Abstract
Ovarian adenocarcinoma is characterized by a late detection, dissemination of cancer cells into the whole peritoneum, and the frequent acquisition of chemoresistance. If these particularities can be explained in part by intrinsic properties of ovarian cancer cells, an increased number of studies show the importance of the tumor microenvironment in tumor progression. Ovarian cancer cells can regulate the composition of their stroma in promoting the formation of ascitic fluid, rich in cytokines and bioactive lipids, and in stimulating the differentiation of stromal cells into a pro-tumoral phenotype. In return, cancer-associated fibroblasts, cancer-associated mesenchymal stem cells, tumor-associated macrophages, or other peritoneal cells, such as adipocytes and mesothelial cells can regulate tumor growth, angiogenesis, dissemination, and chemoresistance. This review focuses on the current knowledge about the roles of stromal cells and the associated secreted factors on tumor progression. We also summarize the different studies showing that targeting the microenvironment represents a great potential for improving the prognosis of patients with ovarian adenocarcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ovarian adenocarcinoma represents the sixth most common cancer in terms of mortality in both European and North American women, and is the leading cause of death from gynecological malignancies. This is due to: (1) the late detection of cancer development (75 % of women present with an advanced disease at diagnosis), therefore ovarian cancer cells have already disseminated into the whole peritoneum; (2) the persistence of ovarian cancer cells in the peritoneum and acquisition of chemoresistant properties, which could be responsible for any recurrences following treatment (up to 18 months after) [1–3]. This is despite efficient surgery and chemotherapy which associates platinum salts and taxanes.
Historically, it was admitted that all epithelial ovarian tumors grew from the coelomic ovarian epithelium. However, recent results showing a great histologic and molecular heterogeneity of ovarian cancers seem to temper this dogma and to indicate that so-called ovarian tumors could be originated from other organs. Indeed, high-grade serous tumors come from the ovary surface but also from the distal part of fallopian tubes. Most of mucinous tumors are in reality metastases coming from gastrointestinal cancer such as colon or stomach cancer. Clear cell and endometrioid tumors are derivated from endometriosis (presence of endometrial tissue outside the uterine cavity). Benign and borderline tumors could present an ovarian origin although initiatory cells of these tumors are poorly known [4].
In addition to this histologic classification, Shih and Kurman proposed to classified epithelial ovarian cancers (types I and II) according to their genetic alterations and tumorigenesis pathway [5]. Thus, type I ovarian cancers are often early diagnosed (confined to ovaries), grew slowly, resist to conventional chemotherapies but may respond to hormonal treatment. These cancers are frequently associated with KRAS, BRAF, phosphatase, and tensin homolog (PTEN) or phosphatidylinositide 3-kinase (PI3K) genes mutation. On the contrary, type II ovarian cancers are frequently diagnosed at late stage (with extra-ovarian dissemination), grew quickly, respond to conventional chemotherapies. Previously described mutations are rarely found in type II ovarian cancers but BRAC1/2 and p53 mutations are frequent.
Ovarian carcinoma usually metastasizes along the peritoneum throughout the pelvic and abdominal cavity, such as pelvic wall, omentum, and mesentery. Other metastatic sites exist such as lung, skin, pleura, mediastinal, and lymph nodes [6]. Metastases have been also found in bone, brain, or gastrointestinal track [7–9]. Concerning the brain, metastatic have been found as solitary lesions located in the cerebellum or as solitary intracranial metastases. Gastrointestinal tract metastasis of ovarian carcinoma is merely limited to serosa. While the typical venous drainage of the ovary is via the ovarian veins which empty into the vena cava, connections with the vertebral veins have been postulated to be involved in these rare metastatic sites. A possible role of the BRCA1 gene has been also been proposed in this rare phenomenon [7]. Anyway, prognosis after documentation of extra-abdominal metastases from epithelial ovarian carcinoma is poor.
Ovarian cancers present specific features different to those exhibited by other human tumor malignancies. These include: (1) the origin of primary tumors as some clinicians have shown that ovarian adenocarcinoma cells can originate from ovarian epithelium, but also from the Fallopian tubes, endometrium, or peritoneum; (2) the way ovarian adenocarcinoma cells can disseminate by exfoliation from the ovaries (or the tubes) and that cancer cells do not randomly migrate throughout the whole peritoneum; and (3) the fact that secondary tumors (disseminated tumors, chemoresistant tumors, etc.) do not present any genetic mutations additional to the primary tumors [4].
An increasing number of studies have revealed that these particularities of ovarian cancers could be attributed to both the intrinsic properties of tumors and to their microenvironment [10–12]. Indeed, ovarian tumors comprise not only malignant cells, but also many other non-malignant cell types (adipocytes, fibroblasts, endothelial cells, migratory hematopoietic cells, stem cells, etc.). This produces a unique stroma that can modify the neoplastic properties of tumor cells.
One of the characteristics of ovarian adenocarcinoma cells is to disseminate into peritoneal sites such as the hepatic hile, omentum, spleen, uterus, etc. Stromal cells, such as mesothelial cells or mesenchymal stem cells, can regulate the extracellular matrix (ECM) composition and produce molecules which could attract ovarian carcinoma cells to these specific sites [13–15].
Ovarian tumors are usually highly vascularized, which correlates with a poor prognosis [16]. The importance of the angiogenic process in this disease could be due to the fact that endothelial cells can be activated by cancer cells as well as stromal cells, contributing to tumor development [11, 17, 18].
Concerning chemoresistance, ovarian cancer cells are typically sensitive to chemotherapy once the treatment has initiated. They become chemoresistant either during the treatment or at various times following therapy (up to several months after the primary treatment) [3]. There has been an almost exclusive experimental focus on the malignant chemoresistant cells that compose tumors. This is probably due to the success of those studies which defined the requirement for several mutational events in epithelial cells for the formation of chemoresistant tumors. Nevertheless, recent studies performed on the paired analysis of primary tumors and their metastatic lesions in ovarian cancer, revealed that metastatic lesions are most similar in their gene expression patterns to normal tissue, when compared to primary tumors. There are genetic differences between primary (mostly chemosensitive) tumors cells and metastatic lesions. However, these genetic differences could not explain the prognosis for tumor development as there was a significantly higher expression level of good prognosis genes than poor prognosis genes in the peritoneal metastasis versus matched primary tumors [19]. The outcome of oncogenic events in epithelial cells can be significantly modified by the nature of surrounding non-malignant cells. Stromal cells could be implicated in the acquisition of a chemoresistant phenotype by the secretion of cytokines allowing an activation of anti-apoptotic pathways and the exchange of drug efflux proteins enabling improved drug metabolism [10].
This review will focus on the involvement of the microenvironment in all processes of ovarian cancer progression: initiation, development, and relapse. We aim to list all the cells that could be further implicated or not, in the processes of cancer progression, and to include their associated secreted molecules. Indeed, it is becoming apparent that the microenvironment plays an important role in allowing the tumor to express its full neoplastic phenotype and that non-malignant cells have potential as therapeutic targets.
2 Secreted molecules
At early stages of the disease (stages I and II), ovarian cancer is restricted to the ovary. At advanced stages (III and IV), the ovarian cancer disseminates into the peritoneal cavity. This phenomenon is often responsible for an inflammatory process that induces the accumulation of a large quantity of liquid called “ascites”. The ascites contains, among others, molecules secreted by both ovarian cancer cells and the peritoneal microenvironment that can influence the proliferation, angiogenesis, and dissemination or metastasis potential of cancer cells [20, 21]. The following section constitutes a non-exhaustive description of the molecules, proteins, or bioactive lipids, found in ovarian ascites that could be involved in tumor progression (Table 1). The activated metabolic pathways of the cells could also favor the implantation of tumor cells as well as their proliferation and chemoresistance acquisition. Studies of the secreted factors that can be found in the ascitic fluid could help to decipher how cells communicate together. These factors could be potential therapeutic targets and indirectly enable the identification of the cells involved in oncogenesis and chemoresistance.
2.1 Bioactive lipids
2.1.1 Lysophosphatidic acid
Among these molecules, lysophosphatidic acid (LPA) is a small bioactive phospholipid found in ascitic fluid and sera of patients and its presence correlates with a poor prognosis of the disease [21, 22]. This molecule is produced by multiple cells, such as mesothelial cells (which form a monolayer lining the peritoneal cavity), macrophages, or ovarian cancer cells. It is involved in several steps of tumor progression: proliferation, invasion, and chemoresistance.
LPA enhances the proliferation of ovarian cancer cells [21, 23] and allows a long-term proliferation of these cells. Indeed, this phospholipid up-regulates telomerase expression and activity in ovarian cancer cells via both hypoxia inducible factor 1α (HIF-1α) and phosphatidylinositol 3-phosphate kinase pathways [24]. The telomerase prevents replicative senescence, allowing long-term proliferation and increased tumor progression.
LPA also promotes ovarian cancer cell invasion either by acting indirectly on cytokine production or indirectly modifying matrix metalloprotease (MMP) secretion and activity. Indeed, LPA enhances the expression of interleukin-8 (IL-8) [25–28] and other inflammatory cytokines such as IL-6 [27, 28] and vascular endothelial growth factor (VEGF) [29]. LPA stimulates proMMP-2 activation [30], increasing the level of active MMP-9 in some ovarian cell lines [31] and down-regulating tissue inhibitor of metalloproteases (TIMPs) [32]. These effects on ECM regulators lead to an increased invasion of ovarian cancer cells. Moreover, in an immunocompetent mouse model of ovarian cancer, LPA stimulates proliferation, migration, invasion, and the metastasis potential [33]. Finally, LPA, via its receptor LPA2, is a major stimulus of urokinase-type plasminogen activator (uPA) expression [34]. This protease converts plasminogen to plasmin, which degrade some components of the ECM and activate members of the metalloproteinase family.
Said et al. demonstrated the involvement of LPA in nuclear factor-κB (NF-κB) activation and production of prostaglandin E2 (PGE2) and isoprostane-8 (an indicator of the production of oxygen reactive species) in ovarian cancer cells [31]. LPA also enhances the expression and activation of ovarian cancer immuno-reactive antigen domain containing 1 (OCIAD1) [35]. OCIAD1 is overexpressed in ovarian metastatic tumors compared to primary tumors. It has been shown that this protein promotes paclitaxel resistance against cell detachment in an LPA-dependent manner [36].
Past studies demonstrated the implication of LPA in protecting the ovarian cancer cell line HEY from programmed cell death induced by cisplatin, which represents one of the conventional therapies in ovarian cancer treatment [37].
2.1.2 Sphingosine-1-phosphate
Sphingosine-1-phosphate (S1P) is a bioactive phospholipid involved in inflammation and tumor progression. Past studies of Hong et al. demonstrated the presence of S1P at micrometric concentrations in the ascitic fluid of patients with ovarian cancer. Hong et al. then showed that S1P was responsible for apoptosis of cells in suspension and cell growth of adherent cells [38]. S1P induces the expression of IL-8 which has been previously described as pro-tumoral in ovarian cancer [26]. Moreover, S1P is also implicated in the invasiveness of ovarian cancer cells with a dual effect depending on the biolipid concentration. Indeed, a low concentration of S1P (0.5 μM) inhibits the invasion of the ovarian cancer cell line Dov13 and a high concentration (20 μM) enhances invasion. This antagonism could be explained by a differential representation of S1P receptors depending on the S1P concentration [39, 40]. These data have been confirmed using the OVCAR-3 cell line with an additional positive effect on cell migration [41].
2.1.3 Cycloxygenase and prostaglandin axis
Cycloxygenases 1 and 2 (COX-1 and COX-2) are responsible for the biosynthesis of prostaglandins (PG) from arachidonic acid. Data obtained from ovarian cancer patients revealed a positive correlation between COX-2 expression and both tumor proliferation and angiogenesis parameters [42]. The expression of this enzyme is also associated with chemoresistance and a poor prognosis in patients with an ovarian adenocarcinoma [43].
Among the metabolites produced, PGE2 is frequently linked to tumoral processes. Indeed, PGE2 stimulates the proliferation and reduces the apoptosis of some ovarian cancer cell lines by activating COX-2 expression [44]. Rask et al. showed an increased expression of COX-1, COX-2, microsomal prostaglandin E synthase (mPGES-1), and prostaglandin receptor 1 and 2 (EP1–2) in ovarian adenocarcinoma compared to the normal ovary, supporting a role of PGE2 in tumor progression [45].
Obermajer et al. showed that PGE2 in ascites activates the stromal derived factor-1α/CXCR4 (SDF-1α/CXCR4) pathway in myeloid-derived suppressor cells (MDSCs) which migrate into ovarian ascites. Moreover, PGE2 enhances COX-2 expression in MDSCs which creates a positive feedback allowing a constant activity of the SDF-1α/CXCR4 axis and their local retention in these cells [46].
2.2 Cytokines and growth factors
2.2.1 Interleukin-6
IL-6 is a pleiotropic cytokine that has both anti- and pro-inflammatory roles [47]. It is a potent pro-angiogenic cytokine [48, 49], secreted by ovarian cancer cells, mesothelial cells fibroblasts or macrophages, and its expression correlates with a poor prognosis in ovarian carcinoma [50–52]. IL-6 is associated with chemoresistance. Indeed, the expression of this cytokine is elevated in the ascites and serum of women with ovarian cancer receiving paclitaxel chemotherapy [53, 54]. Moreover, autocrine IL-6 production leads to the resistance of ovarian cancer cells to cisplatin and paclitaxel [55]. This cytokine promotes tumor cell proliferation and metastasis of ovarian cancer cell lines and prevents their apoptosis [56, 57]. The IL-6 receptor (IL-6R) is overexpressed in ovarian cancer cells compared to normal ovarian cells [56, 58]. IL-6R has potential as a new target for ovarian cancer therapy.
2.2.2 Interleukin-8
Interleukin-8, also known as CXCL8, is a pro-inflammatory chemokine secreted by multiple cell types such as monocytes, mesothelial cells, endothelial cells, and tumor cells [59]. The expression of this cytokine and its receptor by ovarian cancer cells correlates with cell proliferation both in vitro and in vivo [60]. In ovarian cancer, a high expression of IL-8 is associated with an advanced tumor stage, increased ovarian cancer mortality, and poor disease-free survival [61–63]. The inhibition of IL-8 expression in the mouse leads to a diminution of tumor growth due to decreased angiogenesis, and a reduction in metastatic dissemination by a decreased synthesis of metalloproteases MMP-2 and MMP-9 [61]. The NF-κB pathway seems to be responsible for both IL-8 and VEGF expression, which leads to increased angiogenesis and tumorigenicity [64]. In contrast, Lee et al. showed that IL-8 expression in ovarian cancer cells could reduce tumor growth by enhancing the infiltration of neutrophils and macrophages [65].
2.2.3 Vascular endothelial growth factor
VEGF is a pro-angiogenic cytokine that can be synthesized by a large variety of cells (endothelial cells, macrophages, mesenchymal stem cells, tumor cells, etc.). It enhances the proliferation and migration of endothelial cells as well as the permeability of existing vessels [66]. The expression of VEGF in ovarian cancer is associated with poor prognosis [63]. VEGF acts synergistically with CXCL-12 (also called SDF-1α) to induce angiogenesis both in vivo and in vitro [67]. In an in vivo model of ovarian adenocarcinoma, treatment with A4.6.1, a monoclonal antibody directed against human VEGF, significantly inhibits the growth of subcutaneous SKOV-3 tumors. The inhibition of VEGF completely suppresses ascites formation, yet partially inhibits tumor growth in intraperitoneal SKOV-3 tumors [68]. Moreover, combination therapy with inhibitors of VEGF plus paclitaxel markedly reduces both tumor growth and ascites development in ovarian carcinoma compared to paclitaxel treatment alone [69, 70].
In the past decade, targeting angiogenesis has become a promising way to treat cancer. The utilization of bevacizumab (Avastin), a monoclonal antibody targeting VEGF, showed an improvement of patient survival in numerous types of cancer and has been tested in ovarian cancer. Indeed, some phase III studies have shown that this angiogenesis inhibitor, in combination with conventional chemotherapy, induces an increased progression free survival although demonstrated no effect on overall survival. These results were observed in patients harboring an advanced ovarian cancer, with either a platin sensitivity or resistance, in cases of primary treatment or recurrence [71].
2.2.4 Stromal cell-derived factor-1α
Stromal cell-derived factor-1α (SDF-1α) is a CXC chemokine which binds to the receptor CXCR4. It was originally known for its function in trafficking hematopoietic cells although was recently implicated in ovarian tumor progression and dissemination [72]. Kajiyama et al. showed an involvement of the SDF-1α/CXCR4 axis in the peritoneal metastasis of ovarian cancer [73]. Indeed, SDF-1α promotes the adhesion of epithelial ovarian cells to ECM components and to human peritoneal mesothelial cells.
2.2.5 Epidermal growth factor
Epidermal growth factor has been implicated in tumorigenesis of various cancers, especially ovarian cancer. Indeed, an elevated expression level of EGF receptor (EGFR) on ovarian cancer cells has been correlated with a poor outcome [74] and EGFR has become a new target for ovarian cancer therapy [75]. However, the inhibition of EGFR by monoclonal antibodies (cetuximab and erlotinib) did not demonstrate a survival improvement in ovarian cancer patients [76]. EGF, as LPA, stimulates chemoresistance of the ovarian cancer cell line HEY to cisplatin [37]. This growth factor can induce the epithelial–mesenchymal transition (EMT) of ovarian surface epithelium [77, 78].
2.2.6 Tumor necrosis factor-α
Tumor necrosis factor-α (TNF-α) is a key cytokine in inflammatory processes, originally considered as anti-tumoral. However, studies carried out during the past 25 years have highlighted roles for this cytokine in immunosuppression and its capacity to enhance tumor growth [79]. In ovarian cancer, it was notably revealed that the expression of TNF-α is correlated with the tumor grade [80]. Moreover, the autocrine production of this cytokine by ovarian tumor cells allows an increased synthesis of monocyte chemoattractant protein-1 (MCP-1), SDF-1, IL-6, macrophage inhibitory factor (MIF), and VEGF, enhancing tumor angiogenesis and peritoneal dissemination [81]. Recent studies also demonstrate that the production of TNF-α potentiates IL-17 synthesis, thus allowing the recruitment of myeloid cells and the activation of ovarian tumor progression [82]. We can conclude that TNF-α is a key cytokine allowing the formation of a pro-tumoral cytokinic microenvironment.
2.2.7 Transforming growth factor-β
Transforming growth factor-β (TGF-β) is a cytokine presenting both anti- and pro-tumoral effects, especially implicated in the EMT [83]. This cytokine, whose expression is correlated with an increased angiogenesis in ovarian cancer, promotes tumor cell invasion via the activation of MMPs [84, 85].
The complexity of the cytokinic network (number of molecules and their mutual interactions) present in the ascitic fluid constitutes a very large field to investigate in order to define new diagnostic methods, combinations of prognosis factors, and novel treatments (Table 1).
Until now, there has been no global analysis of patient ascitic fluid in order to identify a correlation between the composition of the ascitic fluid and patient prognosis. There is currently no equation between fluid composition, chemoresistance acquisition, and tumor aggressiveness. Both VEGF and IL-6 have been defined as good targets to stop tumor progression and prevent relapse. Anti-VEGF treatment is now combined with the basal treatment of combining platinum salts and taxanes. This targeted treatment allows an improvement in progression-free survival yet does not influence the overall survival of patients [71]. A treatment with an IL-6 inhibitor in association with the VEGF targeted treatment could be conceived to overcome chemoresistance.
Currently, lipids are not targeted molecules for the treatment of ovarian cancer since there has been only one clinical trial showing a correlation between obesity and ovarian cancer, as previously observed for breast cancers. However, there is an increase in the consideration of their use in the literature.
Non-exhaustive table of principal factors, proteins and bioactive lipids, contained in ovarian tumor ascitic fluids, their role, and the cells responsible for their production.
2.3 Exosomes
One general characteristic of tumors is their ability to release or shed intact vesicular portions of membrane material, termed exosomes, which were initially described by Tayor et al. [86]. While the precise mechanism of shedding remains unclear, the rate of shedding is significantly increased in most neoplastic cells and occurs continuously. Exosomes are found both in ascites and in sera from most ovarian cancer patients [87–89]. They interact with other cells and may serve as vehicles for the transfer of proteins as well as RNA (mRNA or miRNA) among cells [90–92]. First, exosomes were postulated to be functional extensions of antigen-presenting cells able to stimulate immune responses, but their direct role in tumor progression has been quickly proposed [86]. Exosomes are internalized by tumor cells from which they are originated as well as by stromal cells via various endocytic pathways, involving proteins of cells and exosomes [93]. Concerning the uptake by immune cells, phosphatidyl-serine on the exosomal surface would be implicated [88].
For Peng et al., these exosomes would have no effect on growth and apoptosis of tumor cells but impaired the cytotoxic activity of peripheral blood mononuclear cell in the presence of dendritic cells (DCs) [87]. In the same way, Taylor et al. showed that exosomes from ascites of ovarian cancer bearing patients suppressed the expression of T-cell activation signaling components, CD3-ζ and JAK3 and induced apoptosis [89]. On the other hand, Liang et al. performed a proteomic analysis on purified exosomes derived from ovarian cancer cell lines. They evidenced vehiculed tissue-specific proteins associated with tumorigenesis and metastasis, suggesting that exosomes may play important roles in ovarian cancer progression or in chemoresistance acquisition [91]. Indeed, Keller et al. showed that malignant ascites-derived exosomes cargo tumor progression-related proteins such as L1-cell adhesion molecule (L1-CAM), CD24, ADAM10, and extracellular matrix metalloproteinase inducer [88]. The administration of these malignant ascite-derived exosomes in tumor-bearing mice enhanced tumor growth. Concerning chemoresistance acquisition, Yin et al. have shown that secretion of annexin A3 from ovarian cancer cells is associated with platinum resistance in ovarian cancer patients [94]. This annexin A3 can be detected in exosomes released from cisplatin ovarian resistant cells.
Several mechanisms responsible for these protumoral effects have been suggested by Keller et al.: (1) exogenous application of exosomes could mediate a direct growth-promoting effect on ovarian cancer; (2) the exosomal proteolytic activity liberates growth factors or is helpful in inducing neovascularization; and (3) exosomes suppress immune functions (CD1 nude mice possess B cells and NK cells) that form a barrier against the xenograft [88]. Such a function may require the uptake of exosomes by immune cells. In summary, exosomes would act on tumor growth through the three mechanisms proposed by Keller.
Concerning their effect on stromal cells, Peng and Taylor evidenced their effect on immune cells and more recently, Cho et al. showed that exosome treatment induced adipose-derived stromal cells (ADSCs) to exhibit the typical characteristics of tumor-associated myofibroblasts, with increased expression of α-smooth active muscle (α-SMA) and tumor-promoting factors (SDF-1 and TGF-β) [87, 90, 92]. This phenomenon was correlated with an increased expression of TGF-β receptors I and II. Analysis of TGF-β receptor-mediated downstream signaling pathways revealed that each exosome activated different signaling pathways depending on the ovarian cells lines. Exosomes are so endowed with functional properties that could be beneficial for tumor growth. They could represent an important new target for therapeutical intervention.
3 Extracellular matrix
Extracellular matrix and adhesion molecules constitute a great part of the tumor microenvironment, especially in ovarian cancer. Indeed ovarian cancer cells disseminate into the whole peritoneum yet have specific sites to induce cancer nodules. These particular sites are the omentum (small and big epiploon), the fallopian tubes, the spleen, the uterus, the hepatic hile, etc. The potential of ovarian cancer cells to disseminate and metastasize into the peritoneal cavity is ruled by, among others, the ECM composition [95]. Variations in its composition can explain why cells disseminate into specific sites.
Some ovarian carcinoma lines (Hey, OVCAR429, ES-2, and HOC-7) express membrane type 1 MMP (MT1-MMP) that degrade collagen and allow invasion. Controversially, both SKOV-3 and OVCAR-3 cells do not express MT1 therefore are unable to invade polymerized collagen I matrices in vitro [96]. Moreover, the expression of MT1-MMP (as well as MMP-2, MMP-9, and TIMP-2) in ovarian cancer is correlated with poor survival [97–99].
Secreted protein acidic and rich in cysteine (SPARC) is a protein transiently secreted to the ECM that interact with growth factors and ECM components and regulate MMP expression and the cell cytoskeleton [100]. SPARC has controversial effects on cancer. It is regarded as a pro-tumoral protein in breast or melanoma cancer, yet in other models, such as ovarian cancer, SPARC expression is correlated with a good prognosis. SPARC is down-regulated in ovarian carcinoma cells compared to normal epithelial ovarian cells and the restoration of its expression reduces growth and tumorigenicity by inducing apoptosis of ovarian cancer cells [101, 102]. Moreover, SPARC reduces the adhesion of ovarian cancer cells (mediated by αvβ3 and αvβ5 integrins) to ECM, in particular to fibronectin and vitronectin [103, 104]. However, SPARC is not restricted to adhesion processes and can interact with other molecules, such as cytokines. Indeed, this protein antagonizes the mitogenic effect of VEGF in ovarian cancer cells [105] and inhibits proliferation, chemotaxis, and invasion induced by IL-6 [103]. SPARC inhibits the LPA-induced chemotactic and pro-invasive effects [103] as well as the LPA-induced secretion of IL-1, IL-6, IL-8, MCP-1 in SKOV-3 cells [103]. In some ovarian cancer cells, the overexpression of SPARC abrogates the proliferative and pro-invasive effects of MCP-1 (which was added to the culture medium) [31]. SPARC has a pivotal role in the regulation of microenvironmental interactions. Indeed, it inhibits the crosstalk established between ovarian cancer cells and either macrophages or mesothelial cells that result in a production of IL-6, uPA, PGE2, and isoprostane-8, in addition to an activation of NF-κB [31].
Mesothelin is a differentiation antigen found on the surface of mesothelial cells and is overexpressed in ovarian cancer. This protein attaches to the cell membrane by a glycophosphatidyl inositol linkage and enhances invasion of ovarian cancer cells by inducing MMP-7 secretion through MAPK/ERK- and JNK-dependent pathways [106].
It has been revealed that a high level of stromal hyaluronan, an extracellular polysaccharide, is correlated with tumor progression and a poor prognosis in ovarian cancer [107]. Versican, a proteoglycan, is increased in ovarian tumors and surrounding malignant stroma and associated with a poor outcome, possibly by acting in synergy with hyaluronan [108]. Ween et al. reviewed the capacities of versican and hyaluronan to promote ovarian cancer cell motility and invasion and the actual therapies developed against these extracellular components [109].
The expression of L1-CAM is correlated with a poor outcome in ovarian cancer [110] which could be explained by enhanced cell migration and tumor dissemination. Moreover, L1-CAM can prevent cisplatin-induced apoptosis in the ovarian carcinoma cell line OVMz [111].
The adhesion of ovarian cancer cells to the ECM via the integrin system is of great importance for cancer progression. This system is central for metastasis because of its interaction with a large panel of ECM proteins. In ovarian cancer cells and stromal cells, αv and β1 are the most expressed integrin subunits [112]. Moreover, the expression of these subunits in cancer cells or stromal cells is closely related to the expression of angiogenic and ECM-regulated proteins. The expression of β1 subunit in fibroblasts is correlated with TIMP-2 expression. In the same way, a high level of αv and β1 integrins in stromal cells is respectively associated with the presence of IL-8 and Ets, a transcription factor implied in tumor progression and particularly in the synthesis of proteases. On the contrary, the β1 subunit level is associated with the expression of IL-8 and FGF-2 in stromal cells [112]. In ovarian cancer cell line NOM-1, the MMP-9 activity is stimulated by the secretion of fibronectin and blocked by an α5 integrin antibody [113]. Hapke et al. showed that the ovarian cancer cell line OV-MZ-6 expresses integrin αvβ3 which, when associated to vitronectin, enhances cell proliferation and motility [114].
Although it has not been proposed to date, it could be of great interest to evaluate the proteins secreted by the matrix in order to predict patient outcome.
4 Endothelial cells and angiogenesis
The arrival of new vessels is a rate-limiting step of tumor development as when the diameter of a tumor becomes greater than 100–200 μm, the diffusion of nutrients and oxygen is not sufficient to reach all cells [115] (for review see [116]). The mechanism by which new vessels are created from those pre-existing is called “neo-angiogenesis” and is stimulated by the secretion of pro-angiogenic factors by tumor cells and their microenvironment [117].
4.1 Ovarian cancer cells and endothelial cells activation
Ovarian cancer cells stimulate neo-angiogenesis through differentiation of endothelial progenitor cells (EPCs). Indeed, the number of circulating EPCs is higher in the blood of ovarian cancer patients than in healthy individuals [118]. These cells are bone-marrow-derived cells which can migrate toward tumoral sites via peripheral blood.
In vitro, ovarian cancer cells from either primary tumors or metastasis, as well as cancer cell lines with mesenchymal properties, can induce differentiation of blood monocytes into CD14+/KDR+ cells through, in part, their secretion of VEGF and pleiotrophin. The CD14+/KDR+ subpopulation of cells are endothelial progenitors and exhibit angiogenic abilities such as differentiation, with the formation of tubular structures on matrigel [119, 120]. Alvero et al. studied an ovarian cancer stem cell population expressing the CD44 marker. Injection of these cells into immunosuppressed mice leads to highly vascular tumors. In vitro, CD44+ cells are able to form vessel-like structures on matrigel and acquire the endothelial cell markers VE-cadherin and CD34. These ovarian cancer stem-like cells can serve as vascular progenitors, able to differentiate in a VEGF-independent and IKKβ-dependent way [121]. The contribution of ovarian cancer stem cells in tumor vasculature is corroborated by another study in which ascitic ovarian cancer cells expressing CD133, a stem cell marker, did not exhibit tumorigenic abilities yet contributed to endothelial vasculature [122].
The study of signaling pathways activated in ovarian cancer cells, and potentially implicated in tumor angiogenesis, reveals the involvement of the PI3K/Akt axis. Indeed, an Akt3-dependent VEGF secretion [123] contributes to the recruitment of EPCs due, in part, to an increase in Id-1 expression, an EPC mobilizing factor [124].
4.2 VEGF and other pro-angiogenic factors
Production of pro-angiogenic growth factors is dependent on the tumor implantation site. Indeed, mice bearing ovarian intraperitoneal xenograft demonstrate higher levels of both plasmatic VEGF and basic fibroblast growth factor (bFGF) than mice bearing subcutaneous xenograft. These data support the significant role of the tumor microenvironment in tumor implantation and development [125].
Among these pro-angiogenic factors, the key molecule VEGF has been extensively analyzed and is previously described in part 2.6. In ovarian cancer, VEGF is expressed in 70 % of cases. In advanced stages, VEGF levels in serum and ascitic fluid correlate with both disease progression and response to chemotherapy [17, 126]. VEGF production is associated with ascites formation and tumor burden and VEGF level is a marker of ascites malignancy [18, 127]. Ovarian cancer cells express VEGFR, (VEGFR1 or VEGFR2, or both, depending on cell line) and 85 % of tumors tested exhibit a strong expression of VEGFR2. The activation of this receptor is linked to angiogenesis but also with ovarian cancer cell activation. Indeed, the inhibition of VEGFR2 decreases ovarian cancer cell migration and invasion in vitro and decreases tumor growth in vivo, which can be explained by decreased tumor vascularization and tumor cell proliferation, as well as increased apoptosis [128].
However, many other factors have been implied in angiogenesis regulation, such as Versican, Ephrin A2 (EphA2), MIF, and the previously described (part 2) TNF-α, IL-6, and IL-8.
Versican (a chondroitin sulfate proteoglycan) is overexpressed by both ovarian cancer cells and peritumoral stroma from human tumor fragments in advanced stages. The expression of this molecule correlates with microvessel density and endothelial cells ability to invade in vitro. Moreover, patients with high Versican expression levels have both lower overall and progression-free survival than patients with low Versican expression levels [129].
A positive correlation between the expression of EphA2 and CD31 (an endothelial cell marker), MMP2, MMP9, and MT1-MMP was revealed by the analysis of patient samples. EphA2 is a tyrosine kinase receptor overexpressed in 75 % of ovarian cancers and potentially involved in oncogenesis through its role in angiogenesis. Moreover, EphA2 overexpression in endothelial cells correlates with a lower patient overall survival [130].
MIF is overexpressed in ovarian cancer cell lines, ovarian carcinomas, and malignant ascites. The depletion of this chemokine leads to less vascularized tumors and a decrease in the endothelial cell proportion of ascites. MIF promotion of angiogenesis may be due to the stimulation of pro-angiogenic factor VEGF and inflammatory chemokines TNF-α and IL-6 [131].
4.3 Matrix metalloproteases
There are several lines of evidence of a relationship between MMPs and angiogenesis. In a model of peritoneal dissemination, VEGF activates MMP2 in ascitic fluid [132]. The VEGF secretion by ovarian cancer cells leads to the synthesis of MMP9 by microenvironment cells, such as endothelial cells [133]. Expression of MMP1 correlates with a poor prognostic outcome [134, 135] and it induces angiogenesis via the activation of protease activated receptor-1 as well as increasing the secretion of IL-8 and growth-regulated α protein [136].
4.4 Microenvironment and angiogenesis
The production of pro-angiogenic factors is regulated by the tridimensional organization of ovarian tumor cells and their microenvironment. Indeed, there is increased production of IL-8 and VEGF in ovarian tumor cells lines when they are organized in a spheroid compared to a monolayer culture [137]. Moreover, the levels of VEGF and fibroblast growth factor 2 (FGF-2) are significantly higher in the plasma of intraperitoneal tumor-bearing mice compared to subcutaneous bearing mice, demonstrating the importance of the peritoneal microenvironment in ovarian tumor vascularization [125].
Neoangiogenesis is consecutive to secretions of pro-angiogenic factors, like cytokines and growth factors, by both tumor cells and microenvironment cells, such as carcinoma-associated fibroblasts (CAFs) and mesenchymal stem cells (MSCs) [137]. Indeed, these cells participate in angiogenesis by promoting endothelial cell migration [138, 139]. Moreover, ovarian tumor cells can stimulate human adipose tissue-derived MSCs (hASCs) via LPA to secrete pro-angiogenic factors such as SDF-1α and VEGF, leading to the stimulation of endothelial cells in vitro [140].
The pro-angiogenic effects of stromal cells will be described in the following sections.
4.5 Anti-angiogenic treatment
Targeting angiogenesis is increasingly attractive for the treatment of ovarian cancer, as previously described (part 2). For a recent review of an anti-angiogenic assay in ovarian cancer see [141]. Two recent phase III clinical trials using bevacizumab (Avastin), a monoclonal antibody blocking VEGF, in combination with both carboplatin and paclitaxel increased the progression-free survival, although not overall survival [142, 143]. These encouraging results with targeted therapies could open the door for new methods of treatment in ovarian cancer.
5 Fibroblasts
Ovarian cancer is an aging disease characterized, among others, by the transformation of microenvironment fibroblasts into senescent fibroblasts. This transformation could be initiated by growth-regulated oncogene 1 (GRO-1) whose expression is activated in Ras-transformed human ovarian epithelial cells and ovarian cancer cells [144].
Senescent fibroblasts are able to trigger the neoplastic transformation of partially transformed ovarian epithelial cells (overexpressing C-myc) via a secreted factor [145]. In this work, Lawrenson et al. discussed the potential candidates secreted by senescent fibroblasts: osteopontin, SDF-1, VEGF, amphiregulin, hepatocyte growth factor (HGF), and IL-6. Although all of these molecules have been identified as regulators of pre-senescent or senescent fibroblasts, nothing is known about the secretory factor responsible for the epithelial cell transformation.
Ovarian cancer cells stimulate the secretion of uPA in fibroblasts, by releasing FGF-2, HB-EGF, HGF, IGF-1, and IL-1α [146]. These cells can also synthesize FGF-2 which acts in an autocrine fashion. Ovarian cancer cells enhance the release of proMMP-2 and TIMP-2 by tumor fibroblasts. The proMMP-2 is then processed by a membrane-bound metalloproteinase (MT-MMP) to form an active MMP-2 which degrades the ECM and allows the invasion of ovarian cancer cells [147, 148]. On the contrary, TIMP-2 produced by fibroblasts acts as a regulator that inhibits the activation of proMMP-2 into MMP-2. Fibroblasts are a key regulator of the ECM composition and dynamics.
A particularity of ovarian cancer cells is their capacity to exfoliate and disseminate into both the peritoneum and omentum. Fibroblasts in the omentum are able to enhance ovarian cancer cell adhesion and invasion by cell–cell contact [149]. Recently, Cai et al. described more precisely the interactions between omentum fibroblasts and ovarian cancer cells. Ovarian cancer cells secrete TGF-β1 and activate normal fibroblasts which then proliferate and express α-SMA. In return, activated fibroblasts secrete MMP-2 and regulate the expression of HGF in epithelial ovarian cancer cells. This crosstalk results in an increased adhesion and invasion of ovarian cancer cells into the omentum [150] (Fig. 1).
Role of fibroblasts and CAFs in the ovarian tumor microenvironment. Non-exhaustive roles of normal fibroblasts, senescent fibroblasts, or cancer-associated fibroblasts (CAFs) in ovarian tumor progression. The senescence of fibroblasts could be induced by the secretion of GRO-1 by ovarian tumor cells and the partially transformed ovarian epithelial cells (Ras mutation). In return, senescent fibroblasts could promote the neoplastic transformation of these transformed cells. Ovarian tumor cells can induce the formation of CAFs either from normal fibroblasts present in the omentum or from adipose-derived stromal cells (ADSCs). These CAFs can modify the composition of the extracellular matrix (ECM) by regulating the MMPs’ activity. Figure made with Microsoft PowerPoint 2010
6 Myofibroblasts or carcinoma-associated fibroblasts
Among the tumor microenvironment cells, myofibroblasts or CAFs are the most abundant. Their origin and precise phenotype have been discussed at length, however most studies demonstrate pro-tumoral effects of these cells [151].
Some ovarian cancer cell types (SKOV-3 and OVCAR-3) are able to secrete exosomes which induce expression of α-SMA, a myofibroblast marker, in ADSCs, as described in part 2.3 [90]. Conditioned medium by ovarian cancer cells can increase the quantity of cellular reactive oxygen species in fibroblasts, resulting in an overexpression of chloride intracellular channel 4 which allows a myofibroblast conversion [152].
Although CAFs are absent in the normal ovary, they are present in benign and borderline ovarian cancer and abundant in epithelial ovarian carcinoma. Since the expression of α-SMA is higher in cases of lymph node and omentum metastasis than in cases without metastasis, Zhang et al. hypothesized that CAFs contribute to metastasis by enhancing angiogenesis, lymphangiogenesis, and tumor cell migration and invasion [138].
Lai et al. revealed that silencing fibroblast activation protein (FAP) expression, a specific CAF marker, in these cells reduces both their growth and stem cell gene expression. Paradoxically, they also demonstrated that silencing FAP in the ovarian cancer cell line SKOV-3, although it does not express this protein, induces ovarian tumors and significantly reduces tumor growth in a xenograft mouse model [153]. These data show that targeting CAFs in cancer has potential for the development of new therapies (Fig. 1).
7 Mesothelial cells
The peritoneal cavity and its organs are lined by a mesothelial cell monolayer [154]. Mesothelial cells express several ECM proteins and adhesion molecules, including hyaluronan, which promotes ovarian carcinoma cell adhesion via CD44 [107, 155–159]. In a mouse model, the inhibition of CD44 by an antibody reduces the adhesion of ovarian cancer cells to the peritoneum as well as their spreading capacities [158]. In the peritoneal cavity, ovarian carcinoma cells form multicellular aggregates called spheroids, which adhere to ECM components and mesothelial cell monolayers [160]. Mesothelial cells induce the motility of human ovarian carcinoma cells [14].
On the contrary, the omentum mesothelial cells are able to inhibit the adhesion and invasion of some ovarian cancer cells in a cell–cell contact fashion [149] and behave like a protective barrier in order to protect the underlying tissue from cancer cells.
Mesothelial cells are also able to secrete several proteins involved in tumor progression. Indeed, these cells secrete a huge quantity of IL-6 previously described as pro-tumoral in ovarian cancer [161] (part 2). Under the effect of IL-1, mesothelial cells secrete bFGF, a protein implicated in mitosis, angiogenesis, and chemotactism [162]. Moreover, mesothelial cells activated by IL-1β potentially secreted by resident macrophages or inflammatory cells, are a source of VEGF present in ascitic fluid [163]. Finally, mesothelial cells constitutively produce LPA that enhances adhesion, migration, and invasion of ovarian cancer cells [164].
Mesothelial cells are able to establish a crosstalk with ovarian cancer cells. Indeed, a coculture of peritoneal mesothelial cells with ovarian cancer cells enhances the production of MCP-1 (in a time-dependent manner), uPA, PGE2, and isoprostane-8 [31].
8 Mesenchymal stem cells
MSCs have long-term viability, a self-renewal capacity, an adherence to plastic, express antigenic markers (CD73, CD90, and CD105) and can differentiate into multiple cell types, such as adipocytes, chondrocytes, or osteocytes [165]. MSCs derived from bone marrow can inhibit the proliferation and enhance the early apoptosis of the ovarian cancer cell line SKOV-3 [166]. On the contrary, SKOV-3 cells can induce the migration of MSCs.
While some data present an anti-tumoral effect of MSCs in certain types of cancer, the majority of studies focus on the pro-tumoral properties of these cells. Ovarian carcinoma cells are able to modify their own microenvironment, notably by secreting LPA. LPA secretion of ovarian carcinoma lines SKOV-3 and OVCAR-3 can activate the expression of α-SMA and SDF-1 in hASCs which differentiate into CAFs [167].
MSCs also play a role in the resistance to treatment in ovarian cancer. Indeed, MSCs secrete CXCL12, also known as SDF-1, which enhances the resistance of ovarian cancer cells to hyperthermia (42 °C) [168]. Recent studies revealed that MSCs activate ovarian tumor cell migration and invasion by IL-6, in a tridimensional model of amniotic membranes [13].
McLean et al. isolated carcinoma-associated MSCs (CA-MSCs) from ovarian tumors. These cells present a normal morphology and maintain their differentiation capacity. However, they exhibit molecular differences such as an increased expression of bone morphogenetic protein (BMP) 2, BMP4, and BMP6 which is responsible for increased tumor growth compared to “normal” MSCs [169].
Rafii et al. showed that CA-MSCs (called Hospicells in their study) are able to confer chemoresistance to ovarian cancer cells by direct cell–cell contact and “oncologic trogocytosis”, the exchange of membrane fragments containing multidrug resistance proteins [170]. These CA-MSCs are also involved in ovarian oncogenesis. Indeed, Pasquet et al. demonstrated that they can promote tumorigenicity in vivo by enhancing angiogenesis. This effect was explained by an induction of both VEGF and hypoxia-induced factor 1α (HIF-1α) expression in ovarian cancer cells [171]. Castells et al. further studied these interactions between ovarian cancer cells and CA-MSCs. They demonstrated that they are able to enhance the secretion of pro-tumoral cytokines IL-6, IL-8, and VEGF in ovarian cancer cells. Moreover, they discovered interactions between CA-MSCs and macrophages. Indeed, CA-MSCs can attract macrophages to tumor sites and trigger their differentiation into a pro-tumoral M2 phenotype via a secreted factor [172]. Hospicells also present immunosuppressive properties by inhibiting the T-cell response [173].
Castells et al. showed that supernatants of CA-MSCs as well as bone marrow MSCs were able to induce development of chemoresistance by reducing apoptosis. They observed that CA-MSC secretions were able to confer carboplatin resistance to ovarian cancer cells by inhibiting the activation of effector caspases and blocking apoptosis. The activation of PI3K/Akt pathway signaling and phosphorylation of the downstream target, X-linked inhibitor of apoptosis (Xiap), underlined their implication in ovarian cancer chemoresistance (Fig. 2).
Roles of MSCs and adipocytes in the ovarian tumor microenvironment. Non-exhaustive roles of mesenchymal stem cells (MSCs) and adipocytes in the ovarian tumor microenvironment. MSCs can promote the resistance of ovarian tumor cells to hyperthermia by the secretion of SDF-1. Under the effect of LPA, MSCs can differentiate into cancer associated fibroblasts. Moreover, cancer-associated MSCs (CA-MSCs) can be found in the ovarian stroma and promote tumor growth by the secretion of BMPs, regulate angiogenesis, enhance chemoresistance by the transfer of drug efflux proteins, and immunosuppress T lymphocytes by the secretion of nitric oxide. The omental adipocytes are able to promote tumor growth by the secretion of fatty acids and potentiate the metastatic process by the secretion of adipokines. Figure made with Microsoft PowerPoint 2010
9 Adipocytes
Adipocytes are cells composing the adipose tissue, which specialize in energy storage and are known to secrete growth factors. The role of these cells in the tumoral microenvironment is poorly studied, especially in ovarian cancer.
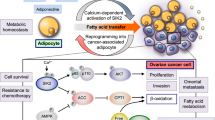
The omentum is a peritoneal structure principally composed by adipocytes and is the main site of metastasis dissemination of advanced ovarian cancer. Adipocytes can induce the homing of ovarian cancer cells by the secretion of cytokines, such as IL-6, IL-8, MCP-1, TIMP-1, and adiponectin. These cells can also enhance ovarian tumor growth by providing fatty acids via fatty acid binding protein 4 [174].
Zhang et al. (cancer res) have shown that obesity can accelerate tumor growth. First, because excess white adipose tissue (WAT) is a source of adipose stromal cells (ASC and adipocytes progenitors) that will be recruited by tumors and secrete adipokines and important angiogenic immunomodulatory and survival factors [175]. These factors will favor cancer progression and will activate infiltrating monocytic cells. Second, a role of WAT-derived stroma in cancer metastasis has also been suggested as CXCL1 and IL-8 secreted by tumor cells and signaling via receptor CXCR1 or CXCR2 are implicated in migration of human omental ASCs.
In different obesity models, and concerning ovarian cancer (abdominal visceral fat) as well as breast cancer, several teams have shown that adipocytes are often surrounded by mast cells, solitary macrophages and macrophages forming crown-like structures (CLS) [176, 177]. As these CLS were associated with activated NFκb and increased levels of proinflammatory mediators (TNF-α, IL-1β, and COX2), their role in cancer progression is warmly suggested [177]. So, there is a potential link between obesity, inflammation, and increased aromatase expression that caused macrophages recruitment. The type of recruited macrophages has still to be determined in these obesity models to really prove the link between obesity and cancer progression even if the axis between WAT, ASCc, and adipocytes is clearly involved in tumor growth, angiogenesis, and metastatic spread. In return, macrophages-derived cytokines have been suggested to stimulate lipolysis in adipocytes leading to increase release of saturated fatty acids. These saturated fatty acids activate TLR4 signaling in macrophages and thereby stimulate the production of proinflammatory mediators involved in chemoresistance acquisition by tumor cells [178].
At last, other publications involve the indirect role of adipocytes in cancer progression. As an example, Sy et al. have shown that ovarian cancer cells expressed a HOXA9 factor that favors ASCs to acquire the features of CAFs [179]. Those CAFs have been described in details by Kidd et al. [180]. They showed that most vascular and fibrovascular stroma (pericytes, αSMA (+) myofibroblasts, and endothelial cells) originates from adipose tissue via, between other, exosomes secretion by tumor cells (part 2.3).
The obesity, strongly associated with the presence of adipocytes and commonly measured by body mass index (BMI), is associated with a poor survival in numerous cancers including breast [181] or endometrial cancer [182]. Studies carried out on the ovarian cancer show contradictory results. Indeed, Fotopoulou et al. exhibit that the BMI does not seems to be associated with ovarian cancer stage or histology and that this marker is not predictive of survival or operative outcome and morbidity at the time of primary debulking [183]. On the contrary, several studies and a recent meta-analysis show that obesity is correlated with increased incidence of ovarian cancer and poor survival [184, 185]. Finally, this correlation seems to be closely associated with the histological subtype. Indeed, Olsen et al. show that a high BMI is correlated with an increased risk of clear cell, invasive endometrioid, invasive mucinous, and low-grade invasive serous ovarian cancer [186, 187].
Although adipocytes were originally considered as simple lipid storage cells, their role in the tumor microenvironment is clearly evident and their study in ovarian cancer constitutes a very promising field.
10 Immune system
The immune system, first characterized as the eradication of pathogens and tumors, can be hijacked by tumor cells and become a tumor-promoting environment. It is composed of DCs, CD8+ lymphocytes (cytotoxic T lymphocytes), γδ lymphocytes, CD4+ lymphocytes (regulatory T cells (Tregs) and T helper cells), monocytes/macrophages, neutrophils, etc. The equilibrium between these cells defines the immunosuppressive and pro-tumoral properties of this microenvironment.
In 2003, Zhang et al. revealed that the presence of CD3+ T lymphocytes is correlated with a better survival in patients with ovarian cancer [188]. More recently, Fialova et al. studied the composition of tumor-infiltrating immune cells in patients with ovarian cancer at different stages. They revealed that early stages were characterized by a strong Th17 immune response followed by Th1 recruitment for stage II. In the advanced stages III and IV, they detected Helio+ (a hematopoietic-specific transcription factor involved in the regulation of lymphocyte development) activated T regulatory cells and high quantities of myeloid dendritic cells and monocytes/macrophages [189]. Finally, Milne et al. showed in 2012 that the absolute lymphocyte count (number of circulating lymphocytes in peripheral blood) recorded at the time of prognosis is strongly associated with both disease burden and prognosis and not the number of CD8+ tumor infiltrating lymphocytes [190].
10.1 Dendritic cells
Dendritic cells are antigen-presenting cells (APCs) responsible for T lymphocyte activation during adaptive immunity and can be divided into two different subtypes: myeloid (mDCs) and plasmacytoid (pDCs).
Wei et al. highlighted that tumor-associated pDCs (TApDCs) are able to modify ovarian tumor immunity by inducing immunosuppressive CD8+ T lymphocytes [191].
Many studies focus on the effects of DCs in tumor immunity. However, some recent data demonstrated a role for DCs in ovarian tumor vascularization with opposite effects according to the dendritic subtype. Indeed, Curiel et al. hypothesized that ovarian tumors can exclude mDCs, which have angiogenesis inhibition properties, and attract pDCs which enhance angiogenesis via TNF-α and IL-8 secretion [192]. In ovarian cancer, some tumor-infiltrating DCs (CD11+ and DEC205+) acquire endothelial and pericyte characteristics, such as SMA or VEGF-A expression and participate in the maintenance of tumor vasculature. Indeed, the depletion of these cells results in vascular apoptosis, tumor necrosis, an enhanced effect of chemotherapies, and a boost of anti-tumor immunity [193].
Labidi-Galy et al. revealed phenotypic and functional differences between TApDCs and ascites pDCs in advanced ovarian cancer. Ascites pDCs exhibit pro-inflammatory properties whereas TApDCs have strong immunosuppressive characteristics and correlate with early relapse and a poor outcome [194, 195].
10.2 CD8+ T lymphocytes
CD8+ T lymphocytes, also known as cytotoxic T lymphocytes (CTLs), are specialized in killing virus-infected cells or tumor cells. They secrete perforin which creates pores in the plasma membrane of target cells, as well as granzyme, a serine protease which activates caspases and leads to apoptosis.
Sato et al. revealed evidence that both intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/Treg ratio correlate with a good outcome in ovarian cancer [196, 197]. The utilization of these cytotoxic cells could be a way to improve the prognosis of patients with an ovarian cancer.
10.3 γδ T lymphocytes
γδ T lymphocytes are CD3+ cells expressing a T cell receptor with γ and δ chains. They constitute a small percentage of T lymphocytes and exhibit anti-tumor properties. Indeed, these cells are able to reduce the proliferation potential of ovarian cancer stem cells and enhance their apoptosis and sensitivity to chemotherapeutic drugs via the production of IL-17 [198].
10.4 Regulatory T cells
Regulatory T cells are a suppressor T cell population responsible for the immune tolerance to self-antigens and the inactivation of the immune system. In 2012, Peng et al. reviewed the different populations of Tregs in ovarian cancer and the potential clinical applications of these cells [199]. As they are strongly subjected to microenvironmental regulation, a strategy of reprogramming Tregs could offer a new way to treat cancer.
Ovarian cancer cells can also modify the phenotype of immune cells. Alvero et al. [200] showed the existence of two subpopulations of ovarian cancer cells with different cytokine profiles: cancer stem cells (type I) and differentiated cancer cells (type II). The type II subpopulation is able to enhance the production of regulatory T cells which creates a tolerant microenvironment and prevents an immune response.
Curiel et al. showed that higher numbers of tumor Treg cells correlates to an increased risk of death and poor survival in ovarian cancer. These cells, characterized by the expression of CD4, CD25, and FoxP3, can be recruited by the chemokine CCL12 secreted by ovarian cancer cells and microenvironment macrophages [201].
10.5 T helper cells
T helper (Th) cells are CD4+ naïve T lymphocytes activated by APCs. The subtype obtained: Th1, Th2, or Th17, is defined by the cytokinic environment and interactions with APCs. These different subtypes activate the immune system, although differ in their cytokine production and target cells.
Th1 cells secrete both IFN-γ and TNF-α and are responsible for the activation of CTLs or anti-tumoral macrophages. On the contrary, Th2 cells are characterized by IL-4 secretion, activation of tumor-associated macrophages and therefore tumor progression [202]. Even though a direct link between Th2 cells and ovarian cancer has not been established, certain cytokines they produce are present in ascites and have been correlated with a bad prognosis in ovarian cancer [203].
Th17 cells secrete the cytokine IL-17, are known to have both anti-tumor and pro-tumor effects, and exhibit strong interactions with Tregs [204]. Miyahara et al. revealed the presence of Th17 cells at sites of ovarian cancer. Ovarian cancer cells and tumor-associated APCs secrete cytokines that could be responsible for the expansion of Th17 cells [205]. Moreover, Leveque et al. demonstrated that IL-2 can trigger the conversion of ovarian cancer-associated CD4+ Tregs into Th17 cells [206]. On the contrary, Fialova et al., as described above, observed a high Th17 immune response only in the early stages of ovarian cancer [189]. Recent data showed that a high density of IL-17-producing cells correlates with the presence of tumor-associated macrophages and a good prognosis in ovarian cancer [207].
Although the presence of Th17 cells has been confirmed in ovarian cancer, the specific effects of these cells in tumor progression remain to be established.
10.6 Macrophages
Monocytes and macrophages derive from myeloid cells located in the bone marrow. After maturation, monocytes circulate in the bloodstream and can migrate into tissues where they differentiate into macrophages. Depending on the environmental context and the tumor development stage, activated macrophages can be separated into two distinct phenotypes: M1 (classically activated) which inhibit tumor growth and M2 (alternatively activated) which are pro-tumoral. Although the exact definition remains controversial, it is clear that tumor-associated macrophages (TAMs) consistently present a highly immunosuppressive M2 profile [208].
Hagemann et al. described the capacities of ovarian cancer cells to regulate the macrophage phenotype. Indeed, cancer cells are able to differentiate macrophages into a TAM phenotype [209]. These interactions are bidirectional as macrophages are able to increase cancer cells invasion potential via the TNF-α and NF-κB pathways. Moreover, macrophages, potentially alongside VEGF, proteases, and secreted growth factors, facilitate ovarian cancer dissemination into the mouse peritoneum [210]. They are also key signaling cells that help to organize the responses of other cells (for e.g., mast cells and neutrophils).
In return, ovarian cancer cells, or its peritoneal microenvironment, regulate the macrophage phenotype. Duluc et al. observed that ovarian cancer ascites differentiate monocytes into TAM-like cells via the actions of leukemia inhibitory factor, IL-6, and macrophage colony-stimulating factor [211]. The two subpopulations of ovarian cancer cells identified by Alvero et al. [200], described previously (cancer stem cells and differentiated cancer cells), are able to differentiate macrophages into cells with either a tumor repair profile or an immunosuppressive profile. In both of these cases, ovarian cancer cells create a pro-tumoral microenvironment.
Finally, macrophages can regulate tumoral immunity. Indeed, macrophages, like ovarian cancer cells, secrete macrophage-derived chemokine (MDC = CCL22) which attract Tregs to the tumor, suppressing T cell immunity and enhancing tumor growth [201]. A subpopulation of ovarian cancer stromal macrophages expresses B7-H4, a co-stimulatory molecule which reduces the proliferation and cytokine production of T cells. These macrophages negatively regulate T cell immunity and their presence correlates with the number of tumor-infiltrating Tregs and a poor outcome in ovarian carcinoma [212, 213]. As there is a clear correlation between an abundance of TAMs and enhanced tumor progression, TAMs could be markers of poor prognosis.
10.7 Neutrophils
Neutrophils are leukocytes specialized in phagocytosis and defense against invading microorganisms. While their antibacterial functions are well described, there is a growing interest in their role in the context of the tumor. Indeed, neutrophils, like macrophages, can be separated into two populations: N1, which are pro-inflammatory and anti-tumorigenic, and N2, which have pro-tumoral and immunosuppressive properties. Tumor-associated neutrophils (TANs) have a distinct phenotype and can exhibit either N1 or N2 characteristics depending on the tumoral context [214, 215].
There have been no studies carried out on the direct role of TANs in ovarian cancer progression. However, we previously described how Lee et al. demonstrated that IL-8 production by ovarian cancer cells reduced tumor growth which could be due, in part, to the recruitment of neutrophils [65]. Neutrophils remain a very exploratory field to investigate in order to develop new therapies against ovarian cancer.
11 Conclusion
Ovarian cancer is the most lethal gynecological malignancy. It is often diagnosed in advanced stages and, despite therapy, 70 % of patients relapse within 2 years with incurable disease [1]. Epithelial ovarian cancer is recognized as a heterogeneous disease and is divided according to histological subtypes: high-grade serous, low-grade serous, clear cell, endometrioid, and mucinous. Each histological subtype is associated with a distinct clinical behavior (response to chemotherapy, pattern of metastasis, and survival) yet has historically been treated as one entity.
Regimens with clinical benefit and minimal toxicity are urgently needed to take into account the ovarian subtype in addition to the surrounding microenvironment. Indeed, ovarian cancer is characterized by a peritoneal dissemination at advanced stages. The peritoneum constitutes a wide and rich microenvironment, composed of numerous factors within ascitic fluid (proteins and bioactive lipids), a dynamic ECM as well as a large panel of stromal cells: inflammatory cells (macrophages, lymphocytes, etc.), mesothelial cells, adipocytes, CAFs, MSCs, etc.
Ovarian cancer cells thus disseminate into an open pro-tumoral stroma, involved in cancer progression, dissemination, and chemoresistance through cellular interactions and the secretion of cytokines and growth factors (Fig. 3).
The ovarian tumor microenvironment. Non-exhaustive representation of the different ovarian tumor microenvironment factors. Ovarian tumor cells are able to regulate the composition of their microenvironment, particularly by promoting the differentation of fibroblasts, mesenchymal stem cells, and adipose-derived stromal cells into cancer-associated fibroblasts. These cells, like mesothelial cells, can degrade the extracellular matrix and potentiate the invasion of tumor cells. The mesothelial cells are also able to promote angiogenesis and regulate the adhesion of tumor cells. Tumor-associated macrophages can regulate angiogenesis, enhance the production of ascitic fluid, activate the recruitment of T regulator lymphocytes, and inhibit cytotoxic T lymphocytes. The omental adipocytes promote tumor growth and the metastatic process by the secretion of adipokines and fatty acids. Figure made with Microsoft PowerPoint 2010
11.1 Therapeutic opportunities
Over the past decade, improvements in ovarian cancer treatment concerned only the schedule of administration of platinum salts and taxanes (intraperitoneal chemo-hyperthermia and liposomal cisplatin) and improvements in surgical procedures. Over the same time frame other cancer malignancies, such as breast or colon cancers, have benefited from a great improvement in targeted therapies. Based on this review, several targets could be envisaged.
Firstly, VEGF is a well-recognized target in ovarian cancer treatment and the majority of countries have proposed the combined treatment of platinum salts with bevacizumab. The addition of bevacizumab to the front-line therapy for ovarian cancer has produced a benefit in progression-free survival in several phase III trials. However, several issues require clarification in the future, especially the optimal patient selection based on predictive biomarkers and the duration of therapy [216]. A combined treatment targeting VEGF as well as IL-6 and IL-8 also involved in angiogenesis via different pathways, is conceivable.
Several tyrosine kinase inhibitors that target VEGF receptors as well as other receptors are under investigation in various trials for ovarian cancer. We can mention nintedanib (FGFR), pazopanib (PDGFR), and cabozantinib (c-Met). These antibodies will target not only the ovarian tumor cells but also stromal cells, enabling a regulation of the dynamic microenvironment.
Finally, Trebananib (AMG 386) is a peptide-Fc fusion protein which prevents interactions between angiopoetin-1 and angiopoetin-2, expressed on vascular endothelial cells with the Tie2 receptor, thereby inhibiting vascular maturation and reducing the impact of VEGF stimulation. In clinical trials so far, trebananib has been administered weekly, in combination with chemotherapy and as maintenance therapy. Efficacy seems to be dose-dependent and the toxicity profile appears to differ from bevacizumab [217].
It is 30 years since the introduction of cisplatin for the treatment of ovarian cancer and the overall survival curves of patients have been poorly modified [4]. Considering the major importance of the stroma in ovarian cancer dissemination, developing new treatments by targeting the microenvironment remains the optimum way to improve the clinical outcome of patients.
11.2 Immunomonitoring and prognostic relevance of stromal cells in clinical trials
The prognostic roles of tumor-infiltrating neutrophils, macrophages, DCs, and MSCs have been separately associated with poor clinical outcome in ovarian cancer. In order to depict the real engagement of each type of stromal cell at every stage of tumor development, immunomonitoring of stromal cells, using both tumor biopsies and peripheral blood could be proposed for each patient. Similarly, ovarian cyst fluid is a rich proteome resource for the detection of new tumor biomarkers (proteins and lipids) with the potential as prognosis markers.
Depending on the evolution of patients, this type of analysis could determine which cells and molecules are involved in tumor development, tumor dissemination, and chemoresistance acquisition. Therefore, it could be envisaged that modeling (multivariate logistic regression) all of these parameters, taking into account the stage of the disease, histological subtype, and the molecular profile of tumors, could aid the development of individual treatments for ovarian adenocarcinoma.
Ovarian cancer is characterized by a strong heterogeneity among patients (origin and mutations), a peritoneal dissemination and the acquisition of a chemoresistance by ovarian tumor cells. We reviewed the multiple interactions between the ovarian stroma (stromal cells and ascitic factors) and tumors. Ovarian tumor cells can modify the composition of their stroma by inducing the differentiation of normal cells into a pro-tumoral phenotype. In return, stromal cells can improve ovarian cancer progression by regulating tumor growth and dissemination, angiogenesis, immunosuppression, and chemoresistance. It is now evident that targeting the microenvironment holds great potential for the adaptation of treatments for each patient and improving prognosis.
References
Colombo, N., Van, G. T., Parma, G., Amant, F., Gatta, G., Sessa, C., et al. (2006). Ovarian Cancer Critical Reviews Oncology/Hematology, 60(2), 159–179.
Hennessy, B. T., Coleman, R. L., & Markman, M. (2009). Ovarian cancer. Lancet, 374(9698), 1371–1382.
Romero, I., & Bast, R. C., Jr. (2012). Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology, 153(4), 1593–1602.
Vaughan, S., Coward, J. I., Bast, R. C., Jr., Berchuck, A., Berek, J. S., Brenton, J. D., et al. (2011). Rethinking ovarian cancer: recommendations for improving outcomes. Nature Reviews Cancer, 11(10), 719–725.
Shih, I., & Kurman, R. J. (2004). Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. The American Journal of Pathology, 164(5), 1511–1518.
Cheng, B., Lu, W., Xiaoyun, W., YaXia, C., & Xie, X. (2009). Extra-abdominal metastases from epithelial ovarian carcinoma: an analysis of 20 cases. International Journal of Gynecological Cancer, 19(4), 611–614.
Sekine, M., Yoshihara, K., Komata, D., Haino, K., Nishino, K., & Tanaka, K. (2013). Increased incidence of brain metastases in BRCA1-related ovarian cancers. The Journal of Obstetrics and Gynaecology Research, 39(1), 292–296.
Ziegler, J., Gliedman, P., Fass, D., Beckman, M., Neophytides, A., & Steinfeld, A. (1987). Brain metastases from ovarian cancer. Journal of Neuro-Oncology, 5(3), 211–215.
Zhou, J. J., & Miao, X. Y. (2012). Gastric metastasis from ovarian carcinoma: a case report and literature review. World Journal of Gastroenterology, 18(43), 6341–6344.
Castells, M., Thibault, B., Delord, J. P., & Couderc, B. (2012). Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. International Journal of Molecular Sciences, 13(8), 9545–9571.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646–674.
Hanahan, D., & Coussens, L. M. (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21(3), 309–322.
Touboul, C., Lis, R., Al, F. H., Raynaud, C. M., Warfa, M., Althawadi, H., et al. (2013). Mesenchymal stem cells enhance ovarian cancer cell infiltration through IL6 secretion in an amniochorionic membrane based 3D model. Journal of Translational Medicine, 11(1), 28.
Rieppi, M., Vergani, V., Gatto, C., Zanetta, G., Allavena, P., Taraboletti, G., et al. (1999). Mesothelial cells induce the motility of human ovarian carcinoma cells. International Journal of Cancer, 80(2), 303–307.
Wang, E., Ngalame, Y., Panelli, M. C., Nguyen-Jackson, H., Deavers, M., Mueller, P., et al. (2005). Peritoneal and subperitoneal stroma may facilitate regional spread of ovarian cancer. Clinical Cancer Research, 11(1), 113–122.
Hollingsworth, H. C., Kohn, E. C., Steinberg, S. M., Rothenberg, M. L., & Merino, M. J. (1995). Tumor angiogenesis in advanced stage ovarian carcinoma. The American Journal of Pathology, 147(1), 33–41.
Duncan, T. J., Al-Attar, A., Rolland, P., Scott, I. V., Deen, S., Liu, D. T., et al. (2008). Vascular endothelial growth factor expression in ovarian cancer: a model for targeted use of novel therapies? Clinical Cancer Research, 14(10), 3030–3035.
Byrne, A. T., Ross, L., Holash, J., Nakanishi, M., Hu, L., Hofmann, J. I., et al. (2003). Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clinical Cancer Research, 9(15), 5721–5728.
Malek, J. A., Martinez, A., Mery, E., Ferron, G., Huang, R., Raynaud, C., et al. (2012). Gene expression analysis of matched ovarian primary tumors and peritoneal metastasis. Journal of Translational Medicine, 10, 121.
Moradi, M. M., Carson, L. F., Weinberg, B., Haney, A. F., Twiggs, L. B., & Ramakrishnan, S. (1993). Serum and ascitic fluid levels of interleukin-1, interleukin-6, and tumor necrosis factor-alpha in patients with ovarian epithelial cancer. Cancer, 72(8), 2433–2440.
Xu, Y., Gaudette, D. C., Boynton, J. D., Frankel, A., Fang, X. J., Sharma, A., et al. (1995). Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clinical Cancer Research, 1(10), 1223–1232.
Xu, Y., Shen, Z., Wiper, D. W., Wu, M., Morton, R. E., Elson, P., et al. (1998). Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA, 280(8), 719–723.
Xu, Y., Fang, X. J., Casey, G., & Mills, G. B. (1995). Lysophospholipids activate ovarian and breast cancer cells. Biochemical Journal, 309(Pt 3), 933–940.
Yang, K., Zheng, D., Deng, X., Bai, L., Xu, Y., & Cong, Y. S. (2008). Lysophosphatidic acid activates telomerase in ovarian cancer cells through hypoxia-inducible factor-1alpha and the PI3K pathway. Journal of Cellular Biochemistry, 105(5), 1194–1201.
So, J., Navari, J., Wang, F. Q., & Fishman, D. A. (2004). Lysophosphatidic acid enhances epithelial ovarian carcinoma invasion through the increased expression of interleukin-8. Gynecologic Oncology, 95(2), 314–322.
Schwartz, B. M., Hong, G., Morrison, B. H., Wu, W., Baudhuin, L. M., Xiao, Y. J., et al. (2001). Lysophospholipids increase interleukin-8 expression in ovarian cancer cells. Gynecologic Oncology, 81(2), 291–300.
Fang, X., Schummer, M., Mao, M., Yu, S., Tabassam, F. H., Swaby, R., et al. (2002). Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochimica et Biophysica Acta, 1582(1–3), 257–264.
Fang, X., Yu, S., Bast, R. C., Liu, S., Xu, H. J., Hu, S. X., et al. (2004). Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. The Journal of Biological Chemistry, 279(10), 9653–9661.
Hu, Y. L., Tee, M. K., Goetzl, E. J., Auersperg, N., Mills, G. B., Ferrara, N., et al. (2001). Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. Journal of the National Cancer Institute, 93(10), 762–768.
Fishman, D. A., Liu, Y., Ellerbroek, S. M., & Stack, M. S. (2001). Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Research, 61(7), 3194–3199.
Said, N. A., Elmarakby, A. A., Imig, J. D., Fulton, D. J., & Motamed, K. (2008). SPARC ameliorates ovarian cancer-associated inflammation. Neoplasia, 10(10), 1092–1104.
Sengupta, S., Kim, K. S., Berk, M. P., Oates, R., Escobar, P., Belinson, J., et al. (2007). Lysophosphatidic acid downregulates tissue inhibitor of metalloproteinases, which are negatively involved in lysophosphatidic acid-induced cell invasion. Oncogene, 26(20), 2894–2901.
Li, H., Wang, D., Zhang, H., Kirmani, K., Zhao, Z., Steinmetz, R., et al. (2009). Lysophosphatidic acid stimulates cell migration, invasion, and colony formation as well as tumorigenesis/metastasis of mouse ovarian cancer in immunocompetent mice. Molecular Cancer Therapeutics, 8(6), 1692–1701.
Wang, G. L., Wen, Z. Q., Xu, W. P., Wang, Z. Y., Du, X. L., & Wang, F. (2008). Inhibition of lysophosphatidic acid receptor-2 expression by RNA interference decreases lysophosphatidic acid-induced urokinase plasminogen activator activation, cell invasion, and migration in ovarian cancer SKOV-3 cells. Croatian Medical Journal, 49(2), 175–181.
Wang, C., Michener, C. M., Belinson, J. L., Vaziri, S., Ganapathi, R., & Sengupta, S. (2010). Role of the 18:1 lysophosphatidic acid-ovarian cancer immunoreactive antigen domain containing 1 (OCIAD1)-integrin axis in generating late-stage ovarian cancer. Molecular Cancer Therapeutics, 9(6), 1709–1718.
Sengupta, S., Michener, C. M., Escobar, P., Belinson, J., & Ganapathi, R. (2008). Ovarian cancer immuno-reactive antigen domain containing 1 (OCIAD1), a key player in ovarian cancer cell adhesion. Gynecologic Oncology, 109(2), 226–233.
Frankel, A., & Mills, G. B. (1996). Peptide and lipid growth factors decrease cis-diamminedichloroplatinum-induced cell death in human ovarian cancer cells. Clinical Cancer Research, 2(8), 1307–1313.
Hong, G., Baudhuin, L. M., & Xu, Y. (1999). Sphingosine-1-phosphate modulates growth and adhesion of ovarian cancer cells. FEBS Letters, 460(3), 513–518.
Smicun, Y., Reierstad, S., Wang, F. Q., Lee, C., & Fishman, D. A. (2006). S1P regulation of ovarian carcinoma invasiveness. Gynecologic Oncology, 103(3), 952–959.
Devine, K. M., Smicun, Y., Hope, J. M., & Fishman, D. A. (2008). S1P induced changes in epithelial ovarian cancer proteolysis, invasion, and attachment are mediated by Gi and Rac. Gynecologic Oncology, 110(2), 237–245.
Park, K. S., Kim, M. K., Lee, H. Y., Kim, S. D., Lee, S. Y., Kim, J. M., et al. (2007). S1P stimulates chemotactic migration and invasion in OVCAR3 ovarian cancer cells. Biochemical and Biophysical Research Communications, 356(1), 239–244.
Ali-Fehmi, R., Morris, R. T., Bandyopadhyay, S., Che, M., Schimp, V., Malone, J. M., Jr., et al. (2005). Expression of cyclooxygenase-2 in advanced stage ovarian serous carcinoma: correlation with tumor cell proliferation, apoptosis, angiogenesis, and survival. American Journal of Obstetrics and Gynecology, 192(3), 819–825.
Ferrandina, G., Lauriola, L., Distefano, M. G., Zannoni, G. F., Gessi, M., Legge, F., et al. (2002). Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. Journal of Clinical Oncology, 20(4), 973–981.
Munkarah, A. R., Morris, R., Baumann, P., Deppe, G., Malone, J., Diamond, M. P., et al. (2002). Effects of prostaglandin E(2) on proliferation and apoptosis of epithelial ovarian cancer cells. Journal of the Society for Gynecologic Investigation, 9(3), 168–173.
Rask, K., Zhu, Y., Wang, W., Hedin, L., & Sundfeldt, K. (2006). Ovarian epithelial cancer: a role for PGE2-synthesis and signalling in malignant transformation and progression. Molecular Cancer, 5, 62.
Obermajer, N., Muthuswamy, R., Odunsi, K., Edwards, R. P., & Kalinski, P. (2011). PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Research, 71(24), 7463–7470.
Naka, T., Nishimoto, N., & Kishimoto, T. (2002). The paradigm of IL-6: from basic science to medicine. Arthritis Research, 4(Suppl 3), S233–S242.
Nash, M. A., Ferrandina, G., Gordinier, M., Loercher, A., & Freedman, R. S. (1999). The role of cytokines in both the normal and malignant ovary. Endocrine-Related Cancer, 6(1), 93–107.
Nilsson, M. B., Langley, R. R., & Fidler, I. J. (2005). Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Research, 65(23), 10794–10800.
Garg, R., Wollan, M., Galic, V., Garcia, R., Goff, B. A., Gray, H. J., et al. (2006). Common polymorphism in interleukin 6 influences survival of women with ovarian and peritoneal carcinoma. Gynecologic Oncology, 103(3), 793–796.
Plante, M., Rubin, S. C., Wong, G. Y., Federici, M. G., Finstad, C. L., & Gastl, G. A. (1994). Interleukin-6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer, 73(7), 1882–1888.
Tempfer, C., Zeisler, H., Sliutz, G., Haeusler, G., Hanzal, E., & Kainz, C. (1997). Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecologic Oncology, 66(1), 27–30.
Penson, R. T., Kronish, K., Duan, Z., Feller, A. J., Stark, P., Cook, S. E., et al. (2000). Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. International Journal of Gynecological Cancer, 10(1), 33–41.
Scambia, G., Testa, U., Benedetti, P. P., Foti, E., Martucci, R., Gadducci, A., et al. (1995). Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. British Journal of Cancer, 71(2), 354–356.
Wang, Y., Niu, X. L., Qu, Y., Wu, J., Zhu, Y. Q., Sun, W. J., et al. (2010). Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Letters, 295(1), 110–123.
Syed, V., Ulinski, G., Mok, S. C., & Ho, S. M. (2002). Reproductive hormone-induced, STAT3-mediated interleukin 6 action in normal and malignant human ovarian surface epithelial cells. Journal of the National Cancer Institute, 94(8), 617–629.
Obata, N. H., Tamakoshi, K., Shibata, K., Kikkawa, F., & Tomoda, Y. (1997). Effects of interleukin-6 on in vitro cell attachment, migration and invasion of human ovarian carcinoma. Anticancer Research, 17(1A), 337–342.
Rath, K. S., Funk, H. M., Bowling, M. C., Richards, W. E., & Drew, A. F. (2010). Expression of soluble interleukin-6 receptor in malignant ovarian tissue. American Journal of Obstetrics and Gynecology, 203(3), 230–238.
Waugh, D. J., & Wilson, C. (2008). The interleukin-8 pathway in cancer. Clinical Cancer Research, 14(21), 6735–6741.
Xu, L., & Fidler, I. J. (2000). Interleukin 8: an autocrine growth factor for human ovarian cancer. Oncology Research, 12(2), 97–106.
Merritt, W. M., Lin, Y. G., Spannuth, W. A., Fletcher, M. S., Kamat, A. A., Han, L. Y., et al. (2008). Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. Journal of the National Cancer Institute, 100(5), 359–372.
Shahzad, M. M., Arevalo, J. M., Armaiz Pena, G. N., Lu, C., Stone, R. L., Moreno-Smith, M., et al (2010). Stress effects on FOSB and interleukin-8 (IL8) driven ovarian cancer growth and metastasis. J Biol Chem
Kassim, S. K., El Salahy, E. M., Fayed, S. T., Helal, S. A., Helal, T., Azzam, E., et al. (2004). Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clinical Biochemistry, 37(5), 363–369.
Huang, S., Robinson, J. B., Deguzman, A., Bucana, C. D., & Fidler, I. J. (2000). Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Research, 60(19), 5334–5339.
Lee, L. F., Hellendall, R. P., Wang, Y., Haskill, J. S., Mukaida, N., Matsushima, K., et al. (2000). IL-8 reduced tumorigenicity of human ovarian cancer in vivo due to neutrophil infiltration. Journal of Immunology, 164(5), 2769–2775.
Ferrara, N., Gerber, H. P., & LeCouter, J. (2003). The biology of VEGF and its receptors. Nature Medicine, 9(6), 669–676.
Kryczek, I., Lange, A., Mottram, P., Alvarez, X., Cheng, P., Hogan, M., et al. (2005). CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Research, 65(2), 465–472.
Mesiano, S., Ferrara, N., & Jaffe, R. B. (1998). Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. The American Journal of Pathology, 153(4), 1249–1256.
Hu, L., Hofmann, J., Zaloudek, C., Ferrara, N., Hamilton, T., & Jaffe, R. B. (2002). Vascular endothelial growth factor immunoneutralization plus Paclitaxel markedly reduces tumor burden and ascites in athymic mouse model of ovarian cancer. The American Journal of Pathology, 161(5), 1917–1924.
Hu, L., Hofmann, J., Holash, J., Yancopoulos, G. D., Sood, A. K., & Jaffe, R. B. (2005). Vascular endothelial growth factor trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clinical Cancer Research, 11(19 Pt 1), 6966–6971.
Heitz, F., Harter, P., Barinoff, J., Beutel, B., Kannisto, P., Grabowski, J. P., et al. (2012). Bevacizumab in the treatment of ovarian cancer. Advances in Therapy, 29(9), 723–735.
Scotton, C. J., Wilson, J. L., Scott, K., Stamp, G., Wilbanks, G. D., Fricker, S., et al. (2002). Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Research, 62(20), 5930–5938.
Kajiyama, H., Shibata, K., Terauchi, M., Ino, K., Nawa, A., & Kikkawa, F. (2008). Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. International Journal of Cancer, 122(1), 91–99.
Psyrri, A., Kassar, M., Yu, Z., Bamias, A., Weinberger, P. M., Markakis, S., et al. (2005). Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clinical Cancer Research, 11(24 Pt 1), 8637–8643.
Gui, T. and Shen, K. (2012). The epidermal growth factor receptor as a therapeutic target in epithelial ovarian cancer. Cancer Epidemiol
Schilder, R. J., Pathak, H. B., Lokshin, A. E., Holloway, R. W., Alvarez, R. D., Aghajanian, C., et al. (2009). Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash. Gynecologic Oncology, 113(1), 21–27.
Ahmed, N., Maines-Bandiera, S., Quinn, M. A., Unger, W. G., Dedhar, S., & Auersperg, N. (2006). Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. American Journal of Physiology. Cell Physiology, 290(6), C1532–C1542.
Colomiere, M., Ward, A. C., Riley, C., Trenerry, M. K., Cameron-Smith, D., Findlay, J., et al. (2009). Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. British Journal of Cancer, 100(1), 134–144.
Balkwill, F. (2009). Tumour necrosis factor and cancer. Nature Reviews Cancer, 9(5), 361–371.
Naylor, M. S., Stamp, G. W., Foulkes, W. D., Eccles, D., & Balkwill, F. R. (1993). Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. Journal Clinical Investigation, 91(5), 2194–2206.
Kulbe, H., Thompson, R., Wilson, J. L., Robinson, S., Hagemann, T., Fatah, R., et al. (2007). The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Research, 67(2), 585–592.
Charles, K. A., Kulbe, H., Soper, R., Escorcio-Correia, M., Lawrence, T., Schultheis, A., et al. (2009). The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. The Journal of Clinical Investigation, 119(10), 3011–3023.
Elliott, R. L., & Blobe, G. C. (2005). Role of transforming growth factor Beta in human cancer. Journal of Clinical Oncology, 23(9), 2078–2093.
Nakanishi, Y., Kodama, J., Yoshinouchi, M., Tokumo, K., Kamimura, S., Okuda, H., et al. (1997). The expression of vascular endothelial growth factor and transforming growth factor-beta associates with angiogenesis in epithelial ovarian cancer. International Journal of Gynecological Pathology, 16(3), 256–262.
Rodriguez, G. C., Haisley, C., Hurteau, J., Moser, T. L., Whitaker, R., Bast, R. C., Jr., et al. (2001). Regulation of invasion of epithelial ovarian cancer by transforming growth factor-beta. Gynecologic Oncology, 80(2), 245–253.
Thery, C., Zitvogel, L., & Amigorena, S. (2002). Exosomes: composition, biogenesis and function. Nature Reviews Immunology, 2(8), 569–579.
Peng, P., Yan, Y., & Keng, S. (2011). Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncology Reports, 25(3), 749–762.
Keller, S., Konig, A. K., Marme, F., Runz, S., Wolterink, S., Koensgen, D., et al. (2009). Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Letters, 278(1), 73–81.
Taylor, D. D., & Gercel-Taylor, C. (2005). Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. British Journal of Cancer, 92(2), 305–311.
Cho, J. A., Park, H., Lim, E. H., Kim, K. H., Choi, J. S., Lee, J. H., et al. (2011). Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecologic Oncology, 123(2), 379–386.
Liang, B., Peng, P., Chen, S., Li, L., Zhang, M., Cao, D., et al. (2013). Characterization and proteomic analysis of ovarian cancer-derived exosomes. Journal of Proteomics, 80C, 171–182.
Taylor, D. D., & Gercel-Taylor, C. (2008). MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecologic Oncology, 110(1), 13–21.
Escrevente, C., Keller, S., Altevogt, P., & Costa, J. (2011). Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer, 11, 108.
Yin, J., Yan, X., Yao, X., Zhang, Y., Shan, Y., Mao, N., et al. (2012). Secretion of annexin A3 from ovarian cancer cells and its association with platinum resistance in ovarian cancer patients. Journal of Cellular and Molecular Medicine, 16(2), 337–348.
Elmasri, W. M., Casagrande, G., Hoskins, E., Kimm, D., & Kohn, E. C. (2009). Cell adhesion in ovarian cancer. Cancer Treatment and Research, 149, 297–318.
Sodek, K. L., Ringuette, M. J., & Brown, T. J. (2007). MT1-MMP is the critical determinant of matrix degradation and invasion by ovarian cancer cells. British Journal of Cancer, 97(3), 358–367.
Davidson, B., Goldberg, I., Gotlieb, W. H., Kopolovic, J., Ben Baruch, G., Nesland, J. M., et al. (1999). High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clinical and Experimental Metastasis, 17(10), 799–808.
Davidson, B., Goldberg, I., Gotlieb, W. H., Kopolovic, J., Ben Baruch, G., Nesland, J. M., et al. (2002). The prognostic value of metalloproteinases and angiogenic factors in ovarian carcinoma. Molecular and Cellular Endocrinology, 187(1–2), 39–45.
Kamat, A. A., Fletcher, M., Gruman, L. M., Mueller, P., Lopez, A., Landen, C. N., Jr., et al. (2006). The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clinical Cancer Research, 12(6), 1707–1714.
Podhajcer, O. L., Benedetti, L. G., Girotti, M. R., Prada, F., Salvatierra, E., & Llera, A. S. (2008). The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Reviews, 27(4), 691–705.
Mok, S. C., Chan, W. Y., Wong, K. K., Muto, M. G., & Berkowitz, R. S. (1996). SPARC, an extracellular matrix protein with tumor-suppressing activity in human ovarian epithelial cells. Oncogene, 12(9), 1895–1901.
Yiu, G. K., Chan, W. Y., Ng, S. W., Chan, P. S., Cheung, K. K., Berkowitz, R. S., et al. (2001). SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. The American Journal of Pathology, 159(2), 609–622.
Said, N. A., Najwer, I., Socha, M. J., Fulton, D. J., Mok, S. C., & Motamed, K. (2007). SPARC inhibits LPA-mediated mesothelial-ovarian cancer cell crosstalk. Neoplasia, 9(1), 23–35.
Said, N., & Motamed, K. (2005). Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. The American Journal of Pathology, 167(6), 1739–1752.
Said, N., Socha, M. J., Olearczyk, J. J., Elmarakby, A. A., Imig, J. D., & Motamed, K. (2007). Normalization of the ovarian cancer microenvironment by SPARC. Molecular Cancer Research, 5(10), 1015–1030.
Chang, M. C., Chen, C. A., Chen, P. J., Chiang, Y. C., Chen, Y. L., Mao, T. L., et al. (2012). Mesothelin enhances invasion of ovarian cancer by inducing MMP-7 through MAPK/ERK and JNK pathways. The Biochemical Journal, 442(2), 293–302.
Anttila, M. A., Tammi, R. H., Tammi, M. I., Syrjanen, K. J., Saarikoski, S. V., & Kosma, V. M. (2000). High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Research, 60(1), 150–155.
Voutilainen, K., Anttila, M., Sillanpaa, S., Tammi, R., Tammi, M., Saarikoski, S., et al. (2003). Versican in epithelial ovarian cancer: relation to hyaluronan, clinicopathologic factors and prognosis. International Journal of Cancer, 107(3), 359–364.
Ween, M. P., Oehler, M. K., & Ricciardelli, C. (2011). Role of versican, hyaluronan and CD44 in ovarian cancer metastasis. International Journal of Molecular Sciences, 12(2), 1009–1029.
Fogel, M., Gutwein, P., Mechtersheimer, S., Riedle, S., Stoeck, A., Smirnov, A., et al. (2003). L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet, 362(9387), 869–875.
Stoeck, A., Gast, D., Sanderson, M. P., Issa, Y., Gutwein, P., & Altevogt, P. (2007). L1-CAM in a membrane-bound or soluble form augments protection from apoptosis in ovarian carcinoma cells. Gynecologic Oncology, 104(2), 461–469.
Davidson, B., Goldberg, I., Gotlieb, W. H., Kopolovic, J., Risberg, B., Ben-Baruch, G., et al. (2003). Coordinated expression of integrin subunits, matrix metalloproteinases (MMP), angiogenic genes and Ets transcription factors in advanced-stage ovarian carcinoma: a possible activation pathway? Cancer Metastasis Reviews, 22(1), 103–115.
Shibata, K., Kikkawa, F., Nawa, A., Suganuma, N., & Hamaguchi, M. (1997). Fibronectin secretion from human peritoneal tissue induces Mr 92,000 type IV collagenase expression and invasion in ovarian cancer cell lines. Cancer Research, 57(23), 5416–5420.
Hapke, S., Kessler, H., Luber, B., Benge, A., Hutzler, P., Hofler, H., et al. (2003). Ovarian cancer cell proliferation and motility is induced by engagement of integrin alpha(v)beta3/Vitronectin interaction. Biological Chemistry, 384(7), 1073–1083.
Folkman, J., Watson, K., Ingber, D., & Hanahan, D. (1989). Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature, 339(6219), 58–61.
Carmeliet, P., & Jain, R. K. (2000). Angiogenesis in cancer and other diseases. Nature, 407(6801), 249–257.
Schmid, M. C., & Varner, J. A. (2010). Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. Journal of Oncology, 2010, 201026.
Su, Y., Zheng, L., Wang, Q., Li, W., Cai, Z., Xiong, S., et al. (2010). Quantity and clinical relevance of circulating endothelial progenitor cells in human ovarian cancer. Journal of Experimental & Clinical Cancer Research, 29, 27.
Peichev, M., Naiyer, A. J., Pereira, D., Zhu, Z., Lane, W. J., Williams, M., et al. (2000). Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood, 95(3), 952–958.
Collino, F., Revelli, A., Massobrio, M., Katsaros, D., Schmitt-Ney, M., Camussi, G., et al. (2009). Epithelial–mesenchymal transition of ovarian tumor cells induces an angiogenic monocyte cell population. Experimental Cell Research, 315(17), 2982–2994.
Alvero, A. B., Fu, H. H., Holmberg, J., Visintin, I., Mor, L., Marquina, C. C., et al. (2009). Stem-like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells, 27(10), 2405–2413.
Kusumbe, A. P., Mali, A. M., & Bapat, S. A. (2009). CD133-expressing stem cells associated with ovarian metastases establish an endothelial hierarchy and contribute to tumor vasculature. Stem Cells, 27(3), 498–508.
Liby, T. A., Spyropoulos, P., Buff, L. H., Eldridge, J., Beeson, C., Hsu, T., et al. (2012). Akt3 controls vascular endothelial growth factor secretion and angiogenesis in ovarian cancer cells. International Journal of Cancer, 130(3), 532–543.
Su, Y., Zheng, L., Wang, Q., Bao, J., Cai, Z., & Liu, A. (2010). The PI3K/Akt pathway upregulates Id1 and integrin alpha4 to enhance recruitment of human ovarian cancer endothelial progenitor cells. BMC Cancer, 10, 459.
Keyes, K. A., Mann, L., Teicher, B., & Alvarez, E. (2003). Site-dependent angiogenic cytokine production in human tumor xenografts. Cytokine, 21(2), 98–104.
Li, L., Wang, L., Zhang, W., Tang, B., Zhang, J., Song, H., et al. (2004). Correlation of serum VEGF levels with clinical stage, therapy efficacy, tumor metastasis and patient survival in ovarian cancer. Anticancer Research, 24(3b), 1973–1979.
Nascimento, I., Schaer, R., Lemaire, D., Freire, S., Paule, B., Carvalho, S., et al. (2004). Vascular endothelial growth factor (VEGF) levels as a tool to discriminate between malignant and nonmalignant ascites. APMIS, 112(9), 585–587.
Spannuth, W. A., Nick, A. M., Jennings, N. B., Armaiz-Pena, G. N., Mangala, L. S., Danes, C. G., et al. (2009). Functional significance of VEGFR-2 on ovarian cancer cells. International Journal of Cancer, 124(5), 1045–1053.
Ghosh, S., Albitar, L., LeBaron, R., Welch, W. R., Samimi, G., Birrer, M. J., et al. (2010). Up-regulation of stromal versican expression in advanced stage serous ovarian cancer. Gynecologic Oncology, 119(1), 114–120.
Lin, Y. G., Han, L. Y., Kamat, A. A., Merritt, W. M., Landen, C. N., Deavers, M. T., et al. (2007). EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer, 109(2), 332–340.
Hagemann, T., Robinson, S. C., Thompson, R. G., Charles, K., Kulbe, H., & Balkwill, F. R. (2007). Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Molecular Cancer Therapeutics, 6(7), 1993–2002.
Yabushita, H., Shimazu, M., Noguchi, M., Kishida, T., Narumiya, H., Sawaguchi, K., et al. (2003). Vascular endothelial growth factor activating matrix metalloproteinase in ascitic fluid during peritoneal dissemination of ovarian cancer. Oncology Reports, 10(1), 89–95.
Belotti, D., Calcagno, C., Garofalo, A., Caronia, D., Riccardi, E., Giavazzi, R., et al. (2008). Vascular endothelial growth factor stimulates organ-specific host matrix metalloproteinase-9 expression and ovarian cancer invasion. Molecular Cancer Research, 6(4), 525–534.
Sood, A. K., Seftor, E. A., Fletcher, M. S., Gardner, L. M., Heidger, P. M., Buller, R. E., et al. (2001). Molecular determinants of ovarian cancer plasticity. The American Journal of Pathology, 158(4), 1279–1288.
Wang, L., Madigan, M. C., Chen, H., Liu, F., Patterson, K. I., Beretov, J., et al. (2009). Expression of urokinase plasminogen activator and its receptor in advanced epithelial ovarian cancer patients. Gynecologic Oncology, 114(2), 265–272.
Agarwal, A., Tressel, S. L., Kaimal, R., Balla, M., Lam, F. H., Covic, L., et al. (2010). Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: implications for antiangiogenic therapy. Cancer Research, 70(14), 5880–5890.
Sonoda, T., Kobayashi, H., Kaku, T., Hirakawa, T., & Nakano, H. (2003). Expression of angiogenesis factors in monolayer culture, multicellular spheroid and in vivo transplanted tumor by human ovarian cancer cell lines. Cancer Letters, 196(2), 229–237.
Zhang, Y., Tang, H., Cai, J., Zhang, T., Guo, J., Feng, D., et al. (2011). Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Letters, 303(1), 47–55.
Castells, M., Thibault, B., Mery, E., Golzio, M., Pasquet, M., Hennebelle, I., et al. (2012). Ovarian ascites-derived Hospicells promote angiogenesis via activation of macrophages. Cancer Letters, 326(1), 59–68.
Jeon, E. S., Heo, S. C., Lee, I. H., Choi, Y. J., Park, J. H., Choi, K. U., et al. (2010). Ovarian cancer-derived lysophosphatidic acid stimulates secretion of VEGF and stromal cell-derived factor-1 alpha from human mesenchymal stem cells. Experimental and Molecular Medicine, 42(4), 280–293.
Schmitt, J., & Matei, D. (2012). Targeting angiogenesis in ovarian cancer. Cancer Treatment Reviews, 38(4), 272–283.
Burger, R. A., Brady, M. F., Bookman, M. A., Fleming, G. F., Monk, B. J., Huang, H., et al. (2011). Incorporation of bevacizumab in the primary treatment of ovarian cancer. The New England Journal of Medicine, 365(26), 2473–2483.
Perren, T. J., Swart, A. M., Pfisterer, J., Ledermann, J. A., Pujade-Lauraine, E., Kristensen, G., et al. (2011). A phase 3 trial of bevacizumab in ovarian cancer. The New England Journal of Medicine, 365(26), 2484–2496.
Yang, G., Rosen, D. G., Zhang, Z., Bast, R. C., Jr., Mills, G. B., Colacino, J. A., et al. (2006). The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America, 103(44), 16472–16477.
Lawrenson, K., Grun, B., Benjamin, E., Jacobs, I. J., Dafou, D., & Gayther, S. A. (2010). Senescent fibroblasts promote neoplastic transformation of partially transformed ovarian epithelial cells in a three-dimensional model of early stage ovarian cancer. Neoplasia, 12(4), 317–325.
Noskova, V., Ahmadi, S., Asander, E., & Casslen, B. (2009). Ovarian cancer cells stimulate uPA gene expression in fibroblastic stromal cells via multiple paracrine and autocrine mechanisms. Gynecologic Oncology, 115(1), 121–126.
Westerlund, A., Hujanen, E., Puistola, U., & Turpeenniemi-Hujanen, T. (1997). Fibroblasts stimulate human ovarian cancer cell invasion and expression of 72-kDa gelatinase A (MMP-2). Gynecologic Oncology, 67(1), 76–82.
Boyd, R. S., & Balkwill, F. R. (1999). MMP-2 release and activation in ovarian carcinoma: the role of fibroblasts. British Journal of Cancer, 80(3–4), 315–321.
Kenny, H. A., Krausz, T., Yamada, S. D., & Lengyel, E. (2007). Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. International Journal of Cancer, 121(7), 1463–1472.
Cai, J., Tang, H., Xu, L., Wang, X., Yang, C., Ruan, S., et al. (2012). Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis, 33(1), 20–29.
Xing, F., Saidou, J., & Watabe, K. (2010). Cancer associated fibroblasts (CAFs) in tumor microenvironment. Frontiers in Bioscience, 15, 166–179.
Yao, Q., Qu, X., Yang, Q., Wei, M., & Kong, B. (2009). CLIC4 mediates TGF-beta1-induced fibroblast-to-myofibroblast transdifferentiation in ovarian cancer. Oncology Reports, 22(3), 541–548.
Lai, D., Ma, L., & Wang, F. (2012). Fibroblast activation protein regulates tumor-associated fibroblasts and epithelial ovarian cancer cells. International Journal of Oncology, 41(2), 541–550.
Cannistra, S. A. (2004). Cancer of the ovary. The New England Journal of Medicine, 351(24), 2519–2529.
Niedbala, M. J., Crickard, K., & Bernacki, R. J. (1985). Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix. An in vitro model system for studying tumor cell adhesion and invasion. Experimental Cell Research, 160(2), 499–513.
Lessan, K., Aguiar, D. J., Oegema, T., Siebenson, L., & Skubitz, A. P. (1999). CD44 and beta1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. The American Journal of Pathology, 154(5), 1525–1537.
Cannistra, S. A., Kansas, G. S., Niloff, J., DeFranzo, B., Kim, Y., & Ottensmeier, C. (1993). Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Research, 53(16), 3830–3838.
Strobel, T., Swanson, L., & Cannistra, S. A. (1997). In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: a novel role for CD44 in the process of peritoneal implantation. Cancer Research, 57(7), 1228–1232.
Yeo, T. K., Nagy, J. A., Yeo, K. T., Dvorak, H. F., & Toole, B. P. (1996). Increased hyaluronan at sites of attachment to mesentery by CD44-positive mouse ovarian and breast tumor cells. The American Journal of Pathology, 148(6), 1733–1740.
Burleson, K. M., Casey, R. C., Skubitz, K. M., Pambuccian, S. E., Oegema, T. R., Jr., & Skubitz, A. P. (2004). Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecologic Oncology, 93(1), 170–181.
Offner, F. A., Obrist, P., Stadlmann, S., Feichtinger, H., Klingler, P., Herold, M., et al. (1995). IL-6 secretion by human peritoneal mesothelial and ovarian cancer cells. Cytokine, 7(6), 542–547.
Cronauer, M. V., Stadlmann, S., Klocker, H., Abendstein, B., Eder, I. E., Rogatsch, H., et al. (1999). Basic fibroblast growth factor synthesis by human peritoneal mesothelial cells: induction by interleukin-1. The American Journal of Pathology, 155(6), 1977–1984.
Stadlmann, S., Amberger, A., Pollheimer, J., Gastl, G., Offner, F. A., Margreiter, R., et al. (2005). Ovarian carcinoma cells and IL-1beta-activated human peritoneal mesothelial cells are possible sources of vascular endothelial growth factor in inflammatory and malignant peritoneal effusions. Gynecologic Oncology, 97(3), 784–789.
Ren, J., Xiao, Y. J., Singh, L. S., Zhao, X., Zhao, Z., Feng, L., et al. (2006). Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Research, 66(6), 3006–3014.
Uccelli, A., Moretta, L., & Pistoia, V. (2008). Mesenchymal stem cells in health and disease. Nature Reviews Immunology, 8(9), 726–736.
Jiang, J., Chen, W., Zhuang, R., Song, T., & Li, P. (2010). The effect of endostatin mediated by human mesenchymal stem cells on ovarian cancer cells in vitro. Journal of Cancer Research and Clinical Oncology, 136(6), 873–881.
Jeon, E. S., Moon, H. J., Lee, M. J., Song, H. Y., Kim, Y. M., Cho, M., et al. (2008). Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells. Stem Cells, 26(3), 789–797.
Lis, R., Touboul, C., Mirshahi, P., Ali, F., Mathew, S., Nolan, D. J., et al (2010). Tumor associated mesenchymal stem cells protects ovarian cancer cells from hyperthermia through CXCL12. Int J Cancer
McLean, K., Gong, Y., Choi, Y., Deng, N., Yang, K., Bai, S., et al. (2011). Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. The Journal of Clinical Investigation, 121(8), 3206–3219.
Rafii, A., Mirshahi, P., Poupot, M., Faussat, A. M., Simon, A., Ducros, E., et al. (2008). Oncologic trogocytosis of an original stromal cells induces chemoresistance of ovarian tumours. PLoS One, 3(12), e3894.
Pasquet, M., Golzio, M., Mery, E., Rafii, A., Benabbou, N., Mirshahi, P., et al. (2010). Hospicells (ascites-derived stromal cells) promote tumorigenicity and angiogenesis. International Journal of Cancer, 126(9), 2090–2101.
Castells, M., Thibault, B., Mery, E., Golzio, M., Pasquet, M., Hennebelle, I., et al (2012). Ovarian ascites-derived Hospicells promote angiogenesis via activation of macrophages. Cancer Lett
Martinet, L., Poupot, R., Mirshahi, P., Rafii, A., Fournie, J. J., Mirshahi, M., et al. (2010). Hospicells derived from ovarian cancer stroma inhibit T-cell immune responses. International Journal of Cancer, 126(9), 2143–2152.
Nieman, K. M., Kenny, H. A., Penicka, C. V., Ladanyi, A., Buell-Gutbrod, R., Zillhardt, M. R., et al. (2011). Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Medicine, 17(11), 1498–1503.
Zhang, Y., Daquinag, A. C., Amaya-Manzanares, F., Sirin, O., Tseng, C., & Kolonin, M. G. (2012). Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Research, 72(20), 5198–5208.
Altintas, M. M., Azad, A., Nayer, B., Contreras, G., Zaias, J., Faul, C., et al. (2011). Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. Journal of Lipid Research, 52(3), 480–488.
Subbaramaiah, K., Howe, L. R., Bhardwaj, P., Du, B., Gravaghi, C., Yantiss, R. K., et al. (2011). Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prevention Research (Philadelphia, Pa.), 4(3), 329–346.
Roodhart, J. M., Daenen, L. G., Stigter, E. C., Prins, H. J., Gerrits, J., Houthuijzen, J. M., et al. (2011). Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell, 20(3), 370–383.
Ko, S. Y., Barengo, N., Ladanyi, A., Lee, J. S., Marini, F., Lengyel, E., et al. (2012). HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. The Journal of Clinical Investigation, 122(10), 3603–3617.
Kidd, S., Spaeth, E., Watson, K., Burks, J., Lu, H., Klopp, A., et al. (2012). Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One, 7(2), e30563.
Protani, M., Coory, M., & Martin, J. H. (2010). Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Research and Treatment, 123(3), 627–635.
Kaaks, R., Lukanova, A., & Kurzer, M. S. (2002). Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiology, Biomarkers and Prevention, 11(12), 1531–1543.
Fotopoulou, C., Richter, R., Braicu, E. I., Kuhberg, M., Feldheiser, A., Schefold, J. C., et al. (2011). Impact of obesity on operative morbidity and clinical outcome in primary epithelial ovarian cancer after optimal primary tumor debulking. Annals of Surgical Oncology, 18(9), 2629–2637.
Reeves, G. K., Pirie, K., Beral, V., Green, J., Spencer, E., & Bull, D. (2007). Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ, 335(7630), 1134.
Protani, M. M., Nagle, C. M., & Webb, P. M. (2012). Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prevention Research (Philadelphia, Pa.), 5(7), 901–910.
Olsen, C. M., Nagle, C. M., Whiteman, D. C., Purdie, D. M., Green, A. C., & Webb, P. M. (2008). Body size and risk of epithelial ovarian and related cancers: a population-based case-control study. International Journal of Cancer, 123(2), 450–456.
Olsen, C. M., Nagle, C. M., Whiteman, D. C., Ness, R., Pearce, C. L., Pike, M. C., et al. (2013). Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocrine-Related Cancer, 20(2), 251–262.
Zhang, L., Conejo-Garcia, J. R., Katsaros, D., Gimotty, P. A., Massobrio, M., Regnani, G., et al. (2003). Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England Journal of Medicine, 348(3), 203–213.
Fialova, A., Partlova, S., Sojka, L., Hromadkova, H., Brtnicky, T., Fucikova, J., et al (2012). Dynamics of T-cell infiltration during the course of ovarian cancer: the gradual shift from a Th17 effector cell response to a predominant infiltration by regulatory T-cells. Int J Cancer
Milne, K., Alexander, C., Webb, J. R., Sun, W., Dillon, K., Kalloger, S. E., et al. (2012). Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. Journal of Translational Medicine, 10, 33.
Wei, S., Kryczek, I., Zou, L., Daniel, B., Cheng, P., Mottram, P., et al. (2005). Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Research, 65(12), 5020–5026.
Curiel, T. J., Cheng, P., Mottram, P., Alvarez, X., Moons, L., Evdemon-Hogan, M., et al. (2004). Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Research, 64(16), 5535–5538.
Huarte, E., Cubillos-Ruiz, J. R., Nesbeth, Y. C., Scarlett, U. K., Martinez, D. G., Buckanovich, R. J., et al. (2008). Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Research, 68(18), 7684–7691.
Labidi-Galy, S. I., Sisirak, V., Meeus, P., Gobert, M., Treilleux, I., Bajard, A., et al. (2011). Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Research, 71(16), 5423–5434.
Labidi-Galy, S. I., Treilleux, I., Goddard-Leon, S., Combes, J. D., Blay, J. Y., Ray-Coquard, I., et al. (2012). Plasmacytoid dendritic cells infiltrating ovarian cancer are associated with poor prognosis. Oncoimmunology, 1(3), 380–382.
Sato, E., Olson, S. H., Ahn, J., Bundy, B., Nishikawa, H., Qian, F., et al. (2005). Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America, 102(51), 18538–18543.
Hamanishi, J., Mandai, M., Iwasaki, M., Okazaki, T., Tanaka, Y., Yamaguchi, K., et al. (2007). Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America, 104(9), 3360–3365.
Lai, D., Wang, F., Chen, Y., Wang, C., Liu, S., Lu, B., et al. (2011). Human ovarian cancer stem-like cells can be efficiently killed by gammadelta T lymphocytes. Immunother: Cancer Immunol.
Peng, D. J., Liu, R., & Zou, W. (2012). Regulatory T cells in human ovarian cancer. Journal of Oncology, 2012, 345164.
Alvero, A. B., Montagna, M. K., Craveiro, V., Liu, L., & Mor, G. (2011). Distinct subpopulations of epithelial ovarian cancer cells can differentially induce macrophages and T regulatory cells toward a pro-tumor phenotype. Immunol: Am J Reprod.
Curiel, T. J., Coukos, G., Zou, L., Alvarez, X., Cheng, P., Mottram, P., et al. (2004). Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine, 10(9), 942–949.
DeNardo, D. G., Barreto, J. B., Andreu, P., Vasquez, L., Tawfik, D., Kolhatkar, N., et al. (2009). CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell, 16(2), 91–102.
Gavalas, N. G., Karadimou, A., Dimopoulos, M. A., & Bamias, A. (2010). Immune response in ovarian cancer: how is the immune system involved in prognosis and therapy: potential for treatment utilization. Clinical and Developmental Immunology, 2010, 791603.
Bettelli, E., Korn, T., Oukka, M., & Kuchroo, V. K. (2008). Induction and effector functions of T(H)17 cells. Nature, 453(7198), 1051–1057.
Miyahara, Y., Odunsi, K., Chen, W., Peng, G., Matsuzaki, J., & Wang, R. F. (2008). Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America, 105(40), 15505–15510.
Leveque, L., Deknuydt, F., Bioley, G., Old, L. J., Matsuzaki, J., Odunsi, K., et al. (2009). Interleukin 2-mediated conversion of ovarian cancer-associated CD4+ regulatory T cells into proinflammatory interleukin 17-producing helper T cells. Journal of Immunotherapy, 32(2), 101–108.
Lan, C., Huang, X., Lin, S., Huang, H., Cai, Q., Lu, J., et al (2013). High density of IL-17-producing cells is associated with improved prognosis for advanced epithelial ovarian cancer. Cell Tissue Res
Biswas, S. K., Sica, A., & Lewis, C. E. (2008). Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. Journal of Immunology, 180(4), 2011–2017.
Hagemann, T., Wilson, J., Burke, F., Kulbe, H., Li, N. F., Pluddemann, A., et al. (2006). Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. Journal of Immunology, 176(8), 5023–5032.
Robinson-Smith, T. M., Isaacsohn, I., Mercer, C. A., Zhou, M., Van, R. N., Husseinzadeh, N., et al. (2007). Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Research, 67(12), 5708–5716.
Duluc, D., Delneste, Y., Tan, F., Moles, M. P., Grimaud, L., Lenoir, J., et al. (2007). Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood, 110(13), 4319–4330.
Kryczek, I., Zou, L., Rodriguez, P., Zhu, G., Wei, S., Mottram, P., et al. (2006). B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. The Journal of Experimental Medicine, 203(4), 871–881.
Kryczek, I., Wei, S., Zhu, G., Myers, L., Mottram, P., Cheng, P., et al. (2007). Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Research, 67(18), 8900–8905.
Fridlender, Z. G., & Albelda, S. M. (2012). Tumor-associated neutrophils: friend or foe? Carcinogenesis, 33(5), 949–955.
Piccard, H., Muschel, R. J., & Opdenakker, G. (2012). On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Critical Reviews in Oncology/Hematology, 82(3), 296–309.
Gonzalez, M. A., Bratos, R., Marquez, R., Alonso, S., & Chiva, L. (2013). Bevacizumab as front-line treatment for newly diagnosed epithelial cancer. Expert Review of Anticancer Therapy, 13(2), 123–129.
Banerjee, S., & Kaye, S. B. (2013). New strategies in the treatment of ovarian cancer—current clinical perspectives and future potential. Cancer Res: Clin.
Acknowledgments
The manuscript was revised by AngloScribe, Nîmes for English language editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thibault, B., Castells, M., Delord, JP. et al. Ovarian cancer microenvironment: implications for cancer dissemination and chemoresistance acquisition. Cancer Metastasis Rev 33, 17–39 (2014). https://doi.org/10.1007/s10555-013-9456-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-013-9456-2