKT/mTOR pathway is required for the anti-tumor action of HER2-targeted therapies and that it can also mediate resistance to anti-HER2 therapies [36–38]. Based on this background, everolimus has been evaluated as treatment of de novo and acquired resistance to trastuzumab in two phase III trials, BOLERO-1 and BOLERO-3. In BOLERO-1 [39], addition of everolimus to trastuzumab and paclitaxel as first-line treatment of HER2-positive advanced breast cancer did not prolong PFS (15 vs 14 months). BOLERO-3 [40] only included patients progressing on trastuzumab-paclitaxel; in this trial, the addition of everolimus to trastuzumab-vinorelbine prolonged PFS compared to trastuzumab-vinorelbine (7.0 vs 5.8 months, p = 0.0067). Both studies showed that patients with ER-negative/HER2-positive tumors derived more benefit from everolimus.
A genomic and immunohistochemical analysis of tumors from 377 patients enrolled in BOLERO-1 and BOLERO-3 showed that the benefit of adding everolimus was restricted to patients with PIK3CA mutations, PTEN loss, or “hyperactive” PI3K pathway, defined as low PTEN expression/mutation and/or known PIK3CA and/or AKT1 E17K mutation [41]. These results are in agreement with preclinical studies that suggest that PI3K/AKT/mTOR activation is causal to resistance to anti-HER2 therapies. For example, GEMs with transgenic mammary tumors HER2 and PIK3CAH1047R are highly resistant to anti-HER2 combinations (trastuzumab/pertuzumab and trastuzumab/lapatinib) with the addition of the pan-PI3K inhibitor buparlisib restoring drug sensitivity [42]. A biomarker analysis in CLEOPATRA (phase III study evaluating trastuzumab/taxane ± pertuzumab in HER2-positive metastatic breast cancer) showed that patients with PTEN loss and PIK3CA mutations derived lower benefit of the anti-HER2 therapy [43]. In the setting of neoadjuvant treatment, a recent meta-analysis including almost 1000 patients [44] suggested that patients with HER2+/PIK3CA mutant tumors had lower rates of pathologic complete response to trastuzumab, lapatinib, or the combination compared to HER2+ breast cancers with wild-type PIK3CA. This detrimental effect was restricted to ER-positive tumors but had no impact in overall survival. These results of the interaction of PI3K activation with prognosis and intrinsic resistance to anti-HER2 therapies in the neoadjuvant and metastatic setting are hard to reconcile with data from the adjuvant setting. Neither PIK3CA hot spot-activating mutation or PTEN loss predicted for lack of benefit from adjuvant trastuzumab in two randomized clinical trials including more than 3000 patients [45, 46].
6 New TORC1/TORC2 inhibitors
Everolimus is an allosteric inhibitor of TORC1 but does not affect TORC2. Blockage of TORC1 relieves a negative-feedback loop between S6K and IRS1, leading to an increase in phosphorylation of AKT on Ser473 by uninhibited TORC2. This has been seen in tumor biopsies of patients on treatment with rapalogs [47, 48], underscoring an intrinsic limitation of the anti-tumor activity of TORC1 inhibitors. Catalytic TORC1/TORC2 inhibitors, such as AZD2014 or MLN0128, are in clinical development with the aim of a more profound and complete blockade of mTOR complexes and avoidance of the compensatory activation of AKT [49, 50]. Laboratory studies have already shown that TORC1/TORC2 inhibitors induce a better blockade of PI3K/AKT/mTOR signaling as measured by inhibition of p-4EBP1, p-S6, and p-AKT compared to rapalogs [51]. These drugs are also active against everolimus-resistant acquired mutations in the rapamycin-binding domain of mTOR [52, 53].
7 PI3K inhibitors in breast cancer
A plethora of compounds have been developed to inhibit PI3K in breast cancer (Table 1). They are classified according to the specificity for each PI3K isoform in (1) pan-PI3K, targeting all class I isoforms, isoform-specific PI3K inhibitors, and dual-PI3K/mTOR inhibitors. As single agents, these drugs are far from the response rates obtained with the inhibition of other oncogenic kinases in other cancer types (such as those targeting mutant EGFR and mutant ALK in lung cancer and mutant BRAF in melanoma). Co-existing genetic alterations in tumors, compensatory feedback loops, and associated toxicity that precludes an adequate dose intensity are possible explanations for this lack of efficacy. Early results with pan-PI3K inhibitors in breast cancer are disappointing, with several studies showing increase toxicity and not a substantial improvement over endocrine therapy alone. Several phase III registration trials with PI3Kα-specific inhibitors are ongoing at the time of this writing (Table 1).
8 Pan-PI3K inhibitors in combination with endocrine therapy
Preclinical studies and retrospective analysis of some clinical trials have suggested that ER+/PIK3CA mutant tumors exhibit a lower response to anti-estrogens compared to ER+/PIK3CA wild-type tumors [54, 55]. In addition, some patients with ER+ breast cancer that progress on anti-estrogen therapy respond clinically to PI3K inhibitors [56]. ER+ human breast cancer cell lines that adapt to estrogen deprivation exhibit amplification of PI3K/AKT/mTOR signaling, and PI3K pathway inhibitors prevent acquired hormone independence [26]. Low levels of estradiol can rescue ER+/PIK3CA mutant cells from the lethal effect of PI3K inhibitors [57]. Further, inhibition of PI3K/AKT results in upregulation of ERα mRNA and protein and ER transcriptional activity [58–60], also suggesting co-regulation of ER and PI3K pathways. Finally, combined inhibition of ER and PI3K is synergistic against ER+/PIK3CA mutant xenografts [60, 61]. Taken together, these data strongly suggest that combined inhibition of ER and PI3K is a robust therapeutic approach to target these cancers.
Pan-PI3K inhibitors such as pictilisib (GDC-0941) or buparlisib (BKM120) target all class I p110 isoforms. Although they provide a potential advantage by broadly targeting multiple oncogenic PI3K isozymes, this approach narrows their therapeutic window by increasing adverse events [62].
Pictilisib (GDC-0941) is an oral ATP-competitive reversible inhibitor of all four class I PI3K isoforms [63, 64]. In a presurgical study by Schmid et al. [65], the combination of picitisilib and anastrozole was superior to anastrozole alone at inhibiting breast cancer cell proliferation as measured by Ki67 IHC. PIK3CA mutations were not predictive of response in this study. The phase II FERGI trial evaluated the addition of pictisilib to fulvestrant in post-menopausal patients progressing on an AI [66]. Pictisilib and fulvestrant did not increase PFS compared to fulvestrant and placebo (hazard ratio of 0·74, p = 0.096). Of note, no difference in outcome was seen in patients with PIK3CA mutant tumors. Patients treated with pictisilib showed a high rate of serious adverse event, mostly rash, pneumonitis, diarrhea, stomatitis, and transaminitis. This toxicity led to dose modifications in 45% of cases and treatment discontinuation in 24%. Of note, only 18% of patients developed hyperglycemia, which is an on-target effect of pictilisib as a result of inhibition of p110α, suggesting that adequate inhibition of PI3K was not achieved in FERGI, thus limiting the evaluability of this study.
Two early-phase clinical trials tested the pan-PI3K inhibitor buparlisib in combination with endocrine therapy, reporting good activity and a toxicity profile characterized by transaminitis, hyperglycemia, diarrhea, and mood disorders (anxiety, depression, irritability) [56, 67]. Both studies showed that the intermittent dose of buparlisib (100 mg 5 days on/2 days off) had lower rates of adverse events than daily dosing (100 mg/day). Buparlisib in combination with fulvestrant was studied in the phase III BELLE-2 trial for post-menopausal patients with metastatic breast cancer progressing on an AI [68]. Patients were randomized to receive fulvestrant plus buparlisib or placebo. Randomization was stratified by PI3K/AKT/mTOR pathway activation (defined by PIK3CA mutation and/or PTEN loss) status. Buparlisib modestly increased the median PFS by 1.9 months (6.9 vs 5.0 months, p < 0.001). For patients with pathway activation, there was no difference in the benefit of buparlisib. In a subset of patients (n = 581) where PIK3CA mutation was assessed in plasma tumor, cell-free DNA at trial entry, buparlisib plus fulvestrant showed a significant increase in PFS when compared to fulvestrant alone (7 vs 3.2 months, HR 0.56, p < 0.001) for PIK3CA mutants. Serious adverse events were reported in 23% of patients, dose reductions in 46%, and dose interruptions in 55%. The toxicity profile was characterized by transaminitis, hyperglycemia, mood disorders, and rash (Table 2).
At this time, the low benefit/toxicity ratio of buparlisib is not encouraging for the further development of this drug. Results of a phase III trial of fulvestrant ± buparlisib in patients with ER+ breast cancer progressing on everolimus (BELLE-3; NCT01633060) will be reported at the end of 2016.
9 PI3K alfa-specific inhibitors in combination with endocrine therapy
PI3Kα is the isoform predominantly mutated in cancer. Its selective inhibition has been shown to block PI3K/AKT signaling in response to different growth factor stimuli [69–71]. The rationale for the development of PIK3α-specific inhibitors is to maximize the inhibition of p110α while sparing the patient from the side effects associated with inhibition of all p110 isozymes. Two PI3Kα inhibitors are in clinical development for breast cancer in combination with endocrine therapy, alpelisib (BYL719) and taselisib (GDC-0032).
Alpelisib has been studied as single agent [72], showing preferential activity against those tumors with PIK3CA mutations and a toxicity profile overall lower than that seen with pan-PI3K inhibitors and consisting of hyperglycemia, nausea, diarrhea, decreased appetite, fatigue, vomiting, and rash. Alpelisib plus fulvestrant is being studied in a phase III trial for patients with metastatic ER-positive breast cancer progressing on an AI (SOLAR1; NCT02437318). Two other phase I trials of alpelisib in combination with AIs have reported also showing preferential activity in patients with PIK3CA mutant cancers [73, 74]. In the study conducted by our group, the clinical benefit rate in patients with PIK3CA mutant vs PIK3CA wild tumors was 41 vs 20%, respectively.
Taselisib is a potent inhibitor of p110α, p110δ, and p110γ, with a 30-fold less potency against p110β active to p110α. It has also a greater selectivity against PIK3CA mutant isoforms than wild type and it is superior in terms of pathway inhibition and induction of apoptosis to other PI3K inhibitors [75, 76]. A phase I trial of taselisib + letrozole [77] reported the following grade ≥3 adverse events: diarrhea 14%, hyperglycemia 7%, stomatitis 4%, and fatigue 4%; the recommended phase II dose was 6 mg/day. A phase Ib trial of taselisib in combination with tamoxifen was presented in ASCO 2016 [78], with a similar toxicity profile. A phase II trial of the combination taselisib + fulvestrant [79] in 60 patients reported colitis (13.3%) and diarrhea (11.7%) as the most common grade ≥3 adverse events and an 18% rate of treatment discontinuation. Clinical response rate was higher in patients with PIK3CA mutant compared to PIK3CA wild-type cancers (41 vs 14%, respectively). Taselisib is currently being evaluated with fulvestrant in a randomized phase III study in patients with metastatic ER+ tumors with and without PIK3CA mutations and with previous exposure to an AI (SANDPIPER; NCT02340221).
10 AKT inhibitors
AKT is a serine/threonine kinase with three isoforms (AKT1, AKT2, and AKT3). It is a downstream target of the PI3K and plays an important role in cancer cell survival, cell cycle entry, and glucose metabolism [2]. E17K-AKT1 is the most common somatic alteration in AKT, occurring in about 2% of all breast cancers. E17K increases lipid affinity to the AKT PH domain, resulting in constitutive membrane localization and activation of AKT [21]. There are several phase I and II clinical trials with allosteric and catalytic AKT inhibitors. In breast cancer, clinical results have been communicated with MK-2206 and AZD5363. A phase I trial of the combination of the allosteric pan-AKT inhibitor MK-2206 with fulvestrant reported grade 3 rash as the dose-limiting toxicity and a clinical benefit rate of 42%. Response was not associated with PI3K mutation status [80]. A recent report by Hyman and colleagues suggests that the E17K-AKT1 mutation could be a predictive marker for the efficacy of the pan-AKT catalytic inhibitor AZD5363. AZD5363 was evaluated in a phase I trial in patients with solid tumors harboring E17K-AKT1; 33 of 41 assessable patients (including 21 with ER-positive breast cancer) had regression of tumors and persistent AKT1-E17K allele decay in plasma tumor DNA [81].
11 Conclusions
The PI3K/AKT/mTOR pathway is the most frequently mutated network in breast cancer, providing multiple molecular targets that can be exploited with a therapeutic intent. However, biomarkers which identify PI3K-dependent cancers, that as a result of that dependence would be more likely to respond to these drugs, are not yet established. Following the approval of TORC1 inhibitors, which disable one of the key hubs downstream PI3K, PI3Kα inhibitors are poised to be the next ones to be approved for use in breast cancer. Large ongoing randomized registration trials with PI3Kα inhibitors include an adequate number of patients with cancers with activating “hot spot” PIK3CA mutations in order to determine if the benefit of these drugs, if present, will be limited to against cancers of that genotype. Although manageable, on-target toxicities induced by these drugs are not insignificant. Despite early signals of clinical activity, significant challenges for the therapeutic targeting of PI3K/AKT remain. These include mechanisms of compensation in tumor and host tissues upon treatment with these drugs that limit their optimal biological dosing and anti-tumor effect but also the incomplete knowledge about targeted drugs that should be used in combination with PI3K pathway inhibitors. It is clear that the benefit of these drugs will require development of rational combinations before they become a significant component of the anti-cancer portfolio in breast cancer.
References
Engelman, J. A., Luo, J., & Cantley, L. C. (2006). The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature Reviews. Genetics, 7(8), 606–619.
Engelman, J. A. (2009). Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature Reviews. Cancer, 9(8), 550–562.
Thorpe, L. M., Yuzugullu, H., & Zhao, J. J. (2015). PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nature Reviews. Cancer, 15(1), 7–24.
Agoulnik, I. U., Hodgson, M. C., Bowden, W. A., Ittmann, M. M., Agoulnik, I. U., Hodgson, M. C., et al. (2011). INPP4B: the new kid on the PI3K block. Oncotarget, 2(4), 321–328.
Salmena, L., Carracedo, A., & Pandolfi, P. P. (2008). Tenets of PTEN tumor suppression. Cell, 133(3), 403–414.
Ciriello, G., Gatza, M. L., Beck, A. H., Wilkerson, M. D., Rhie, S. K., Pastore, A., et al. (2015). Comprehensive molecular portraits of invasive lobular breast cancer. Cell, 163(2), 506–519.
Huang, C.-H., Mandelker, D., Schmidt-Kittler, O., Samuels, Y., Velculescu, V. E., Kinzler, K. W., et al. (2007). The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science, 318(5857), 1744–1748.
Hao, Y., Wang, C., Cao, B., Hirsch, B. M., Song, J., Markowitz, S. D., et al. (2013 13). Gain of interaction with IRS1 by p110α-helical domain mutants is crucial for their oncogenic functions. Cancer Cell, 23(5), 583–593.
Burke, J. E., Perisic, O., Masson, G. R., Vadas, O., & Williams, R. L. (2012). Oncogenic mutations mimic and enhance dynamic events in the natural activation of phosphoinositide 3-kinase p110α (PIK3CA). Proceedings of the National Academy of Sciences of the United States of America, 109(38), 15259–15264.
Zhao, J. J., Liu, Z., Wang, L., Shin, E., Loda, M. F., & Roberts, T. M. (2005). The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proceedings of the National Academy of Sciences of the United States of America, 102(51), 18443–18448.
Isakoff, S. J., Engelman, J. A., Irie, H. Y., Luo, J., Brachmann, S. M., Pearline, R. V., et al. (2005). Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Research, 65(23), 10992–11000.
Kang, S., Bader, A. G., & Vogt, P. K. (2005). Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proceedings of the National Academy of Sciences of the United States of America, 102(3), 802–807.
Tikoo, A., Roh, V., Montgomery, K. G., Ivetac, I., Waring, P., Pelzer, R., et al. (2012). Physiological levels of Pik3ca(H1047R) mutation in the mouse mammary gland results in ductal hyperplasia and formation of ERα-positive tumors. PloS One, 7(5), e36924.
Yuan, W., Stawiski, E., Janakiraman, V., Chan, E., Durinck, S., Edgar, K. A., et al. (2013). Conditional activation of Pik3ca(H1047R) in a knock-in mouse model promotes mammary tumorigenesis and emergence of mutations. Oncogene, 32(3), 318–326.
Adams, J. R., Xu, K., Liu, J. C., Agamez, N. M. R., Loch, A. J., Wong, R. G., et al. (2011). Cooperation between Pik3ca and p53 mutations in mouse mammary tumor formation. Cancer Research, 71(7), 2706–2717.
Network, T. C. G. A. (2012 4). Comprehensive molecular portraits of human breast tumours. Nature, 490(7418), 61–70.
Loi, S., Haibe-Kains, B., Majjaj, S., Lallemand, F., Durbecq, V., Larsimont, D., et al. (2010). PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proceedings of the National Academy of Sciences of the United States of America, 107(22), 10208–10213.
Kalinsky, K., Jacks, L. M., Heguy, A., Patil, S., Drobnjak, M., Bhanot, U. K., et al. (2009). PIK3CA mutation associates with improved outcome in breast cancer. Clinical Cancer Research, 15(16), 5049–5059.
Sabine, V. S., Crozier, C., Brookes, C. L., Drake, C., Piper, T., van de Velde, C. J. H., et al. (2014). Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study. Journal of Clinical Oncology, 32(27), 2951–2958.
Gewinner, C., Wang, Z. C., Richardson, A., Teruya-Feldstein, J., Etemadmoghadam, D., Bowtell, D., et al. (2009). Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell, 16(2), 115–125.
Carpten, J. D., Faber, A. L., Horn, C., Donoho, G. P., Briggs, S. L., Robbins, C. M., et al. (2007). A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature, 448(7152), 439–444.
Jaiswal, B. S., Janakiraman, V., Kljavin, N. M., Chaudhuri, S., Stern, H. M., Wang, W., et al. (2009). Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell, 16(6), 463–474.
Sun, M., Hillmann, P., Hofmann, B. T., Hart, J. R., & Vogt, P. K. (2010 Aug 31). Cancer-derived mutations in the regulatory subunit p85alpha of phosphoinositide 3-kinase function through the catalytic subunit p110alpha. Proceedings of the National Academy of Sciences of the United States of America, 107(35), 15547–15552.
Laplante, M., & Sabatini, D. M. (2012). mTOR signaling in growth control and disease. Cell, 149(2), 274–293.
Sanchez, C. G., Ma, C. X., Crowder, R. J., Guintoli, T., Phommaly, C., Gao, F., et al. (2011). Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Research, 13(2), R21.
Miller, T. W., Hennessy, B. T., González-Angulo, A. M., Fox, E. M., Mills, G. B., Chen, H., et al. (2010). Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. The Journal of Clinical Investigation, 120(7), 2406–2413.
deGraffenried, L. A., Friedrichs, W. E., Russell, D. H., Donzis, E. J., Middleton, A. K., Silva, J. M., et al. (2004 Dec 1). Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clinical Cancer Research, 10(23), 8059–8067.
Baselga, J., Campone, M., Piccart, M., Burris, H. A., Rugo, H. S., Sahmoud, T., et al. (2012). Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. The New England Journal of Medicine, 366(6), 520–529.
Bachelot, T., Bourgier, C., Cropet, C., Ray-Coquard, I., Ferrero, J.-M., Freyer, G., et al. (2012). Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. Journal of Clinical Oncology, 30(22), 2718–2724.
Wolff, A. C., Lazar, A. A., Bondarenko, I., Garin, A. M., Brincat, S., Chow, L., et al. (2013). Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. Journal of Clinical Oncology, 31(2), 195–202.
Rugo HS, Seneviratne L, Beck JT, Glaspy JA, Peguero JA, Pluard TJ, et al. Prevention of everolimus/exemestane (EVE/EXE) stomatitis in postmenopausal (PM) women with hormone receptor-positive (HR+) metastatic breast cancer (MBC) using a dexamethasone-based mouthwash (MW): results of the SWISH trial. Journal of Clinical Oncology, 34 (suppl; abstr 525).
Hortobagyi, G. N., Chen, D., Piccart, M., Rugo, H. S., Burris, H. A., Pritchard, K. I., et al. (2016). Correlative analysis of genetic alterations and everolimus benefit in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from BOLERO-2. Journal of Clinical Oncology, 34(5), 419–426.
(2016). Correlation of PIK3CA mutations in cell-free DNA (cfDNA) and efficacy of everolimus (EVE) in metastatic breast cancer: results from BOLERO-2. Journal of Clinical Oncology, 34(suppl; abstr 519).
Voss, M. H., Hakimi, A. A., Pham, C. G., Brannon, A. R., Chen, Y.-B., Cunha, L. F., et al. (2014). Tumor genetic analyses of patients with metastatic renal cell carcinoma and extended benefit from mTOR inhibitor therapy. Clinical Cancer Research, 20(7), 1955–1964.
Iyer, G., Hanrahan, A. J., Milowsky, M. I., Al-Ahmadie, H., Scott, S. N., Janakiraman, M., et al. (2012 Oct 12). Genome sequencing identifies a basis for everolimus sensitivity. Science, 338(6104), 221.
Miller, T. W., Forbes, J. T., Shah, C., Wyatt, S. K., Manning, H. C., Olivares, M. G., et al. (2009). Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clinical Cancer Research, 15(23), 7266–7276.
Nagata, Y., Lan, K.-H., Zhou, X., Tan, M., Esteva, F. J., Sahin, A. A., et al. (2004). PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell, 6(2), 117–127.
Berns, K., Horlings, H. M., Hennessy, B. T., Madiredjo, M., Hijmans, E. M., Beelen, K., et al. (2007). A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell, 12(4), 395–402.
Hurvitz, S. A., Andre, F., Jiang, Z., Shao, Z., Mano, M. S., Neciosup, S. P., et al. (2015). Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. The Lancet Oncology, 16(7), 816–829.
André, F., O’Regan, R., Ozguroglu, M., Toi, M., Xu, B., Jerusalem, G., et al. (2014). Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet Oncology, 15(6), 580–591.
André, F., Hurvitz, S., Fasolo, A., Tseng, L.-M., Jerusalem, G., Wilks, S., et al. (2016). Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2-overexpressing metastatic breast cancers: combined exploratory biomarker analysis from BOLERO-1 and BOLERO-3. Journal of Clinical Oncology, 34(18), 2115–2124.
Hanker, A. B., Pfefferle, A. D., Balko, J. M., Kuba, M. G., Young, C. D., Sánchez, V., et al. (2013). Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proceedings of the National Academy of Sciences of the United States of America, 110(35), 14372–14377.
Baselga, J., Cortés, J., Im, S. A., Clark, E., Ross, G., Kiermaier, A., et al. (2014) Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 32(33), 3753–61.
Loibl, S., Majewski, I., Guarneri, V., Nekljudova, V., Holmes, E., Bria, E., et al. (2016). PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Annals of Oncology, 27(8), 1519–1525.
Pogue-Geile, K. L., Song, N., Jeong, J.-H., Gavin, P. G., Kim, S.-R., Blackmon, N. L., et al. (2015). Intrinsic subtypes, PIK3CA mutation, and the degree of benefit from adjuvant trastuzumab in the NSABP B-31 trial. Journal of Clinical Oncology, 33(12), 1340–1347.
Perez, E. A., Dueck, A. C., McCullough, A. E., Chen, B., Geiger, X. J., Jenkins, R. B., et al. (2013). Impact of PTEN protein expression on benefit from adjuvant trastuzumab in early-stage human epidermal growth factor receptor 2-positive breast cancer in the north central cancer treatment group N9831 trial. Journal of Clinical Oncology, 31(17), 2115–2122.
Tabernero, J., Rojo, F., Calvo, E., Burris, H., Judson, I., Hazell, K., et al. (2008). Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. Journal of Clinical Oncology, 26(10), 1603–1610.
Cloughesy, T. F., Yoshimoto, K., Nghiemphu, P., Brown, K., Dang, J., Zhu, S., et al. (2008). Antitumor activity of rapamycin in a phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Medicine, 5(1), e8.
Basu, B., Dean, E., Puglisi, M., Greystoke, A., Ong, M., Burke, W., et al. (2015). First-in-human pharmacokinetic and pharmacodynamic study of the dual m-TORC 1/2 inhibitor AZD2014. Clinical Cancer Research, 21(15), 3412–3419.
Gökmen-Polar, Y., Liu, Y., Toroni, R. A., Sanders, K. L., Mehta, R., Badve, S., et al. (2012). Investigational drug MLN0128, a novel TORC1/2 inhibitor, demonstrates potent oral antitumor activity in human breast cancer xenograft models. Breast Cancer Research and Treatment, 136(3), 673–682.
Guichard, S. M., Howard, Z., Heathcote, D., Roth, M., Hughes, G., Curwen, J., et al. (2012). Abstract 917: AZD2014, a dual mTORC1 and mTORC2 inhibitor is differentiated from allosteric inhibitors of mTORC1 in ER+ breast cancer. Cancer Research, 72(8 Supplement), 917–917.
Wagle, N., Grabiner, B. C., Van Allen, E. M., Amin-Mansour, A., Taylor-Weiner, A., Rosenberg, M., et al. (2014 Oct 9). Response and acquired resistance to everolimus in anaplastic thyroid cancer. The New England Journal of Medicine, 371(15), 1426–1433.
Rodrik-Outmezguine, V. S., Okaniwa, M., Yao, Z., Novotny, C. J., McWhirter, C., Banaji, A., et al. (2016). Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature, 534(7606), 272–276.
Ellis, M. J., Lin, L., Crowder, R., Tao, Y., Hoog, J., Snider, J., et al. (2009). Phosphatidyl-inositol-3-kinase alpha catalytic subunit mutation and response to neoadjuvant endocrine therapy for estrogen receptor positive breast cancer. Breast Cancer Research and Treatment, 119(2), 379.
Miller, T. W., Balko, J. M., & Arteaga, C. L. (2011). Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. Journal of Clinical Oncology, 29(33), 4452–4461.
Mayer, I. A., Abramson, V. G., Isakoff, S. J., Forero, A., Balko, J. M., Kuba, M. G., et al. (2014). Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. Journal of Clinical Oncology, 32(12), 1202–1209.
Crowder, R. J., Phommaly, C., Tao, Y., Hoog, J., Luo, J., Perou, C. M., et al. (2009). PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Research, 69(9), 3955–3962.
Fox, E. M., Kuba, M. G., Miller, T. W., Davies, B. R., & Arteaga, C. L. (2013). Autocrine IGF-I/insulin receptor axis compensates for inhibition of AKT in ER-positive breast cancer cells with resistance to estrogen deprivation. Breast Cancer Res BCR., 15(4), R55.
Creighton, C. J., Fu, X., Hennessy, B. T., Casa, A. J., Zhang, Y., Gonzalez-Angulo, A. M., et al. (2010). Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Research, 12(3), R40.
Bosch, A., Li, Z., Bergamaschi, A., Ellis, H., Toska, E., Prat, A., et al. (2015). PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Science Translational Medicine, 7(283), 283ra51.
Miller, T. W., Balko, J. M., Fox, E. M., Ghazoui, Z., Dunbier, A., Anderson, H., et al. (2011). ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discovery, 1(4), 338–351.
Rodon, J., Dienstmann, R., Serra, V., & Tabernero, J. (2013). Development of PI3K inhibitors: lessons learned from early clinical trials. Nature Reviews. Clinical Oncology, 10(3), 143–153.
O’Brien, C., Wallin, J. J., Sampath, D., GuhaThakurta, D., Savage, H., Punnoose, E. A., et al. (2010). Predictive biomarkers of sensitivity to the phosphatidylinositol 3′ kinase inhibitor GDC-0941 in breast cancer preclinical models. Clinical Cancer Research, 16(14), 3670–3683.
Sarker, D., Ang, J. E., Baird, R., Kristeleit, R., Shah, K., Moreno, V., et al. (2015). First-in-human phase I study of pictilisib (GDC-0941), a potent pan–class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clinical Cancer Research, 21(1), 77–86.
Schmid, P., Pinder, S. E., Wheatley, D., Macaskill, J., Zammit, C., Hu, J., et al. (2016). Phase II randomized preoperative window-of-opportunity study of the PI3K inhibitor pictilisib plus anastrozole compared with anastrozole alone in patients with estrogen receptor-positive breast cancer. Journal of Clinical Oncology, 34(17), 1987–1994.
Krop, I. E., Mayer, I. A., Ganju, V., Dickler, M., Johnston, S., Morales, S., et al. (2016). Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet Oncology, 17(6), 811–821.
Ma, C. X., Luo, J., Naughton, M., Ademuyiwa, F., Suresh, R., Griffith, M., et al. (2016). A phase I trial of BKM120 (buparlisib) in combination with fulvestrant in postmenopausal women with estrogen receptor–positive metastatic breast cancer. Clinical Cancer Research, 22(7), 1583–1591.
Baselga J, Im S-A, Iwata H, Clemons M, et al. (2015). PIK3CA status in circulating tumor DNA (ctDNA) predicts efficacy of buparlisib (BUP) plus fulvestrant (FULV) in postmenopausal women with endocrine-resistant HR+/HER2– advanced breast cancer (BC): first results from the randomized, phase III BELLE-2 trial. SABCS.
Utermark, T., Rao, T., Cheng, H., Wang, Q., Lee, S. H., Wang, Z. C., et al. (2012). The p110α and p110β isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes & Development, 26(14), 1573–1586.
Zhao, J. J., Cheng, H., Jia, S., Wang, L., Gjoerup, O. V., Mikami, A., et al. (2006). The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proceedings of the National Academy of Sciences of the United States of America, 103(44), 16296–16300.
Foukas, L. C., Claret, M., Pearce, W., Okkenhaug, K., Meek, S., Peskett, E., et al. (2006). Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature, 441(7091), 366–370.
Juric D, Burris H, Schuler M, Schellens J, Berlin J, Seggewiß-Bernhardt R, et al. Phase I study of the pi3kα inhibitor byl719, as a single agent in patients with advanced solid tumors (AST). Ann Oncol. 2014;25(suppl 4):iv150.
Shah PD, Moynahan ME, Modi S, et al. (2014). Phase I trial: PI3Kα inhibitor BYL719 plus aromatase inhibitor (AI) for patients with hormone receptor-positive (HR+) metastatic breast cancer (MBC). SABCS.
Mayer IA, Abramson V, Formisano L, Balko JM, Estrada MV, Sanders M, et al. (2016). A phase Ib study of alpelisib (BYL719), a PI3Kα-specific inhibitor, with letrozole in ER+/HER2-negative metastatic breast cancer. Clinical Cancer Research.
Ndubaku, C. O., Heffron, T. P., Staben, S. T., Baumgardner, M., Blaquiere, N., Bradley, E., et al. (2013). Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1 H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1 H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a β-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. Journal of Medicinal Chemistry, 56(11), 4597–4610.
Olivero, A. G., Heffron, T. P., Baumgardner, M., Belvin, M., Ross, L. B., Blaquiere, N., et al. (2013). Abstract DDT02-01: discovery of GDC-0032: a beta-sparing PI3K inhibitor active against PIK3CA mutant tumors. Cancer Research, 73(8 Supplement), DDT02-01.
Saura C, Sachdev J, Patel MR, et al. (2014). Ph1b study of the PI3K inhibitor taselisib (GDC-0032) in combination with letrozole in patients with hormone receptor-positive advanced breast cancer. SABCS.
Baird R, Van Rossum A, Oliveira M,et al. (2016). POSEIDON trial phase 1b results: safety and preliminary efficacy of the isoform selective PI3K inhibitor taselisib (GDC-0032) combined with tamoxifen in hormone receptor (HR) positive, HER2-negative metastatic breast cancer (MBC) patients (pts)—including response monitoring by plasma circulating tumor (ct) DNA. Journal of Clinical Oncology, 34(suppl; abstr 2520).
Dickler M, Saura C, Donald A, et al. (2016). A phase II study of the PI3K inhibitor taselisib (GDC-0032) combined with fulvestrant (F) in patients (pts) with HER2-negative (HER2−), hormone receptor-positive (HR+) advanced breast cancer (BC). Journal of Clinical Oncology, 34 (suppl; abstr 520).
Ma, C. X., Sanchez, C., Gao, F., Crowder, R., Naughton, M., Pluard, T., et al. (2016). A phase I study of the AKT inhibitor MK-2206 in combination with hormonal therapy in postmenopausal women with estrogen receptor-positive metastatic breast cancer. Clinical Cancer Research, 22(11), 2650–2658.
Hyman, D. M., Smyth, L., Bedard, P. L., Oza, A., Dean, E., Armstrong, A., et al. (2015). Abstract B109: AZD5363, a catalytic pan-Akt inhibitor, in Akt1 E17K mutation positive advanced solid tumors. Am Assoc Cancer Res, 14(12 Supplement 2), B109.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guerrero-Zotano, A., Mayer, I.A. & Arteaga, C.L. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev 35, 515–524 (2016). https://doi.org/10.1007/s10555-016-9637-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-016-9637-x