Abstract
Despite the recent advances in the diagnosis of bladder cancer, recurrence after surgical intervention for muscle invasive disease is still problematic as nearly half of the patients harbor occult distant metastases and this, in turn, is associated with poor 5-year survival rate. We have recently identified Rho family GDP dissociation inhibitor 2 (RhoGDI2) protein as functional metastasis suppressor and a prognostic marker in patients after cystectomy. In identifying the mechanisms underlying metastasis suppression by RhoGDI2, we found this protein to be associated with the c-Src kinase in human tumors, where the expression of both is diminished as a function of stage. Interestingly, c-Src bound to and phosphorylated RhoGDI2 resulting in enhanced metastasis suppressive potency. In this review, we will discuss the established roles of c-Src and RhoGDI2 in bladder cancer and speculate on their therapeutic relevance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bladder cancer is a common malignancy affecting the genitourinary system. In the United States, 70,980 estimated new cases, and approximately 14,330 deaths are expected in 2009 [1]. Most cases are of urothelial (formerly named “transitional”) histology and, of these, 75% of patients present with non-muscle invasive and 20–30% present with muscle invasive disease [2]. Despite good prognosis for patients with non-muscle invasive disease, recurrence is common and associated with development of muscle invasive disease in up to 30% [2]. Nearly half of the patients presenting with muscle invasive disease or progressing to this state from non-muscle invasive cancer already harbor occult distant metastases and a poor 5-year survival rate [3]. Numerous factors, including chromosomal markers, genetic polymorphisms, and genetic and epigenetic alterations are involved in tumorigenesis, progression, and metastasis of bladder cancer [4].
Our laboratory has identified the Rho family GDP dissociation inhibitor 2 (RhoGDI2) protein as functional metastasis suppressor in human bladder cancer models and a prognostic marker in patients after cystectomy where diminished tumor expression is associated with decreased patient survival [4–8]. Further examination of these findings revealed that a subset of patients with tumors having RhoGDI2 expression levels similar to those found in non-muscle invasive disease still developed metastasis. This led us to investigate whether mechanisms, other than the expression level, regulate RhoGDI2 function. We have recently identified the c-Src kinase as a novel binding partner of RhoGDI2, phosphorylating it at a specific tyrosine residue and thus mediating its metastasis suppressor function. Surprisingly, decreased c-Src and RhoGDI2 expression levels appeared mutually exclusive in individual tumors, indicating shared signaling pathways leading to metastasis suppression [9]. These findings broaden the possible roles of c-Src in cancer and raise the possibility that some of these are mediated in a tissue specific manner. In this review, we will focus on the roles of c-Src and RhoGDI2 in bladder carcinogenesis and metastasis.
1.1 The Src tyrosine kinase
Src belongs to a family of proto-oncogenic non-receptor tyrosine kinases, called Src family kinases (SFK) [10–12]. Although Rous sarcoma virus (RSV) was first discovered in 1911 by Francis Peyton Rous [13] as a virus causing chicken sarcoma, Bishop, Varmus, and colleagues highlighted the significance of this transforming virus decades later. They demonstrated that the v-Src chicken oncogene (a member of RSV) originated from a cellular proto-oncogene, c-Src [10–12], which is implicated in physiological processes including cell proliferation, apoptosis, cell cycle control, angiogenesis, and cell-cell adhesion and communication [14 and the references cited therein]. Consistent with its role as a proto-oncogene, c-Src has a poor transforming ability, that when activated, becomes highly oncogenic [15–18].
In humans, nine members of the SFKs have been recognized; all share four conserved peptide domains, Src homology (SH) domains that not only allow SFK participation in a myriad of signaling complexes, but also regulate SFK kinase activity through intermolecular and intramolecular interactions [19–23]. Src homology domain-1 (SH-1) is the enzymatic domain that possesses intrinsic tyrosine kinase activity. SH-2 domain facilitates intermolecular interactions between Src and its own C-terminal tyrosine residues resulting in autophosphorylation at more than one tyrosine residues. Autophosphorylation of Tyr527 negatively regulates SFKs, and is catalyzed by C-terminal Src kinase (Csk), resulting in an enzymatically inactive, clamped conformation with SH-1 [24, 25]. Phosphatases are capable of removing the Tyr 527 phosphate group from the C-terminal tyrosine, releasing the clamp [22]. Point mutations or deletions in the C-terminus that alter Tyr527, as occur in v-Src, result in a transforming protein with constitutive enzymatic activity [26, 27]. On the other hand, autophosphorylation at Tyr416 on the SH1 increases the specific activity of c-Src [27]. The SH-3 domain recognizes a pro-x-x-pro motif on many signaling and structural molecules [19, 28], whereas the SH-4 or the NH2-terminal domain is myristoylated and is responsible for membrane association of SFKs [29] (Fig. 1)
Structure and activation of Src Proteins. a Both avian and human c-Src protein are composed of a C-terminal tail containing a negative regulatory tyrosine residue (Tyr527, chicken; Tyr530, human), four Src homology (SH) domains, and a unique amino-terminal domain. The SH1 is the kinase domain and contains the autophosphorylation site (Tyr416, chicken; Tyr419, human); the SH2 domain, interacts with the negative regulatory; the SH3 domain promotes intramolecular contact with the kinase domain in the inactive form of the protein; and the SH4 domain contains a myristoylation site that is important for membrane localization. Chicken v-Src lacks the carboxy-terminal negative-regulatory domain and contains 12 substituted carboxy-terminal amino acids, as well as numerous point mutations throughout the molecule, explaining the high level of activity of this protein. b Inactivation of human c-SRC occurs when its C-terminal Tyr530 is phosphorylated and it binds back to the SH2 domain. This interaction and an interaction between the SH3 domain and the kinase domain result in a closed molecular structure with diminished access of substrates to the kinase domain. Conversely, c-Src activation occurs with removal of the C-terminal phosphotyrosine, displacement of inhibitory intramolecular interactions, and opening of the c-SRC molecular structure. Full activation involves phosphorylation at Tyr419. M myristoylation, P phosphorylation (adapted from [28])
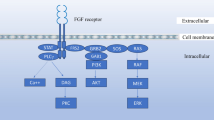
The expression of individual SFKs (or their splice variants) is both tissue and cell specific with c-Src, Fyn, and Yes ubiquitously expressed (reviewed in [14, 23, 24, 27]). SFKs vary in their subcellular locations, such as in caveolae, focal adhesions, endosomes, perinuclear membranes, and the cytoplasmic face of the plasma membrane [14, 23, 24, 27]. Activity of SFKs is regulated by tyrosine kinase receptors (such as epidermal growth factor receptor (EGFR), platelet-derived growth factors (PDGFR), and fibroblast growth factor receptors (FGFRs)), integrins, G-protein coupled receptors, antigen- and Fc-coupled receptors, cytokine receptors, and steroid hormone receptors [27]. SFKs work in concert with a large number of substrates, and upon activation, they signal to a variety of downstream effectors and transcription factors (reviewed in [27]).
1.2 The role of Src in cancer
c-Src is the SFK member most implicated either by overexpression or activation in a wide variety of cancers including colorectal, hepatocellular, pancreatic, gastric, esophageal, breast, prostate, head and neck, ovarian, lung, neuronal cancers, and melanoma as well as leukemia and lymphoma [28, 30, 31]. Being that c-Src is the oldest and best-studied proto-oncogene, its role in human cancer development and progression is multi-faceted. When activated through various mechanisms, from stimulation by growth factors to activating mutations, c-Src results in transformed phenotype with increased cellular proliferation, invasion, and motility, as well as decreased intercellular and cell–matrix adhesion and angiogenesis [28]. Correlative and experimental evidences demonstrated that the expression and activity of c-Src was positively correlated with tumor grade and stage in several cancers [28, 30, 31]. In hepatocellular and colon carcinomas, the overexpression of c-Src is concurrent with underexpression of its negative-regulatory c-Src tyrosine kinase (Csk) protein, leading to higher levels of c-Src activation even in the presence of relatively normal levels of c-Src protein expression [28]. Discrepancies between the levels of c-Src protein expression and activity and the grade and stages of the tumor cells have been found in some colorectal tumors and have been explained by the presence of overexpressed receptor tyrosine kinases such as EGFR in poorly differentiated tumors, which can activate c-Src compensating for the low levels of c-Src [28, 32–35]. Additionally, experimental evidence supported the use of SFK-selective small molecule inhibitors in early phase clinical trials for advanced tumors and other pathologic conditions such as osteoporosis and inflammatory conditions with promising outcomes [34, 36–39].
1.3 Src in bladder cancer
The expression and kinase activity of phosphorylated c-Src in urothelial tumors and cell lines have not received much attention. The earliest study by Rosen and colleagues [40] reported low Src level and activity in T24, TCCSUP, and 5637 human bladder cancer cell lines. The tyrosine kinase activity of pp60c-Src is elevated in human bladder carcinomas over normal bladder mucosa [40]. The increased level and kinase activity were mainly in low-grade bladder cancer cell lines and tumors compared to high-grade counterparts [41]. Novel phosphotyrosyl substrates were identified in human bladder cancer cell lines and tumors displaying elevated pp60c-Src kinase activity, suggesting an inverse association for the Src protooncogene in urothelial cancer differentiation and progression [40, 41]. Furthermore, gene expression profiling and immunohistochemistry of human tumors revealed that Src levels diminish as a function of bladder cancer stage [9].
In addition, c-Src has been shown to be specifically and transiently activated in low-density rat bladder cancer NBT-II cells in response to growth factor (EGF, FGF-1, and cMet) and chemokine stimulation and the dominant-negative c-Src expressed in these cells inhibited scattering activity. In growth-arrested confluent cultures stimulated by the same growth factors, c-Src activation was associated with cell cycle entry and mitogenic pathway activation that were not inhibited by expressing the dominant negative mutant. In either situation, growth factor stimulation and phosphorylation of the cognate receptors on NBT-II cells resulted in activation of the Ras pathway [42, 43] indicating that the two pathways act independently, with c-Src being necessary only in scattering or epithelial mesenchymal transition (EMT) [44]. These observations also suggested that c-Src activation might correspond to an early event in the growth factor-triggered signaling, diverging the signaling cascade into EMT but not to mitogenesis [42, 43]. Consistently, although c-Src activity was not required for growth of tumors derived from NBT-II cells injected into nude mice, the presence of micrometastases was strictly dependent on c-Src as evidenced by the dramatic reduction of metastases by the expression of a dominant-negative mutant of Src (SrcK-) or of Csk, the natural inhibitor of Src [42].
Taken together, these observations suggest that in bladder cancer, the low level and activity of c-Src may be compensated by overexpressed receptor tyrosine kinases as EGFR or FGFRs, or more unusually, c-Src may either activate tumor or metastasis suppressors or inactivate other oncogenes or metastasis promoters.
1.4 The relationship of EGFR and Src
The importance of Src and EGFR interaction in bladder cancer highlighted in a study by Simeonova and colleagues [45] who were first to report that c-Src activity was induced by arsenic in UROtsa cells in vitro, and was found to be a prerequisite for the EGFR and ERK activation. In vivo exposure of mice to arsenic in drinking water was associated with epithelial proliferation, EGFR, and ERK activation in the urinary bladder concomitant with an increase in c-Src levels interacting with EGFR. Src activity was also involved in arsenic-induced, EGFR-independent ERK phosphorylation [45]. Pharmacological or mutational inhibition of Src prevented arsenic-induced but not EGF-induced EGFR or ERK phosphorylation in the uroepithelial cell line. Therefore, it was concluded that Src can activate the ERK pathway either by phosphorylating EGFR or by phosphorylating molecules, such as Shc or FAK, creating binding sites for Grb2, both of which link to the MAPK pathway. In another study, using pharmacological inhibitors and UROtsa cells acutely or chronically exposed to arsenic, Src was shown to mediator of arsenic-induced Cox2 expression and activity, anchorage-independent growth, cell proliferation as well as inhibition of apoptosis [46]. Both studies support the contribution of Src in bladder carcinogenesis. Furthermore, EGFR and SFKs (Src and Yes) were found to be activated in response to serum-starvation in human 5637 bladder cancer cells [47]. Abrogation of EGFR-SFK activation by PP2 or the tyrosine phosphorylation of p145met promoted cell death concurrent with activation of caspase-like proteases. This study concluded that SFK activity is specifically required for serum-independent growth of 5637 cells, as a downstream target of EGFR, but independent of dephosphorylation of the C-terminal tyrosine residue (Y529) [47]. Together with prior observations on the level of Src in normal-, low-, and high-grade bladder cancer [41], these data suggests that Src may be involved in carcinogenesis and/or maintenance of the low grade non-invasive lesion while its role may be unnecessary or different in high-grade invasive lesions [47]. The interactions of Src with the RhoGDI2 metastasis suppressor provide support to the latter possibility. This will be discussed further below.
1.5 The Rho family GDP dissociation inhibitors (RhoGDIs) in human cancer
RhoGDI2 also known as D4-GDI, Ly-GDI, and ARHGDIB belongs to a family of related proteins that also includes RhoGDI1 and RhoGDI3 (Fig. 2). RhoGDIs bind to Rho GTPases, sequester them in the cytosol keeping Rho proteins in the GDP-bound inactive state and preventing their interactions with effectors or other regulatory proteins, namely GTPase activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs; Fig. 3). RhoGDI2 is ubiquitously expressed [7], shares a 78% amino acid identity with RhoGDI1, and has been shown to interact in similar ways with RhoA, Rac1, and Rac2, [48]. The flexible N-terminal domain (1–69) is implicated in the binding of RhoGDI1 to Rac1 and Cdc42, and the binding of RhoGDI2 to Rac2 [49–51]. In some cells, the N-terminal domain of RhoGDI2 is cleaved by caspases with nuclear translocation of the cleaved protein during apoptosis [52–54]. Overexpression of truncated C-terminus of RhoGDI2 in SW480 colorectal cancer cells induced membrane localization of the truncated protein and was associated with induction of metastasis [55].
Structure, function, and phosphorylation of RhoGDIs. a RhoDGI proteins exhibit a high degree of primary sequence conservation as indicated by the percent identity to the right. The three GDIs share two major conserved domains: an immunoglobulin-like domain at the C-terminus and a regulatory arm at the N-terminus (residue 74 marks the beginning of the first β strand in the immunoglobulin like domain of RhoGDI). In addition, RhoGDI3 has a unique helical region at the N-terminus. b Alignment of human RhoGDI1 and RhoGDI2 protein sequences was performed using CLUSTAL FORMAT for T-COFFEE Version_7.71 (http://www.tcoffee.org). c-Src tyrosine phosphorylation sites (red letters) were predicted by KinasePhos (http://kinasephos.mbc.nctu.edu.tw). Note that the predicted tyrosine phosphorylation residues (red) were identical in RhoGI1 and RhoGDI2, including Tyr 153 of RhoGDI2 and Tyr 156 in RhoGDI1 (dark red arrow) a suggesting conserved sites
Mammalian GTPase cycle. Cycle between active (GTP-bound) and an inactive (GDP-bound) GTPase conformation. In the active state, GTPases interact with target proteins (effector). Cycle is regulated by three classes of proteins: nucleotide exchange factors (GEFs) catalyze nucleotide exchange and mediate activation; GTPase-activating proteins (GAPs) stimulate GTP hydrolysis leading to inactivation; and guanine nucleotide exchange inhibitors (GDIs) extract the inactive GTPase from membranes
RhoGDI2 has been shown to be a metastasis suppressor in different cancers, as its expression was decreased or even lost in metastatic cancers, including bladder cancer and Hodgkin’s lymphoma [7, 56]. It has also been shown to promote metastasis in other cancers [57–62]. These functional differences may be due to cell type specificity, variations in the experimental approaches, patient populations, and statistical analyses. Clearly, more work is needed to identify the signaling pathways that RhoGDI2 regulates and their relevance across various tumor types.
1.6 RhoGDI2 in bladder cancer
RhoGDI2 was originally identified as a metastasis suppressor in bladder cancer during studies of the differential invasive and metastatic properties of isogenic human bladder carcinoma cell lines, T24 (non-metastatic), and T24T (highly invasive and metastatic) using experimental metastasis models and comparative genomic studies [5, 6, 8]. Reduced expression of RhoGDI2 correlated with increasing invasive and metastatic activity in T24T cells. In human bladder tumors, the RhoGDI2 level inversely correlated with development of metastatic disease, and multivariate analysis identified RhoGDI2 as an independent prognostic marker of tumor recurrence following radical cystectomy [4, 7]. However, approximately 35% of patients with moderate or high levels of RhoGDI2 protein-developed metastatic disease suggesting that not only the expression level, but other mechanisms might regulate the metastasis suppressor effect of RhoGDI2. Phosphorylation, binding to specific partners, truncations, proteolytic cleavage, or change in subcellular localization was considered given the existing data on the other members of the RhoGDI family [52, 53, 63, 64].
Moissoglu and colleagues [65] recently reported the distinct functional mechanisms of GDI1 and GDI2 in bladder cancer cell lines independent of their affinity and inhibition of GTPase activity. Rho GDI2 was found to have a low affinity and a weak effect on RhoGTPase function, however, GDI2 bound with highest affinity to Rac1 [65] which can also act as a metastasis suppressor [66]. Mutations that altered the affinity of GDI2 for Rac1 abolished metastasis [65], suggesting that the metastasis suppressor mechanism of RhoGDI2 might be due its association with Rac1 promoting its interaction with a specific unidentified GEF.
A role of RhoGDI2 in Src mediated tumor progression was first observed in studies of Ota and colleagues [55] who showed that deletion of the N-terminus of RhoGDI2 suppressed metastasis of 1-1src cells derived from BALB/c 3T3 A31-1-1 cells, not 1-1ras1000 despite suppression of tumorigenicity in the latter [55]. RhoGDI1 and RhoGDI2 were found to be tyrosine-phosphorylated by Src in genetically modified murine cancer cells [64]. Moreover, within individual tumors, the concurrent diminution of Src levels and RhoGDI2 levels were rarely observed suggesting their involvement in common pathways suppressing metastasis [9].
Src phosphorylation has been shown to modulate RhoGDI1- and RhoGDI2–RhoGTPase complex formation, and the Src-phosphorylation sites appeared to be conserved between RhoGDI1 and RhoGDI2 [64]. Computational, proteomic, and experimental approaches identified the association of Src with RhoGDI2 in human bladder cancer cell lines with four potential Src phosphorylation sites, Tyr-24, Tyr-125, Tyr-153, and Tyr-172 in RhoGDI2 [9]. Mutation studies revealed that Tyr-153 is the major site of phosphorylation in UMUC3 and 293T cell lines; a site which is comparable to Tyr-156 phorphorylated by Src in RhoGDI1 overexpressing cells [64]. Phosphomimetic mutants of RhoGDI2 at Tyr153 enhanced the metastasis-suppressor ability of this protein in experimental lung metastasis models of human bladder cancer [9]. RhoGDI2 phosphorylation by Src decreased its association with Rac1 as confirmed by exogenous expression of wild-type RhoGDI2 or mutated substrate tyrosine residues (Y153F, constitutively active or Y153E, inactive). WT RhoGDI2 moderately decreased Rac1 membrane targeting and inhibition, Y153F had a more profound inhibitory effect on Rac1, whereas Y153E released Rac1 inhibition and was associated with a nearly absent lung tumor burden compared with that of GFP-GDI2 [9]. Using computational studies, Tyr-153 of RhoGDI2 was also predicted to phosphorylate and/or bind receptor tyrosine kinases as EGFR and PDGFR [9]. These studies provided the first evidence that Src phosphorylates RhoGDI2 at Tyr153 and this may affect its metastasis-suppressor function possibly via alternations of membrane-bound Rac1.
2 Conclusion
Although c-Src is the oldest and best-studied proto-oncogene, its role in bladder cancer carcinogenesis, progression, and metastasis appears different than that in other cancers. The pathways that impact Src levels and activity, as well as the net outcome of its interactions with substrates and regulators are still being unraveled in this tumor type. Given the observed Src expression and activation in human bladder tumors as a function of stage and its effect on the metastasis suppressive potency of RhoGDI2, one begins to wonder whether Src may be a metastasis-suppressor protein that can also act via RhoGDI2-independent pathways.
References
Jemal, A., et al. (2009). Cancer statistics, 2009. CA: A Cancer Journal for Clinicians, 59(4), 225–249.
Dinney, C. P., et al. (2004). Focus on bladder cancer. Cancer Cell, 6(2), 111–116.
Stein, J. P., et al. (2001). Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1, 054 patients. Journal of Clinical Oncology, 19(3), 666–675.
Theodorescu, D. (2006). Molecular biology of invasive and metastatic urothelial cancer. In S. Lerner, M. Schoenberg, & C. Sternberg (Eds.) Textbook of Bladder Cancer. Taylor and Francis. pp. 147–156.
Gildea, J. J., et al. (2002). RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Research, 62(22), 6418–6423.
Seraj, M. J., et al. (2000). The relationship of BRMS1 and RhoGDI2 gene expression to metastatic potential in lineage related human bladder cancer cell lines. Clinical & Experimental Metastasis, 18(6), 519–525.
Theodorescu, D., et al. (2004). Reduced expression of metastasis suppressor RhoGDI2 is associated with decreased survival for patients with bladder cancer. Clinical Cancer Research, 10(11), 3800–3806.
Titus, B., et al. (2005). Endothelin axis is a target of the lung metastasis suppressor gene RhoGDI2. Cancer Research, 65(16), 7320–7327.
Wu, Y., et al. (2009). Src phosphorylation of RhoGDI2 regulates its metastasis suppressor function. Proceedings of the National Academy of Sciences of the United States of America, 106(14), 5807–5812.
Stehelin, D. (1976). The transforming gene of avian tumor viruses. Pathology and Biology (Paris), 24(8), 513–515.
Stehelin, D., et al. (1976). Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. Journal of Molecular Biology, 101(3), 349–365.
Stehelin, D., et al. (1976). DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature, 260(5547), 170–173.
Rous, P. (1983). Landmark article (JAMA 1911;56:198). Transmission of a malignant new growth by means of a cell-free filtrate. By Peyton Rous. The Journal of the American Medical Association, 250(11), 1445–1449.
Roskoski, R., Jr. (2004). Src protein-tyrosine kinase structure and regulation. Biochemical and Biophysical Research Communications, 324(4), 1155–1164.
Takeya, T., & Hanafusa, H. (1983). Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell, 32(3), 881–890.
Takeya, T., et al. (1981). Comparison between the viral transforming gene (src) of recovered avian sarcoma virus and its cellular homolog. Molecular and Cellular Biology, 1(11), 1024–1037.
Iba, H., et al. (1984). Rous sarcoma virus variants that carry the cellular src gene instead of the viral src gene cannot transform chicken embryo fibroblasts. Proceedings of the National Academy of Sciences of the United States of America, 81(14), 4424–4428.
Takeya, T., & Hanafusa, H. (1982). DNA sequence of the viral and cellular src gene of chickens. II. Comparison of the src genes of two strains of avian sarcoma virus and of the cellular homolog. Journal of Virology, 44(1), 12–18.
Moarefi, I., et al. (1997). Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature, 385(6617), 650–653.
Sicheri, F., & Kuriyan, J. (1997). Structures of Src-family tyrosine kinases. Current Opinion in Structural Biology, 7(6), 777–785.
Sicheri, F., Moarefi, I., & Kuriyan, J. (1997). Crystal structure of the Src family tyrosine kinase Hck. Nature, 385(6617), 602–609.
Xu, W., et al. (1999). Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Molecular Cell, 3(5), 629–638.
Manning, G., et al. (2002). The protein kinase complement of the human genome. Science, 298(5600), 1912–1934.
Brown, M. T., & Cooper, J. A. (1996). Regulation, substrates and functions of src. Biochimica et Biophysica Acta, 1287(2–3), 121–149.
Irby, R. B., & Yeatman, T. J. (2000). Role of Src expression and activation in human cancer. Oncogene, 19(49), 5636–5642.
Levinson, A. D., et al. (1980). The purified product of the transforming gene of avian sarcoma virus phosphorylates tyrosine. Journal of Biological Chemistry, 255(24), 11973–11980.
Thomas, S. M., & Brugge, J. S. (1997). Cellular functions regulated by Src family kinases. Annual Review of Cell and Developmental Biology, 13, 513–609.
Yeatman, T. J. (2004). A renaissance for SRC. Nature Reviews. Cancer, 4(6), 470–480.
Alland, L., et al. (1994). Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. Journal of Biological Chemistry, 269(24), 16701–16705.
Summy, J. M., & Gallick, G. E. (2003). Src family kinases in tumor progression and metastasis. Cancer Metastasis Reviews, 22(4), 337–358.
Summy, J. M., et al. (2005). c-Src regulates constitutive and EGF-mediated VEGF expression in pancreatic tumor cells through activation of phosphatidyl inositol-3 kinase and p38 MAPK. Pancreas, 31(3), 263–274.
Irby, R., et al. (1997). Overexpression of normal c-Src in poorly metastatic human colon cancer cells enhances primary tumor growth but not metastatic potential. Cell Growth & Differentiation, 8(12), 1287–1295.
Irby, R. B., et al. (1999). Activating SRC mutation in a subset of advanced human colon cancers. Nature Genetics, 21(2), 187–190.
Johnson, F. M., & Gallick, G. E. (2007). SRC family nonreceptor tyrosine kinases as molecular targets for cancer therapy. Anticancer Agents in Medical Chemistry, 7(6), 651–659.
Mao, W., et al. (1997). Activation of c-Src by receptor tyrosine kinases in human colon cancer cells with high metastatic potential. Oncogene, 15(25), 3083–3090.
Chiang, G. J., et al. (2005). The src-family kinase inhibitor PP2 suppresses the in vitro invasive phenotype of bladder carcinoma cells via modulation of Akt. Journal of the British Association of Urological Surgeons, 96(3), 416–422.
Kopetz, S., et al. (2009). Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Research, 69(9), 3842–3849.
Park, S. I., et al. (2008). Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Research, 68(9), 3323–3333.
Sen, B., et al. (2009). Sustained Src inhibition results in signal transducer and activator of transcription 3 (STAT3) activation and cancer cell survival via altered Janus-activated kinase-STAT3 binding. Cancer Research, 69(5), 1958–1965.
Rosen, N., et al. (1986). Analysis of pp 60c-src protein kinase activity in human tumor cell lines and tissues. Journal of Biological Chemistry, 261(29), 13754–13759.
Fanning, P., et al. (1992). Elevated expression of pp 60c-src in low grade human bladder carcinoma. Cancer Research, 52(6), 1457–1462.
Boyer, B., Bourgeois, Y., & Poupon, M. F. (2002). Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene, 21(15), 2347–2356.
Rodier, J. M., et al. (1995). pp 60c-src is a positive regulator of growth factor-induced cell scattering in a rat bladder carcinoma cell line. Journal of Cell Biology, 131(3), 761–773.
Thiery, J. P., & Chopin, D. (1999). Epithelial cell plasticity in development and tumor progression. Cancer Metastasis Reviews, 18(1), 31–42.
Simeonova, P. P., et al. (2002). c-Src-dependent activation of the epidermal growth factor receptor and mitogen-activated protein kinase pathway by arsenic. Role in carcinogenesis. Journal of Biological Chemistry, 277(4), 2945–2950.
Eblin, K. E., et al. (2007). Mitogenic signal transduction caused by monomethylarsonous acid in human bladder cells: role in arsenic-induced carcinogenesis. Toxicological Sciences, 95(2), 321–330.
Yamamoto, N., et al. (2006). Tyrosine phosphorylation of p145met mediated by EGFR and Src is required for serum-independent survival of human bladder carcinoma cells. Journal of Cell Science, 119(Pt 22), 4623–4633.
DerMardirossian, C., & Bokoch, G. M. (2005). GDIs: central regulatory molecules in Rho GTPase activation. Trends in Cell Biology, 15(7), 356–363.
Golovanov, A. P., et al. (2001). Structure-activity relationships in flexible protein domains: regulation of rho GTPases by RhoGDI and D4 GDI. Journal of Molecular Biology, 305(1), 121–135.
Olofsson, B. (1999). Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal, 11(8), 545–554.
Ota, T., et al. (2006). RhoGDIbeta lacking the N-terminal regulatory domain suppresses metastasis by promoting anoikis in v-src-transformed cells. Clinical & Experimental Metastasis, 23(7–8), 323–334.
Krieser, R. J., & Eastman, A. (1999). Cleavage and nuclear translocation of the caspase 3 substrate Rho GDP-dissociation inhibitor, D4-GDI, during apoptosis. Cell Death and Differentiation, 6(5), 412–419.
Kwon, K. B., et al. (2002). D4-GDI is cleaved by caspase-3 during daunorubicin-induced apoptosis in HL-60 cells. Experimental and Molecular Medicine, 34(1), 32–37.
Zhou, X., et al. (2004). Nuclear translocation of cleaved LyGDI dissociated from Rho and Rac during Trp53-dependent ionizing radiation-induced apoptosis of thymus cells in vitro. Radiation Research, 162(3), 287–295.
Ota, T., et al. (2004). LyGDI functions in cancer metastasis by anchoring Rho proteins to the cell membrane. Molecular Carcinogenesis, 39(4), 206–220.
Ma, L., et al. (2007). Loss of expression of LyGDI (ARHGDIB), a rho GDP-dissociation inhibitor, in Hodgkin lymphoma. British Journal of Haematology, 139(2), 217–223.
Tapper, J., et al. (2001). Changes in gene expression during progression of ovarian carcinoma. Cancer Genetics and Cytogenetics, 128(1), 1–6.
Hu, L. D., et al. (2007). Biphasic expression of RhoGDI2 in the progression of breast cancer and its negative relation with lymph node metastasis. Oncology Reports, 17(6), 1383–1389.
Zhang, B. (2006). Rho GDP dissociation inhibitors as potential targets for anticancer treatment. Drug Resistance Updates, 9(3), 134–141.
Zhang, B., et al. (2005). Rho GDP dissociation inhibitor protects cancer cells against drug-induced apoptosis. Cancer Research, 65(14), 6054–6062.
Zhang, Y., et al. (2009). Silencing of D4-GDI inhibits growth and invasive behavior in MDA-MB-231 cells by activation of Rac-dependent p38 and JNK signaling. Journal of Biological Chemistry, 284(19), 12956–12965.
Wang, Y., et al. (2005). Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet, 365(9460), 671–679.
Essmann, F., et al. (2000). GDP dissociation inhibitor D4-GDI (Rho-GDI 2), but not the homologous rho-GDI 1, is cleaved by caspase-3 during drug-induced apoptosis. Biochemical Journal, 346(Pt 3), 777–783.
DerMardirossian, C., et al. (2006). Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Molecular Biology of the Cell, 17(11), 4760–4768.
Moissoglu, K., et al. (2009). Rho GDP dissociation inhibitor 2 suppresses metastasis via unconventional regulation of RhoGTPases. Cancer Research, 69(7), 2838–2844.
Uhlenbrock, K., et al. (2004). The RacGEF Tiam1 inhibits migration and invasion of metastatic melanoma via a novel adhesive mechanism. Journal of Cell Science, 117(Pt 20), 4863–4871.
Acknowledgments
This study was supported by NIH grant R01CA075115 to DT. The authors wish to thank Dr. Michael Harding for helpful suggestions. None of the authors have any financial conflict of interest that might be construed to influence the results or interpretation of the manuscript.
Competing interest statement
The authors declare that they have no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Said, N., Theodorescu, D. Pathways of metastasis suppression in bladder cancer. Cancer Metastasis Rev 28, 327–333 (2009). https://doi.org/10.1007/s10555-009-9197-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-009-9197-4