Abstract
Colorectal cancer is the third most common cancer worldwide with a 5-year survival of 50%. Current chemotherapeutic regimens used for advanced colorectal cancer provide an average survival of approximately 20 months. Non-toxic agents such as nutraceutics and supplements have been shown to aid in the prevention and adjuvant treatment of colorectal cancer. This article will discuss the epidemiology, progression, prevention, treatment, and recurrence of colorectal cancer and the role of nutraceutics and supplements in the treatment process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introductions
Cancer is the number two cause of death in the USA, accounting for 23% of all deaths. Colorectal cancer (CRC) is currently the third most common cancer worldwide. The geographic distribution of colorectal cancer varies dramatically. It is more common in westernized countries, which is thought to be due to the accompanying diet and lifestyle changes. Worldwide, the highest incidence rates are in North America, Australia/New Zealand, Western Europe, and Japan, especially in men. Incidence is lower in Africa and Asia, which is why screening for CRC is not typically recommended in these areas [1]. There were nearly 147,000 new cases of colorectal cancer in the USA in 2009, leading to approximately 50,000 deaths. Men and women were almost equally affected with 75,590 new cases for men and 71,380 new cases for women. The age adjusted incidence rate in the USA for men and women is currently 61.2 and 44.8 per 100,000 age adjusted population, respectively, from 2001 to 2005 [2]. Despite these dramatic numbers, the incidence of CRC has been decreasing over the past 20 years. This is the result of increased public awareness, new and improved screening modalities, and identifying those who are at high risk of the disease [2].
The current 5-year survival for all subjects diagnosed with CRC is 65%. This has steadily increased from 52% in the mid-1970s to 59% in the mid-1980s, to the current 5-year survival rate. Survival is proportional to the stage of disease at diagnosis. Localized, regional, and distant metastatic diseases have a respective 5-year survival of 90, 68, and 11% in the USA [2].
2 Screening and surveillance of colon cancer
Screening for colorectal cancer has decreased the incidence of this malignancy. The American College of Gastroenterology currently recommends a screening colonoscopy at the age of 45 for African Americans and 50 for all other races in the USA [3]. African Americans tend to develop CRC at a younger age, have a significantly higher incidence and mortality from CRC, and have more proximal colon lesions [2, 4]. If normal, the colonoscopy should be repeated every 10 years. The reason for the 10-year interval is because that is the time for the progression of normal mucosa to form an advanced adenoma or CRC [4]. Depending on the number, size, and histology of polyps if found and removed, the surveillance intervals are shortened to a few months for the piecemeal removal of a polyp to 5 years for less than three adenomatous polyps [3]. These shortened screening intervals are of utmost importance as studies have demonstrated an increased risk of CRC with an increasing number of adenomas detected and the number of polyps was found to increase linearly with age [5]. Colorectal cancer is an age-related disease. As the world’s elderly population expands, so will the development of colonic adenomas and malignancy; this is why screening is so important.
Although colonoscopy is still considered the gold standard for CRC screening, other modalities can be used. Flexible sigmoidoscopy, double contrast barium enema, and CT colonography are all other methods of CRC screening. Alternative cancer detection tests include guaiac based fecal occult blood test, fecal immunochemical test, and fecal DNA tests. The reason colonoscopy is still the gold standard despite having non-invasive tests available is because if there are any abnormalities on these other tests, a colonoscopy must be done to evaluate endoscopically and the sensitivities of the alternative screening and detection tests are comparatively poor. CT colonography, on the other hand, can produce comparable sensitivities to colonoscopy, but only for large polyps >1 cm and may miss high risk flat lesions [6].
3 Multistage progression of colorectal cancer
Colorectal cancer develops through a multistage progression of normal tissue eventually transforming into invasive cancer via genetic and environmental events. The complex combination of genetic, epigenetic, and post-genetic alterations involves the activation of oncogenes and the inactivation of tumor suppressor genes. Gastrointestinal cancers, including colorectal cancer, typically develop via a series of pathologic events preceded by benign dysplastic intermediates. There are two general pathways of colorectal carcinogenesis. The first involves chromosomal instability and the second involves microsatellite instability. Chromosomal instability accounts for about 85% of sporadic cases of CRC and all cases of familial adenomatous polyposis (FAP). This occurs when entire portions of DNA are deleted leading to abnormal amounts of DNA per cell, or aneuploidy. Microsatellite instability, the second general pathway of CRC carcinogenesis, occurs due to defects in the mismatch repair system. This accounts for 15% of sporadic cases and most cases of hereditary non-polyposis colorectal cancer (HNPCC) [7].
The earliest known lesion in the carcinogenesis of CRC is the aberrant crypt focus (ACF). This is seen endoscopically when the colonic mucosa is stained with methylene blue. ACF are large thick crypts, with an increased luminal diameter having a serrated or slit-like opening. This is followed by the formation of an adenoma. As epithelial cells acquire abnormal growth patterns, the adenoma is formed. This is a tumor or mass that often protrudes into the lumen of the colon, but may be flat as well. More recent data show that the flat adenomas are actually more likely to be located on the right side of the colon and confer a greater risk of neoplastic transformation. In time, these adenomas may enlarge and a subset of cells can acquire additional abnormal growth factors which allow them to invade into the colorectal wall thus becoming malignant and eventually metastasize.
In the multistep process of colon carcinogenesis, somatic mutations develop in key genes such as the adenomatous polyposis coli (APC) gene, p53 tumor suppressor genes, K-Ras oncogene, and various genes that mediate DNA mismatch repair. Adenoma formation begins with a loss of function mutation involving the APC tumor suppressor gene, followed by activation of the K-Ras oncogene [8]. The APC gene is considered the gatekeeper of colonic carcinogenesis as its mutation is responsible for the progression of normal colonic tissue to an early adenoma. Germline mutations of APC are responsible for FAP [9]. The APC gene is responsible for differentiation, adhesion, polarity, migration, development, apoptosis, and even chromosomal segregation [9]. A malfunctioning APC gene allows for the accumulation of β-catenin which then enters the nucleus to trigger the cell cycle [10]. The APC mutation is present in approximately 5% of ACF, 50% of sporadic adenomas and 75% of sporadic colorectal cancers [8, 10].
K-ras is a proto-oncogene that is found to be mutated in approximately 50% of CRC. It is involved in the transduction of mitogenic signals across the cell membrane. K-ras activation has the ability to transform an early adenoma into an intermediate or larger adenoma. K-ras mutations are found in only 9% of small adenomas compared with 58% of larger adenomas >1 cm and 47% of colon cancers.
Subsequent loss of function of genes on chromosome 18q, identified in up to 70% of primary CRCs, and inactivation of p53 brings upon the malignant transformation [7]. The loss of p53 is critical for the transition from adenoma to early carcinoma. The p53 gene, located on the short arm of chromosome 17, normally has the ability to arrest the cell cycle to permit DNA repair, or if the damage to the DNA is too great, cause apoptosis of the cell. The p53 deletion is detected in 50% of lesions displaying high-grade dysplasia and 75% of CRCs [7].
This second major molecular pathway of colorectal carcinogenesis involves microsatellite instability, or the mutation of enzymes involved in DNA mismatch repair (MMR). This is found in 15% of sporadic CRCs, but most of the CRCs associated with HNPCC. Mutations in the MMR genes lead to accumulations of uncorrected replication errors that can affect cell growth. Microsatellites such as poly-A tracts or CA-repeats are particularly vulnerable targets.
The progression from normal mucosa to adenoma and finally adenocarcinoma typically takes 7–10 years, as depicted in Fig. 1. This multistage progression of carcinogenesis is the basis for many chemotherapeutic interventions [9].
Schematic representation of adenoma-to-carcinoma sequence (Reproduced from Kanwar SS, Nautiyal J, Majumdar AP [13], by permission)
4 Prevention
Primary prevention involves three distinct interventions, the aim of which is the prevention of polyp or adenoma formation, since adenomas may progress or transform into malignancy. Firstly, the avoidance of harmful substances like dietary fat. Secondly, is the use of safe medications or nutraceutics/supplements to prevent polyp formation. Lastly, prophylactic colectomy in patients at a high risk of CRC like those with FAP. The problem with primary prevention is the lifestyle changes that accompany a healthier lifestyle. Whether it involves diet, exercise, avoidance of harmful substances like smoking and alcohol use, medications to take on a daily basis, or at the more extreme end, a surgical colectomy if absolutely needed, these changes require compliance from the patient. These changes to one’s life are difficult for the general population to undertake which is why the primary prevention of adenomas is so difficult [11–13].
Secondary prevention, on the other hand, involves halting the transformation of an adenoma into malignancy. This is accomplished with screening colonoscopy to remove any polyps visualized. Currently, most of the CRC prevention rests in the hands of the endoscopist, but colonoscopy, although standard of care, is not a perfect test. Depending on the prep quality and the experience of the gastroenterologist, up to 24% of polyps can be missed on colonoscopy [12, 14]. Additionally, it has been reported that cancer stem cells are present in adenomas and normal mucosa of the elderly [5]. These cancer stem cells, as evidenced by the expression of their markers, were also found to be about 2-fold higher in subjects with 3–4 polyps compared with those with only 1–2 polyps [5]. These and other relevant observations raise the necessity for primary prevention of CRC.
5 Diagnosis and staging of colon cancer
The diagnosis of colorectal cancer is based on histology. The symptoms leading to the diagnostic work up vary. Some of the more common signs and symptoms include abdominal pain, change in bowel habits, hematochezia or melena, weakness, anemia, and weight loss. The symptoms typically begin a diagnostic work up that lead to an evaluation for GI malignancy.
The TNM staging system is preferred for colorectal cancer. Stages 0, I, and II have tumors with varying degrees of invasion without lymph node involvement or metastatic lesions. Stage III colon cancer has lymph node involvement without metastasis. Stage IV colon cancer encompasses any size of tumor and any number of lymph node involvement, but has metastatic lesions.
As seen in Fig. 2 below, most cases of CRC are sporadic. Less than 5% of the cases have an inherited genetic predisposition, such as FAP or HNPCC. Patients may have an increased risk of CRC by having family members with CRC, especially first-degree family members diagnosed with CRC at a young age (<60 years old) [14].
The genetic make-up of colorectal cancer, adapted from Colorectal cancer screening [14]
6 Treatment/recurrence of colon cancer
The backbone of colorectal cancer treatment is surgery, which provides the only hope of a cure. The cure rates and survival are directly proportional to the stage of the malignancy at the time of diagnosis. The treatment for stages 0, I, II, and III involves surgery, with or without chemotherapy. Stage IV is treated mainly with chemotherapy, sometimes with palliative surgery, and has an average survival of approximately 20 months with the use of 5-fluorouracil, oxaliplatin and/or leucovorin (FOLFOX), which is the mainstay for CRC chemotherapy. The other current chemotherapeutic agents for advanced colon cancer are 5-fluorouracil (5-FU) alone, 5-FU/leucovorin, 5-FU/levamisole, 5-FU/leucovorin/levamisole, and more recently oxaliplatin/irinotecan [15]. Despite surgical resection of the primary tumor, if the cancer invades the colonic serosa or involves lymph nodes, the risk of relapse following surgery is 20–30% for stage II and 50–80% for stage III disease [15]. Rectal cancer differs in that radiation therapy is added to the treatments and is often done prior to surgery. Rectal cancer has similar recurrence rates with stage II recurring at a rate of 25–30% after primary resection and stage III at a rate of 50% or greater [15]. Overall, approximately 50% of advanced colon cancers will recur [15].
The reason for colon cancer recurrence is much debated. One theory is the concept of cancer stem cells (CSCs). Cancer stem cells are found in many epithelial cancers, including CRC. They possess the ability for self-renewal, have a long life, and have the potential to repopulate the tumor by developing into any cell in the tumor population. In the colon, the mucosal stem cells are located at the base of the crypts and may accumulate mutations leading to tumor production [10]. We have recently reported that the age-related increase in adenomatous polyps is accompanied by the concomitant increase in cancer stem cells in macroscopically normal mucosa indicating a predisposition of the organ to developing CRC [5]. Cancer stem cells by nature are slower to divide and therefore resistant to chemotherapeutic agents that target rapidly growing and differentiating cells. This resistance to adjuvant chemotherapy can then promote the evolution of resistant clonal cancer cells [10]. In studies done by Yu et al., colon cancer cell lines HCT-116 and HT-29 that were exposed to FOLFOX did inhibit the cell line growth as expected, but enhanced the CSC phenotype as evidenced by the rise in the proportion of CD133-, CD44-, and/or CD166- positive cells and epidermal growth factor receptor levels suggesting the CSC’s were resistant to the chemotherapy [16]. The failure to eliminate the CSCs may be contributing to CRC recurrence. However, continued use of chemotherapy can lead to additional toxicities, some of which are fatal. Therefore, validation of a non-toxic agent(s) that could improve upon the current chemotherapeutic regimen(s) would be highly desirable. To this end, efforts are being made to use a number of dietary supplements or nutraceutics to target this unique subpopulation of cancer cells.
7 Nutraceutics
Nutraceutics, just as the term implies, combines concepts of nutrition and pharmaceutics. The term was first coined in 1989 by Stephen DeFelice, MD [17]. This broad term refers to any product derived from food that can be used for health and medicine. Nutraceutics are now a multi-billion dollar industry that, with the advent of the internet, has exploded over the past 15 years. They are often incorrectly lumped in the same category as supplements. However nutraceuticals differ from supplements in that nutraceutics must not only supplement the diet, but also aid in the prevention or treatment of a disease [17]. Nutraceutics have gained attention over the past few years regarding the prevention and adjuvant treatment of cancer as well.
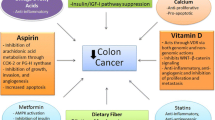
Although surgery is the mainstay for treatment of most colorectal cancers, chemotherapy is often added to surgery or used as a single treatment modality for more advanced malignancies. Chemotherapy, whether used alone or with surgery, has limitations regarding toxicity and effectiveness. Despite the use of surgery and chemotherapy, CRC recurrence remains a major issue. Recurrence is likely related to cancer stem cells and the inability of currently used chemotherapeutic agents to eradicate them. Adding additional chemotherapeutic agents would be ideal; however, given the toxicity of chemotherapeutic agents, this approach is limited. Table 1 lists the nutraceutics and supplements that are associated with the prevention or treatment of various malignancies [18–25]. The perfect addition would be a non-toxic agent that could improve on the current chemotherapeutic regimen. Below, we will discuss how three nutraceutics (folate, vitamin D, and calcium) and one supplement (curcumin) may play a role in the prevention and/or treatment of colorectal cancer.
8 Folate
Folate is a naturally occurring water soluble B vitamin found in many green leafy vegetables like asparagus, broccoli, citrus fruits, and legumes. Since 1998, folate has been fortified into uncooked grain cereals and flour in the USA. Folate is an essential nutrient that cannot be synthesized by the human body and therefore must be ingested from an exogenous source. Folate is different from folic acid. Folates are naturally occurring and rather unstable. The nutritional gain of folate is typically lost in the harvesting, preparation, and cooking of food. Folic acid, on the other hand, has a high bioavailability when ingested as a medication. The dose of folic acid fortification is 140 μg/100 g, which will typically provide on average 400 μg of folic acid per day. Approximately half of this 400 μg comes from naturally occurring folate while the other half from enriched fortified products [26]. Humans need to absorb approximately 50–100 μg of folate per day to replenish the daily degradation and loss through urine and bile [18].
The role of folate in the human body is to mediate and chemically activate the transfer of one-carbon units. Folate is essential for the synthesis of DNA, RNA, and the metabolism of various amino acids [27, 28]. It is absorbed across the lumen of the proximal jejunum via active and passive transport. Folate is present in the plasma mainly as 5-methyltetrahydrofolate (5-methyl tetrahydrofolic acid (THFA). Once inside the cells, 5-methyl THFA is demethylated to THFA, which is the biologically active form participating in folate dependent enzymatic reactions [18].
Folate is used in two general pathways. The first is the production of purine precursors for DNA biosynthesis and the second is the production and utilization of the methyl group donor S-adenosylmethionine for cellular methylation reactions [19]. S-adenosylmethionine is the primary methyl donor for most biologic methylation reactions in the human body including the methylation of DNA and RNA [18, 20, 26].
An inadequate supply of folate may increase the risk of CRC. There are currently two leading hypotheses for this association. First, a low folate level may interfere with gene expression leading to impaired DNA repair. The second hypothesis is that a low folate pool may cause uracil mis-incorporation into the DNA leading to DNA breaks and instability.
The role of folate in the genesis of colorectal cancer was first suggested in patients with ulcerative colitis (UC). Patients with UC have a 10–40-fold increased risk over the general population of developing colorectal cancer. These UC patients were noted to have low folate levels due to a combination of sulfasalazine use (which is a folate antagonist), decreased oral intake, or intestinal losses from chronic inflammation. Although retrospective, Lashner et al. found a relationship between low folate levels and an increased risk of colorectal dysplasia. The results were not significant, but did demonstrate a trend that has been further studied [21].
In rat models, Cravo et al. showed that 100% of male rats fed a folate deficient diet developed microscopic CRC 20 weeks after the injection of dimethylhydrazine, a well known chemical carcinogen that causes CRC. This was compared with only 29% of the rats fed a high folate diet (8 mg/kg). The incidence of macroscopic colorectal neoplasm in the folate sufficient group was 43% compared with 86% in the folate deficient group [22]. This has been further evaluated in human studies. Several retrospective and prospective studies have evaluated the relationship between folate and CRC in the general population, which have generally shown an inverse relationship. However, some results have shown the opposite. In a meta-analysis done in 2004 evaluating seven cohort and nine case control studies, Larsson et al. found that among the cohort studies there was a statistically significant 25% lower risk of CRC among those in the highest category of folate supplementation compared with those with the lowest amount of folate intake. Among the case control studies, there was a 5% lower risk of CRC, however there was a greater amount of heterogeneity among these studies [23].
This concept that folate may act as a chemopreventive agent has also been applied to the prevention of colonic adenomas, the precursors to colorectal cancer. Although a smaller study, in a double-blind, placebo controlled trial, Jaszewski et al. found that high-dose folic acid supplementation (5 mg daily) was associated with a significant reduction in the recurrence of colonic adenomas after 3 years [24]. Specifically, the recurrence of adenoma at the 3-year follow-up colonoscopy was twice as high in the placebo group compared with the folic acid treatment group receiving 5 mg of folic acid per day. Breaking down the types of adenomas, the treatment group also had a statistically significant decline in advanced adenomas (adenomas >1 cm or high-grade dysplasia) compared with placebo (p value 0.02335). In this study, Jaszewski et al. observed that high-dose folic acid supplementation was associated with a significant reduction in the recurrence of colonic adenomas suggesting that folic acid may be an effective chemopreventive agent for colorectal neoplasia [24]. This is further supported by Mennan et al. who measured folate levels in adenoma, carcinoma and normal appearing adjacent mucosa. He found the levels of folate were lower in adenomas and carcinomas compared with normal appearing mucosa [25].
As noted above, there are conflicting results regarding the use of folic acid for the prevention of adenomas. In a double-blind, placebo controlled study by Cole et al., folic acid at a dose of 1 mg/day was not found to reduce the risk of adenoma recurrence and might actually increased the risk of CRC; but the results were not statistically significant [29]. The difference between the study of Jaszewski et al. and Cole et al. was that whereas Cole et al. used a dose of 1 mg folic acid per day, Jaszeweski et al. used a five times higher dose of folic acid. This could partly explain the differences in observation. Further studies are undoubtedly needed to clarify this issue.
Folic acid has also been studied as an adjuvant in the treatment of colon cancer. These studies, however, have shown an acceleration of colorectal carcinogenesis with folic acid supplementation [22]. As studied in animal models by Kim, the timing of the folate supplementation is of utmost importance. Folate has the potential to promote rather than suppress colorectal carcinogenesis when given after a neoplastic focus is already present. Rodents given exceptionally high supplemental folate levels after known microscopic colorectal neoplastic focus was present had acceleration and promotion of CRC [22].
Given this information, folate may be effective for primary prophylaxis but not secondary prophylaxis for the treatment of CRC. If folate accelerates carcinogenesis once there is a neoplastic focus, it should not be used as an adjuvant to chemotherapy or in the prevention of recurrence, but may be useful for chemoprevention. Additional prospective studies in patients at higher risk for CRC, polyps, or alcoholics would be helpful.
9 Calcium
Western diet and lifestyle contribute to an increased incidence of colorectal adenomas and carcinoma. This diet allows excess luminal fatty acids and secondary bile acids within the colon. Fatty acids and free bile acids can irritate colonic epithelium causing cell loss and enhanced proliferation [30]. Calcium may protect colonic mucosa by several proposed mechanisms. Calcium binds fatty acids and bile acids to form insoluble soaps and block colonic epithelial injury [27]. Calcium intake decreases proliferation of colonic epithelial cells in animal models fed a high fat diet [28]. Calcium supplements decreased colonic epithelial cell proliferation frequency and activity in patients at risk for familial colon cancer [31]. Impaired apoptosis is involved in adenoma development. Calcium supplementation enhances differentiation and apoptosis in normal human colonocytes [32, 33].
Calcium’s effect on colonic epithelial cells may include additional mechanisms such as through the human parathyroid calcium sensing receptor, CaSR, located within colonic crypt cells. This receptor is inversely associated with the degree of differentiation in colon cancer cell lines [34]. Calcium supplementation stimulates E-cadherin expression and favors differentiation with less malignant progression in cultured colon cancer cells. Calcium influx, measured in normal and neoplastic colonic cells, differs in cardiac L-type calcium channel activity [35].
Calcium supplementation has shown to play a role in chemoprevention of colorectal neoplasia. Reports have found inadequate calcium intake is associated with an increased risk of multiple cancer types including colon cancer [36]. Baron and colleagues showed in a key trial a 0.76 adjusted risk reduction of adenomatous polyp recurrence in patients given 1,200 mg of elemental calcium daily for 4 years [29]. Further follow-up of patients who completed this trial showed the benefit of calcium supplements persisted for an additional 5 years [37]. Randomized trials in patients with calcium supplementation show some benefit over placebo in cancer risk reduction but results are mixed [38, 39]. High intake of milk or milk products is not clearly protective [40]. Meta-analysis of calcium supplementation to prevent additional colon adenomas does reveal some benefit with an odds ratio of 0.80 [41]. Interest in a high calcium to magnesium ratio in the western diet reveals that calcium and magnesium may interact so that magnesium intake may suppress adenoma incidence where Ca:Mg intake is low [42].
Little data exists to show that calcium may play a role in treatment of colorectal cancer. Neuropathy is a significant toxicity of oxaliplatin chemotherapy. Gamelin and colleagues have shown that calcium and magnesium infusion significantly reduces the frequency and severity of oxaliplatin associated acute peripheral neurotoxicity [43]. Further studies to verify no loss in efficacy of chemotherapy will be important [44]. The potential benefit of calcium added to colorectal cancer treatment strategies is largely unexplored and deserves future investigation.
10 Vitamin D
The role of vitamin D protection against colonic neoplasm began with the inverse association of colon cancer mortality with higher UV-B exposure with residence at higher latitude [32]. Prospective studies show an inverse relationship with serum 25(OH)-vitamin D and colorectal neoplasm incidence [45, 46]. Survival with colorectal cancer is higher in patients with higher initial levels of plasma 25-(OH)-vitamin D3 [33]. Yet, in one prospective randomized trial, Vitamin D and calcium supplementation for 7 years failed to lower colorectal cancer incidence in postmenopausal woman [47]. Whether cancer prevention requires longer duration for supplements and observation is unresolved. Vitamin D may reduce colon cancer risk associated with diet and lifestyle by inhibiting the cyclooxygenase-2 (COX-2) enzyme pathway [12]. If chronic inflammation and vitamin D deficiency concur, adequate vitamin D levels may be protective. Cantorna and colleagues showed that the vitamin D deficient IL-10 knockout mice rapidly developed colitis and related symptoms that could be prevented by vitamin D supplementation [48]. Calcium and vitamin D benefit in prevention of colorectal cancer may include dependent and independent mechanisms [49, 50]. Interestingly, 1,25 dihydroxy vitamin D (1,25(OH)2D3) along with calcium stimulated CaSR expression in several colon cancer cell lines [47]. 1,25(OH)2D3 enhanced cyclin-dependent kinase inhibitors. The potential for a chemopreventative and therapeutic effect on CaSR in colon carcinoma warrants more investigation.
The polyp prevention trial provides evidence for an inverse relationship between total Vitamin D intake (dietary and supplements) and overall adenoma recurrence. Although no significant association was found for total calcium intake, adenoma recurrence was inversely associated with calcium and vitamin D supplementation [51]. Lieberman found an intake more than 645 IU/day of vitamin D is protective against advanced colorectal neoplasms in a prospective cross-sectional study of 3121 asymptomatic veteran patients who underwent colonoscopy [37].
Vitamin D holds promise in the treatment of colorectal cancer. One-alpha,25-dihydroxyvitamin D3 inhibits growth and promotes colon cancer cell differentiation in culture by induction of apoptosis [38]. Use of such active metabolites of vitamin D3 or the analogue EB1089 pose another therapeutic strategy in colorectal cancer. Low doses of 1alpha,25-dihydroxyvitamin D3 enhance the chemotherapy sensitizing benefit of secreted protein acidic rich in cysteine gene and protein in cell cultures of chemotherapy resistant human colorectal cancer [39].
Calcium supplementation offers a modest benefit in prevention of colorectal cancer and reduction in subsequent colonic adenoma recurrence. Vitamin D provides protection of colonic mucosal inflammation and adenoma recurrence as well. More study of its role in the prevention of colorectal cancer is needed. Future study, with higher vitamin D supplemental dosing to a serum level of 30 ng/ml, may be valuable [12]. The future of calcium and active vitamin D metabolites in colon cancer treatment to lessen adverse side effects or synergize with specific chemotherapy holds promise.
11 Curcumin
Curcumin is a lipophilic molecule developed from the plant Curcuma Longa Linn, commonly called turmeric [52]. The turmeric plant, a member of the ginger family, has been used for thousands of years. In India, it was traditionally used for arthritis and muscular disorders whereas in China, it was used as a topical analgesic and treatment for flatulence, colic, hepatitis, and chest pain [53]. Curcumin is used in the coloring and flavoring of many South Asian cuisines and has no known discernable toxicity. Curcumin is the major antioxidant and anti-inflammatory component within turmeric [42]. Studies have shown that curcumin inhibits the growth of transformed cells and has been shown to suppress initiation, promotion, and progression of colon carcinogenesis in carcinogen-induced rodent models [54].
Curcumin, like folate, has a low bioavailability. Curcumin and its metabolites were measured by high-performance liquid chromatography in the tissues, plasma, and feces of Min+/- mice after long-term ingestion of dietary curcumin in doses that reduced adenoma multiplicity by approximately 40%. The majority of the ingested curcumin was present in the feces as authentic curcumin sulfate, 20–25% was measured in the colonic mucosa, and 5–10% was present in the mucosa of small intestine [45, 46]. After termination of dietary curcumin intake, tissue curcumin levels declined rapidly to unquantifiable amounts (within 3–6 h) while fecal levels declined more slowly (with a 23 h half-life). These studies suggest that orally administered curcumin may exert its carcinogenic inhibitory effects primarily via luminal and/or intra-mucosal routes, as negligible levels were absorbed into the circulation [46]. Therefore, poor systemic availability does not preclude its use in prevention/treatment of GI malignancies [47].
Curcumin seems to play a role in both chemoprevention and chemotherapy for CRC. The chemopreventive effect of curcumin is mainly due to the inhibition of COX-2, which leads to inhibition of prostaglandin E that normally causes subsequent colon cancer growth. These chemopreventive effects of curcumin resemble those of NSAIDs and COX-2 inhibitors and thus seem to act strongly via inhibition of arachidonate metabolism, reducing cell proliferation, and inducing apoptosis [55].
Curcumin has also been shown to inhibit the growth of transformed cells, as a chemotherapeutic agent. In a Phase I clinical trial, curcumin was found to be effective in inhibiting the growth of a variety of tumors [56]. Our in vitro studies demonstrate that curcumin, in combination with either 5-FU or FOLFOX (5-FU + Oxaliplatin) causes a greater growth inhibition of HCT-116 or HT-29 colon cancer cells than either agent/regimen alone [57]. Furthermore, in studies comparing capecitabine and curcumin to either agent alone, Kunnumakkara et al. found that the combination inhibited the proliferation of human CRC cell lines, potentiated apoptosis induced by capecitabine, and inhibited nuclear factor-kappaB (NF-κB) activation. In mice, the combination of curcumin and capecitabine were more effective than either agent alone in reducing tumor volume and was highly effective in suppressing distant metastasis. The authors believe that much of the effect of curcumin in synergizing with capecitabine was due to the suppression of NF-κB, which has been implicated in the resistance of various chemotherapeutic agent [58]. NF-κB upregulation is involved in cell survival, proliferation, invasion, angiogenesis, and metastasis in multiple tumor types due to the promotion of anti-apoptotic genes [54, 58]. Despite this, the mechanism by which curcumin exerts its anti-neoplastic activity is not fully understood, but there are over 20 different potential molecular pathways that are currently being studied.
Two additional mechanisms by which curcumin synergizes with chemotherapeutic agents, specifically FOLFOX in the treatment of CRC, both involve growth receptors. The EGF-receptor (EGFR) and/or its family members (referred to as EGFRs) play a crucial role in regulating a number of pathways that affect tumor cell survival, angiogenesis, motility, and invasiveness. Recent data have also implicated the insulin-like growth factor (IGF)/IGF-receptor-1 (IGF-1R) system in the development and progression of CRC [59, 60]. Therefore, agents that would target multiple signaling pathways, such as those associated with both EGFRs and IGF-1R, may be more effective than narrowly focused therapies as they are likely to impact several aspects of tumor progression. Frequently, when a chemotherapeutic agent blocks one malignant pathway, other pathways are amplified, which is why a single chemotherapeutic agent is rarely effective in the treatment of advanced cancer.
Curcumin has not only been studied in the primary prevention and treatment of CRC, but also in preventing recurrence. Curcumin can inhibit the growth of chemo-surviving colon cancer cells, also called CSC, discussed earlier. Cancer stem cells possess the capacity for self-renewal, show the potential to develop into any cell in the overall tumor population, have the ability to drive continued expansion of the population of malignant cells, and invade and metastasize [61, 62]. Failure to eliminate these cells is thought to be one of the underlying causes of cancer recurrence. Curcumin cannot only synergize with FOLFOX to inhibit the growth of colon cancer cells but also attenuate the expression of several colon cancer stem cell markers. Colon cancer cell lines treated with FOLFOX alone showed survival in the range of 60–70%. These chemo-surviving colon cancer cells were then treated with FOLFOX and curcumin which resulted in a reduction in the expression of EGFR and IGF-1R which were previously activated after just the FOLFOX treatment [63]. The authors hypothesized that curcumin has the ability to down-regulate the growth factor receptor-mediated survival signals seen in chemo-surviving cells. It is here that nutraceutics and chemotherapeutic agents can both play a role in the eradication and prevention of recurrent colorectal cancer [63].
12 Conclusion
The future of nutraceutics and supplements is ever expanding. The maximal and most durable therapeutic benefit against tumor growth will be achieved with combination therapies that affect several targets in the carcinogen pathway. The treatment of cancer has drastically improved over the past 30 years to the point that some advanced cancers are now considered chronic diseases as patients are living so much longer with the disease. The addition of non-toxic agents that are well tolerated and aid in the prevention, treatment, and prevention of recurrence is not just an idea, but may be a reality.
References
Parkin, D. M., Bray, F., Ferlay, J., et al. (2005). Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians, 55, 74–108.
Jemal, A., Siegel, R., Ward, E., et al. (2009). Cancer statistics, 2009. CA: A Cancer Journal for Clinicians, 59, 225–249.
Rex, D. K., Johnson, D. A., Anderson, J. C., et al. (2009). American college of gastroenterology guidelines for colorectal cancer screening 2008. The American Journal of Gastroenterology, 104, 739–750.
Winawer, S., Fletcher, R., Rex, D., et al. (2003). Colorectal cancer screening and surveillance: clinical guidelines and rationale—Update based on new evidence. Gastroenterology, 124, 544–560.
Patel, B. B., Yu, Y., Du, J., et al. (2009). Age-related increase in colorectal cancer stem cells in macroscopically normal mucosa of patients with adenomas: a risk factor for colon cancer. Biochemical and Biophysical Research Communications, 378, 344–347.
Davila, R. E., Rajan, E., Baron, T. H., et al. (2006). ASGE guideline: colorectal cancer screening and surveillance. Gastrointestinal Endoscopy, 63, 546–557.
Robbins, D. H., & Itzkowitz, S. H. (2002). The molecular and genetic basis of colon cancer. The Medical Clinics of North America, 86, 1467–1495.
Kanwar, S. S., Nautiyal, J., & Majumdar, A. P. (2010). EGFR(s) inhibitors in the treatment of gastro-intestinal cancers: what’s new? Current Drug Targets, 11, 682–698.
Pino, M. S., & Chung, D. C. (2010). The chromosomal instability pathway in colon cancer. Gastroenterology, 138, 2059–2072.
Salama, P., & Platell, C. (2009). Colorectal cancer stem cells. ANZ Journal of Surgery, 79, 697–702.
Friedlich, M. S. S. (2002). Prevention of colorectal cancer. Canadian Journal of CME, 14, 112–119.
Chan, A. T., & Giovannucci, E. L. (2010). Primary prevention of colorectal cancer. Gastroenterology, 138, 2029–2043.
Nautiyal, J., Kanwar, S. S., & Majumdar, A. P. (2010). EGFR(s) in saging and carcinogenesis of the gastrointestinal tract. Current Protein & Peptide Science, 11, 436–450.
Winawer, S. J. (2007). Colorectal cancer screening. Best Practice & Research. Clinical Gastroenterology, 21, 1031–1048.
Sleisenger, M. H. (2006). Sleisenger & Fordtran’s gastrointestinal and liver disease Pathophysiology, diagnosis, management. St. Louis, Mo: W.B. Saunders.
Yu, Y., Kanwar, S. S., Patel, B. B., et al. (2009). Elimination of colon cancer stem-like cells by the combination of curcumin and FOLFOX. Transl Oncol, 2, 321–328.
Kalra, E. (2003). Nutraceutical-definition and introduction. The AAPS Journal, 5, 27–28.
Majumdar, A. P., Kodali, U., & Jaszewski, R. (2004). Chemopreventive role of folic acid in colorectal cancer. Frontiers in Bioscience, 9, 2725–2732.
Laanpere, M., Altmae, S., Stavreus-Evers, A., et al. (2010). Folate-mediated one-carbon metabolism and its effect on female fertility and pregnancy viability. Nutrition Reviews, 68, 99–113.
Vecchia, C. L., Negri, E., Pelucchi, C., et al. (2002). Dietary folate and colorectal cancer. International Journal of Cancer, 102, 545–547.
Lashner, B. A., Heidenreich, P. A., Su, G. L., Kane, S. V., & Hanauer, S. B. (1989). Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis: A case-control study (97th ed.). New York: Elsevier. ETATS-UNIS.
Kim, Y. I. (2003). Role of folate in colon cancer development and progression. Journal of Nutrition, 133(11 Suppl 1), 3731S–3739S.
Sanjoaquin, M. A., Allen, N., Couto, E., et al. (2005). Folate intake and colorectal cancer risk: a meta-analytical approach. International Journal of Cancer, 113, 825–828.
Jaszewski, R., Misra, S., Tobi, M., et al. (2008). Folic acid supplementation inhibits recurrence of colorectal adenomas: a randomized chemoprevention trial. World Journal of Gastroenterology, 14, 4492–4498.
Jaszewski, R., Khan, A., Sarkar, F. H., et al. (1999). Folic acid inhibition of EGFR-mediated proliferation in human colon cancer cell lines. The American Journal of Physiology, 277, C1142–C1148.
Kim, Y. I. (2007). Folate and colorectal cancer: an evidence-based critical review. Molecular Nutrition & Food Research, 51, 267–292.
Van der Meer, R., & De Vries, H. T. (1985). Differential binding of glycine- and taurine-conjugated bile acids to insoluble calcium phosphate. The Biochemical Journal, 229, 265–268.
Pence, B. C., & Buddingh, F. (1988). Inhibition of dietary fat-promoted colon carcinogenesis in rats by supplemental calcium or vitamin D3. Carcinogenesis, 9, 187–190.
Baron, J. A., Beach, M., Mandel, J. S., et al. (1999). Calcium supplements for the prevention of colorectal adenomas. Calcium polyp prevention study group. The New England Journal of Medicine, 340, 101–107.
Newmark, H. L., Wargovich, M. J., & Bruce, W. R. (1984). Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. Journal of the National Cancer Institute, 72, 1323–1325.
Lipkin, M., & Newmark, H. (1985). Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colonic cancer. The New England Journal of Medicine, 313, 1381–1384.
Garland, C. F., & Garland, F. C. (2006). Do sunlight and vitamin D reduce the likelihood of colon cancer? International Journal of Epidemiology, 35, 217–220.
Ng, K., Meyerhardt, J. A., Wu, K., et al. (2008). Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. Journal of Clinical Oncology, 26, 2984–2991.
Chakrabarty, S., Wang, H., Canaff, L., et al. (2005). Calcium sensing receptor in human colon carcinoma: interaction with Ca(2+) and 1, 25-dihydroxyvitamin D(3). Cancer Research, 65, 493–498.
Wang, X. T., Nagaba, Y., Cross, H. S., et al. (2000). The mRNA of L-type calcium channel elevated in colon cancer: protein distribution in normal and cancerous colon. The American Journal of Pathology, 157, 1549–1562.
Peterlik, M., Grant, W. B., & Cross, H. S. (2009). Calcium, vitamin D and cancer. Anticancer Research, 29, 3687–3698.
Lieberman, D. A., Prindiville, S., Weiss, D. G., et al. (2003). Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA, 290, 2959–2967.
Diaz, G. D., Paraskeva, C., Thomas, M. G., et al. (2000). Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Research, 60, 2304–2312.
Taghizadeh, F., Tang, M. J., & Tai, I. T. (2007). Synergism between vitamin D and secreted protein acidic and rich in cysteine-induced apoptosis and growth inhibition results in increased susceptibility of therapy-resistant colorectal cancer cells to chemotherapy. Molecular Cancer Therapeutics, 6, 309–317.
Martinez, M. E., & Willett, W. C. (1998). Calcium, vitamin D, and colorectal cancer: a review of the epidemiologic evidence. Cancer Epidemiology, Biomarkers & Prevention, 7, 163–168.
Shaukat, A., Scouras, N., & Schunemann, H. J. (2005). Role of supplemental calcium in the recurrence of colorectal adenomas: a metaanalysis of randomized controlled trials. The American Journal of Gastroenterology, 100, 390–394.
Hanif, R., Qiao, L., Shiff, S. J., et al. (1997). Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. The Journal of Laboratory and Clinical Medicine, 130, 576–584.
Gamelin, L., Boisdron-Celle, M., Delva, R., et al. (2004). Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-Fluorouracil and leucovorin for advanced colorectal cancer. Clinical Cancer Research, 10, 4055–4061.
Kurniali, P. C., Luo, L. G., & Weitberg, A. B. (2010). Role of calcium/magnesium infusion in oxaliplatin-based chemotherapy for colorectal cancer patients. Oncology (Williston Park), 24, 289–292.
Perkins, S., Verschoyle, R. D., Hill, K., et al. (2002). Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiology, Biomarkers & Prevention, 11, 535–540.
Barnes, C. J., & Lee, M. (1998). Chemoprevention of spontaneous intestinal adenomas in the adenomatous polyposis coli Min mouse model with aspirin. Gastroenterology, 114, 873–877.
Aggarwal, B. B., Ichikawa, H., Garodia, P., et al. (2006). From traditional Ayurvedic medicine to modern medicine: identification of therapeutic targets for suppression of inflammation and cancer. Expert Opinion on Therapeutic Targets, 10, 87–118.
Cantorna, M. T., Munsick, C., Bemiss, C., et al. (2000). 1, 25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. The Journal of Nutrition, 130, 2648–2652.
Fedirko, V., Bostick, R. M., Flanders, W. D., et al. (2009). Effects of vitamin d and calcium on proliferation and differentiation in normal colon mucosa: a randomized clinical trial. Cancer Epidemiology, Biomarkers & Prevention, 18, 2933–2941.
Newmark, H. L., Yang, K., Kurihara, N., et al. (2009). Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis, 30, 88–92.
Hartman, T. J., Albert, P. S., Snyder, K., et al. (2005). The association of calcium and vitamin D with risk of colorectal adenomas. The Journal of Nutrition, 135, 252–259.
Villegas, I., Sanchez-Fidalgo, S., & Arcon de la Lastra, C. (2008). New mechanisms and therapeutic potential of curcumin for colorectal cancer. Molecular Nutrition & Food Research, 52, 1040–1061.
Grant, K. L. P., & Schneider, C. D. M. D. (2000). Turmeric. [Miscellaneous Article]. American Journal of Health-System Pharmacy, 57, 1121–1122.
Patel, B. B., & Majumdar, A. P. N. (2009). Synergistic role of curcumin with current therapeutics in colorectal cancer: minireview. Nutrition and Cancer, 61, 842–846.
Kawamori, T., Lubet, R., Steele, V. E., et al. (1999). Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Research, 59, 597–601.
Sharma, R. A., Euden, S. A., Platton, S. L., et al. (2004). Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clinical Cancer Research, 10, 6847–6854.
Patel, B. B., Sengupta, R., Qazi, S., et al. (2008). Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. International Journal of Cancer, 122, 267–273.
Kunnumakkara, A. B., Diagaradjane, P., Anand, P., et al. (2009). Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. International Journal of Cancer, 125, 2187–2197.
Adachi, Y., Lee, C. T., Coffee, K., et al. (2002). Effects of genetic blockade of the insulin-like growth factor receptor in human colon cancer cell lines. Gastroenterology, 123, 1191–1204.
Hakam, A., Yeatman, T. J., Lu, L., et al. (1999). Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Human Pathology, 30, 1128–1133.
Wang, J. C., & Dick, J. E. (2005). Cancer stem cells: lessons from leukemia. Trends in Cell Biology, 15, 494–501.
Dick, J. E. (2008). Stem cell concepts renew cancer research. Blood, 112, 4793–4807.
Patel, B. B., Gupta, D., Elliott, A. A., et al. (2010). Curcumin targets FOLFOX-surviving colon cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Research, 30, 319–325.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markle, B., May, E.J. & Majumdar, A.P.N. Do nutraceutics play a role in the prevention and treatment of colorectal cancer?. Cancer Metastasis Rev 29, 395–404 (2010). https://doi.org/10.1007/s10555-010-9234-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-010-9234-3