Abstract
There are ten mitogen-activated protein kinase (MAPK) phosphatases (MKPs) that act as negative regulators of MAPK activity in mammalian cells and these can be subdivided into three groups. The first comprises DUSP1/MKP-1, DUSP2/PAC1, DUSP4/MKP-2 and DUSP5/hVH-3, which are inducible nuclear phosphatases. With the exception of DUSP5, these MKPs display a rather broad specificity for inactivation of the ERK, p38 and JNK MAP kinases. The second group contains three closely related ERK-specific and cytoplasmic MKPs encoded by DUSP6/MKP-3, DUSP7/MKP-X and DUSP9/MKP-4. The final group consists of three MKPs DUSP8/hVH-5, DUSP10/MKP-5 and DUSP16/MKP-7 all of which preferentially inactivate the stress-activated p38 and JNK MAP kinases. Abnormal MAPK signalling will have important consequences for processes critical to the development and progression of human cancer. In addition, MAPK signalling also plays a key role in determining the response of tumour cells to conventional cancer therapies. The emerging roles of the dual-specificity MKPs in the regulation of MAPK activities in normal tissues has highlighted the possible pathophysiological consequences of either loss (or gain) of function of these enzymes as part of the oncogenic process. This review summarises the current evidence implicating the dual-specificity MKPs in the initiation and development of cancer and also on the outcome of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 Mitogen-activated protein kinases and cancer
Mitogen-activated protein kinase (MAPK) pathways constitute a highly conserved family of kinase modules that serve to relay information from extracellular signals to the effectors that control diverse cellular processes such as proliferation, differentiation, migration and apoptosis (reviewed in [1–5]). MAPKs are activated by phosphorylation on both the threonine and tyrosine residues of a conserved signature T-X-Y motif within the activation loop of the kinase. This is mediated by a dual-specificity MAPK kinase (MKK or MEK), which is turn regulated by phosphorylation on serine/theonine residues by a MAPK kinase kinase (MKKK or MEKK [6]). Three major groups of MAPK have been characterised in mammalian cells based on sequence similarity, differential regulation by agonists and substrate specificity. These are the classical p42 and p44 MAPKs otherwise known as extracellular signal-regulated kinases ERK2 and ERK1 respectively, the c-Jun amino-terminal kinases JNK1, 2 and 3 and the four p38 MAPKs (α, β, δ and γ) [7].
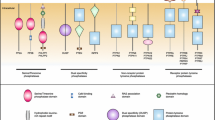
Because MAPK activities impinge on many of the processes involved in the initiation and genesis of cancer, abnormalities in MAPK signalling pathways have been implicated in a wide range of human malignancies. The classical ERK pathway has long been associated with the ability of cancer cells to grow independently of normal proliferation signals and is deregulated in approximately 30% of human tumours (Fig. 1). Oncogenic abnormalities are found in upstream components of the ERK MAPK signalling pathways and include the over-expression or activating mutation of receptor tyrosine kinases, activating mutations in the Ras GTPase and mutations in the serine/threonine MAPK kinase kinase B-raf (reviewed in [8]).
With respect to the stress activated MAP kinases their role in cancer can be complex and, in the case of JNK, somewhat controversial (Fig. 1). For instance, there is evidence implicating JNK activity in Ras-induced cell transformation in vitro (reviewed in [9]). However, tumourigenesis studies using Ras-transformed fibroblasts derived from mice lacking JNK1 and 2 indicate that loss of JNK does not prevent tumour formation on injection of these cells into nude mice. Furthermore, lung metastases were actually larger when JNK was ablated indicating that JNK acts as a tumour suppressor in vivo [10]. Studies of fibroblasts derived from p38α knockout mice also support a role for this MAPK as a tumour suppressor. These cells are more susceptible to H-RasV12-induced transformation, show decreased levels of apoptosis and form tumours more readily when injected into nude mice [11]. Furthermore, the deletion of p38α in adult mice leads to an immature and hyperproliferative lung epithelium that is highly sensitized to K-Ras(G12V)-induced tumourigenesis [12]. The tumour suppressive function of p38 MAPK could be mediated in a number of different ways. The p38 pathway may be involved in the activation of p53 and p53-mediated apoptosis and recent work has also implicated p38 in the promotion of cellular senescence as a means of evading oncogene-induced transformation [13, 14]. Finally, because of the key role that these MAPK pathways play in mediating stress-induced apoptosis, both p38 and JNK signalling are important determinants of cellular responses to conventional cancer therapies including chemotherapy and radiation (Fig. 1).
A major determinant of the biological outcome of MAPK signalling is the duration and magnitude of kinase activation [15]. This reflects a balance between the activities of upstream activators and various negative regulatory mechanisms, which oppose pathway activation. It is now clear that a major point of control occurs at the level of the MAPK itself through the activities of specific MAPK phosphatases. The requirement for phosphorylation on both threonine and tyrosine residues in order to activate the MAPK means that dephosphorylation of either residue is sufficient for kinase inactivation. This can be achieved by serine/threonine phosphatases, tyrosine specific phosphatases or by dual-specificity phosphatases and studies in a wide variety of model organisms from yeast to man have demonstrated that all three major classes of protein phosphatase can perform this task in vivo [16, 17]. However, by far the largest group of protein phosphatases that act to specifically regulate the phosphorylation and activity of mammalian MAPKs are the dual-specificity MAPK phosphatases (MKPs)
1.2 Dual-specificity MAPK phosphatases
The MKPs constitute a distinct subgroup of ten catalytically active enzymes within the larger family of cysteine-dependent dual-specificity protein phosphatases (DUSPs) [18]. They share a common structure that comprises a C-terminal catalytic domain, which shares sequence similarity with the prototypic VH-1 DUSP encoded by vaccinia virus, and an N-terminal non-catalytic domain. This latter region contains both a conserved cluster of basic amino acid residues involved in MAPK recognition known as the kinase interaction motif (KIM) and also sequences which determine the subcellular localisation of these enzymes [19, 20]. On the basis of sequence similarity, gene structure, substrate specificity and subcellular localisation the ten MKPs can be subdivided into three distinct groups (Table 1). The first of these contains DUSP1/MKP-1, DUSP2/PAC-1, DUSP4/MKP-2 and DUSP5/hVH-3. All of these proteins are encoded by highly inducible genes, which are rapidly up-regulated in response to both mitogenic and/or stress stimuli at the transcriptional level. They are all nuclear proteins and with the exception of DUSP5/hVH-3, which appears to specifically target the ERK1/2 MAPKs, they exhibit broad substrate specificity and can inactivate ERK, JNK and p38 MAPKs. The second group of MKPs consists of DUSP6/MKP-3, DUSP7/MKP-X and DUSP9/MKP-4. All three proteins are cytoplasmic enzymes and exhibit a degree of selectivity towards the classical ERK1/2 MAPKs. This is most marked in DUSP6/MKP-3, which has little or no activity towards either JNK or p38 when over-expressed in mammalian cells. The final group of MKPs comprises DUSP8/hVH-5, DUSP10/MKP-5 and DUSP16/MKP-7. These proteins are found in both the cytoplasm and the nucleus and selectively dephosphoryate the stress-activated MAPKs p38 and JNK, while showing little or no activity towards ERK1/2.
Despite a fairly detailed knowledge of the biochemical properties and catalytic mechanism employed by the MKPs [20, 21], our knowledge of their physiological functions is somewhat less complete. However, recent gene targeting experiments in mice have been instrumental in revealing a diverse range of physiological functions for these enzymes [22]. DUSP1/MKP-1 plays an essential role in regulating cytokine biosynthesis in response to bacterial lipopolysaccharide (LPS) and also mediates, at least in part, the anti-inflammatory response to glucocorticoids [23–26]. In addition, DUSP1/MKP-1 plays a key role in metabolic homeostasis, as mice lacking this gene are resistant to obesity induced by a high fat diet [27]. Interestingly, the effects of DUSP1/MKP-1 deletion on metabolic control appear to result from the deregulation of more than one MAPK, indicating that this enzyme acts as an integration point in the control of these signalling pathways. DUSP10/MKP-5 also plays a significant role in mediating innate and adaptive immunity, primarily by regulating the JNK pathway in immune effector cells [28]. In contrast, DUSP2/PAC-1 is a positive regulator of inflammatory responses as DUSP2/PAC-1 knockout mice are resistant in experimental models of immune arthritis [29]. As was the case with DUSP1/MKP-1 these effects stem from complex changes in the activities of more then one MAPK pathway. Of the cytoplasmic MKPs so far analysed in this way, DUSP6/MKP-3 is responsible for regulating ERK activity in response to fibroblast growth factor (FGF) signalling during early mouse development and DUSP9/MKP-4 plays an essential role in the regulation of MAPK activity in extraembryonic tissues [30–32].
Given the diverse range of physiological endpoints which are regulated by the MKPs it would be surprising if they were not involved in the regulation of MAPK activities during processes of direct relevance to the initiation and development of human cancer. The evidence linking MKP function to the process of cell transformation and tumour development is summarised below. In addition the involvement of MKPs in mediating resistance to conventional cancer therapies is also discussed.
2 MAP kinase phosphatases and cancer
2.1 DUSP1/MKP-1
Soon after the characterisation of DUSP1/MKP-1 as a mitogen and stress inducible MAP kinase phosphatase, the levels of DUSP1/MKP-1 mRNA and protein were investigated in human cancers. These initial studies revealed significant over-expression of DUSP1/MKP-1 in a range of human epithelial tumours including prostate, colon and bladder [33]. Interestingly, over-expression was seen only in the early phases of disease with levels of DUSP1/MKP-1 expression falling progressively in tumours of higher histological grade and in metastases (Table 2). This pattern of expression was reinforced by the results of a serial analysis of gene expression (SAGE) study in which DUSP1/MKP-1 was identified as one of 20 transcripts which showed the largest decreases in level in colorectal cancer when compared with normal tissue [34]. No loss of chromosome 5q34-ter, which contains the DUSP1 locus [35], was detected in tumours, nor were any mutations found within the catalytic domain of DUSP1/MKP-1 [33]. The mechanisms by which DUSP1/MKP-1 levels are altered in tumours may be varied and complex. The DUSP1/MKP-1 gene is a transcriptional target of the p53 tumour suppressor [36] and is also up-regulated in response to a variety of cellular stress conditions including oxidative stress and DNA-damaging agents [37]. Interestingly, DUSP1/MKP-1 is also up-regulated in response to hypoxia at levels found in solid tumours, suggesting that it may play a key role in the regulation of MAPK activities within the tumour microenvironment [38].
With respect to prostate cancer, increased DUSP1/MKP-1 expression was also seen in the Noble rat model of sex hormone-induced prostatic dysplasia and carcinoma [39]. Furthermore, the increased levels of DUSP1/MKP-1 expression observed in human prostate cancer were inversely correlated with both JNK activity and markers of apoptosis, indicating that MKP-1 might be anti-apoptotic in these tumours via its activity towards JNK [40, 41]. Consistent with this idea, human DU145 prostatic cancer cells that over-express DUSP1/MKP-1 are resistant to Fas ligand-induced mitochondrial perturbation and apoptosis [42].
DUSP1/MKP-1 levels were also studied in primary ovarian tumours where moderate to strong expression of DUSP1/MKP-1 was detected in 57.6% of invasive ovarian carcinomas and MKP-1 expression was a prognostic marker for shorter progression-free survival (Table 2) [43]. In contrast to the loss of DUSP1/MKP-1 expression noted in prostate, colon and bladder cancer, breast carcinomas showed significant MKP-1 expression even when poorly differentiated or in the late stages of the disease [33]. As was the case in prostate cancer, high levels of MKP-1 correlated with reduced JNK activity suggesting that therapeutic intervention to reduce the expression or activity of DUSP1/MKP-1 might enable the expression of the pro-apoptotic signals from JNK in malignant cells [44]. In support of this idea the over-expression of MKP-1 in breast carcinoma cell lines reduced JNK activity, caspase activation and DNA fragmentation and enhanced cell viability after treatment with alkylating agents (mechlorethamine), anthracyclines (doxorubicin), and microtubule inhibitors (paclitaxel) [45]. In contrast, reduction of DUSP1/MKP-1 levels using a small interfering RNA enhanced sensitivity to these drugs, and this was associated with increased JNK activity.
The idea that DUSP1/MKP-1 might mediate chemoresistance in cancer cells first came to prominence with the realisation that the stress-activated p38 and JNK MAPKs were bona fide substrates for this MKP. Preliminary studies found that over-expression of DUSP1/MKP-1 enhanced cellular resistance to cisplatin and that this correlated with reduced levels of JNK rather than p38 activity [46]. Confirmation of a key role for DUSP1/MKP-1 in mediating resistance to this drug came with the observation that mouse embryo fibroblasts (MEFs) derived from DUSP1/MKP-1 knockout mice were sensitive to cisplatin and that cisplatin-induced cell death could be inhibited by blocking JNK but not ERK and p38 activities in these cells [47]. Another human tumour which shows elevated levels of DUSP1/MKP-1 expression is non-small-cell lung cancer (NSCLC) [48, 49]. Cisplatin is often used to treat this disease, but often the late stage at which this cancer is diagnosed coupled with drug resistance results in a very poor prognosis with median survival of approximately 1 year. Analysis of NSCLC tumour tissues found strong nuclear staining for DUSP1/MKP-1 whereas in normal bronchial epithelium this protein was localized in the cytoplasm as well as the nucleus. In NSCLC cell lines DUSP1/MKP-1 was constitutively expressed and siRNA-mediated down-regulation of the gene caused a tenfold increase in sensitivity to cisplatin [50]. Xenograft experiments in nude mice using these siRNA-expressing cell lines also resulted in tumours that displayed slower growth rates and increased susceptibility to cisplatin. Taken together, these studies indicate that at least in a subset of human cancers, MKP-1 expression may mediate chemoresistance. Given the poor response to conventional chemotherapy in NSCLC and the ability of MKP-1 to mediate resistance to novel anticancer agents such as proteasome inhibitors [51], DUSP1/MKP-1 may be an important target as part of future strategies to improve tumour cell response to chemotherapy.
2.2 Other inducible nuclear MKPs and cancer
Compared with DUSP1/MKP-1, relatively little is known about the involvement of the other inducible nuclear MKPs in human malignancy. The most closely related enzyme to DUSP1/MKP-1 is DUSP4/MKP-2 and there is some evidence that this phosphatase is over-expressed in serous borderline tumours (SBT) of the ovary (Table 2). Compared with serous carcinomas (SCAs) these tumours present a more benign phenotype with a lack of stromal invasion. The expression levels of DUSP4/MKP-2 were found to be much higher in SBT when compared with SCAs and it was speculated that this may play some role in the more benign behaviour of SBT via the suppression of ERK-dependent events associated with degradation of the extracellular matrix [52]. MEK-dependent over-expression of DUSP4/MKP-2 has also been reported in pancreatic cancer cell lines harbouring activating mutations in K-Ras [53] and also in breast cancer where it appears to be co-expressed with DUSP1/MKP-1 [44]. Of the remaining members of this family, expression of DUSP2/PAC-1 has been observed in serous carcinomas of the ovary and its presence was associated with a poor outcome in terms of overall survival [54]. Loss of DUSP2/PAC-1 expression has also been associated with elevated levels of ERK activation in acute leukemias (Table 2) [55].
2.3 Cytoplasmic ERK-specific MKPs and cancer
Of the three cytoplasmic ERK-specific phosphatases encoded by DUSP6/MKP-3, DUSP7/MKP-X and DUSP9/MKP-4, the most widely studied and best characterized is DUSP6/MKP-3. The first hint that DUSP6/MKP-3 might be involved in the pathogenesis of human cancer came from studies of its possible role in pancreatic cancers associated with frequent allelic loss at 12q21. Although no gene mutations were detected, levels of DUSP6/MKP-3 mRNA were significantly reduced in a number of pancreatic cancer cell lines [56]. The vast majority (around 90%) of human pancreatic tumours carry activating mutations in K-Ras. Interestingly, DUSP6/MKP-3 was identified as one of only three genes which are uniquely expressed in myeloma cells harbouring a constitutively active mutant N-ras gene and is also overexpressed in human breast epithelial cells stably expressing H-ras and in human melanoma cell lines with potent activating mutations in B-raf [57–59]. These studies suggest that the over-expression of DUSP6/MKP-3 seen in response to activated Ras might represent a compensatory increase in the negative feedback control of the ERK1/2 MAPK pathway, which lies downstream of these activated oncogenes. In support of this, the tetracycline-induced expression of a functional fusion protein between DUSP6/MKP-3 and green fluorescent protein (GFP) in H-ras transformed fibroblasts following injection into nude mice resulted in a large delay in tumour emergence and growth as compared to the untreated control group [60]. In pancreatic cancer, the subsequent loss of DUSP6/MKP-3 expression might synergise with K-ras resulting in increased activation of ERK1/2 MAP kinase and thus contribute to the development and expression of the full malignant and invasive phenotype. An initial immunohistochemical study of primary pancreatic cancer tissues detected up-regulation in mildly as well as severely dysplastic/in situ carcinoma cells and down-regulation in invasive carcinoma, especially in the poorly differentiated type (Table 2). Furthermore, the reintroduction of active DUSDP6/MKP-3 into cultured pancreatic cancer cells resulted in suppression of cell growth and increased levels of apoptosis [61].
A more extensive study of the expression of DUSP6/MKP-3 in pancreatic intraepithelial neoplasia and/or intraductal papillary-mucinous neoplasms, both of which are considered to be precursor lesions of invasive carcinoma of the pancreas, revealed that the expression of DUSP6/MKP-3 was selectively abrogated in invasive ductal carcinoma cells [62]. In contrast, expression was retained in the majority of precursor lesions. Interestingly, most of the intraductal adenoma/borderline lesions that had lost DUSP6/MKP-3 expression also harboured mutations of K-ras2. These results strongly suggest that loss of DUSP6/MKP-3 is associated exclusively with progression from pancreatic intraepithelial neoplasia to invasive ductal carcinoma. Loss of DUSP6/MKP-3 expression was found to correlate with methylation of CpG sequences in intron 1 of the DUSP6/MKP-3 gene in both pancreatic cell lines and in five of eight cases of pancreatic cancer, four of which were poorly differentiated adenocarcinoma [63]. This suggests that hypermethylation with modification of histone deacetylation play an important role in transcriptional suppression of DUSP6/MKP-3 in this disease. The recent generation of mice lacking the murine DUSP6/MKP-3 gene [30] should allow a systematic evaluation of its potential role in the development and progression of pre-neoplastic pancreatic lesions by intercrossing these animals with recently established murine models of pancreatic cancer, which employ tissue specific expression of mutant K-ras in the pancreatic epithelium [64].
With regard to the other members of this subfamily of cytoplasmic MKPs, much less is known about either their normal physiological functions or any potential roles in cell transformation and cancer. A micro-array study identified DUSP7/MKP-X (Pyst2) as over-expressed in acute leukemia [65] and this result was subsequently confirmed by RT-PCR and Northern blot analysis [66]. A more extensive study showed that DUSP7/MKP-X mRNA and protein were highly expressed in leukocytes obtained from acute myeloid leukemia (AML) patients. High levels of mRNA were also expressed in bone marrow (BM) and peripheral leukocytes from nine AML and acute lymphoblastic leukemia (ALL) patients (Table 2). In contrast, BM from healthy individuals expressed very low levels of DUSP7/MKP-X [67]. While these studies point towards a link with abnormal MAPK signalling in these acute leukemias it is not yet clear if this observation has any pathological or prognostic significance.
DUSP9/MKP-4 the third member of this group of MKPs is a cytosolic MKP which, in contrast to DUSP6/MKP-3, shows a more relaxed substrate specificity displaying significant activity towards stress-activated MAPKs in addition to the classical ERK1/2 MAPKs [68, 69]. Gene targeting experiments in mice have recently shown that DUSP9/MKP-4 plays an essential role in placental development and function but its role in adult animals is as yet unclear [32]. In recent study DUSP9/MKP-4 was identified in an Affymetrix GeneChip analysis as an mRNA which was down-regulated in a non-Ras model of epithelial carcinogenesis. Loss of DUSP9/MKP-4 was also observed in tumours independently generated by the application of chemical or physical carcinogens in mice [70]. Interestingly, reconstitution experiments in which DUSP9/MKP-4 was re-expressed led to increased levels of tumour cell death. In contrast, expression of the related ERK-specific phosphatase DUSP6/MKP-3 did not increase levels of tumour cell apoptosis. Finally, lentiviral mediated expression of DUSP9/MKP-4 in tumourigenic cells led to almost total suppression of tumour formation following sub-cutaneous injection into BALB/c neonatal mice when compared with uninfected cells and conditional (tetracycline-inducible) expression of MKP-4 in established NSCLC cell line induced tumours also caused tumour stasis [70]. Taken together these results are suggestive of a tumour suppressor function for DUSP9/MKP-4 and indicate that its activity towards p38 and JNK in addition to inactivation of ERK may be responsible for this effect. Due to the embryonic lethality of the DUSP9/MKP-4 gene knockout, confirmation of this role in vivo will require carcinogenesis studies in conjunction with conditional loss of DUSP9/MKP-4 activity in adult tissues. This study should also prompt an extensive examination of the levels of DUSP9/MKP-4 mRNA and protein in human skin tumours.
3 Conclusions and perspectives
MAPKs play diverse and sometimes apparently contradictory roles in the initiation and development of human cancers. In the case of the protein phosphatases that negatively regulate MAPKs, there is increasing evidence that these enzymes may be abnormally regulated in certain tumours. However, whether either over-expression or loss of expression is a cause of, or actually contributes to, the malignant phenotype rather than simply being a consequence of cell transformation is as yet unclear. The increasing availability of genetic knockout models for individual MKPs should allow a direct test in cases where loss of expression is associated with the development of certain cancers. These animals can also be used as experimental models to dissect out the roles of MKP in mediating the resistance of tumours to chemical and physical agents used in both conventional and novel cancer therapies. The next few years should bring significant advances in our understanding of how abnormalities in the regulation of MAPK pathways can be exploited as novel therapeutic targets in the war against cancer.
Abbreviations
- ALL:
-
acute lymphocytic leukaemia
- AML:
-
acute myeloid leukaemia
- BM:
-
bone marrow
- DUSP:
-
dual-specificity protein phosphatase
- ERK:
-
extracellular-signal regulated kinase
- JNK:
-
c-Jun amino-terminal kinase
- MAPK:
-
mitogen-activated protein kinase
- MEF:
-
mouse embryo fibroblast
- MKK or MEK:
-
MAPK kinase
- MKKK or MEKK:
-
MAPK kinase kinase
- MKP:
-
MAP kinase phosphatase
- NSCLC:
-
non-small cell lung cancer
- SCA:
-
serous ovarian carcinoma
- SAGE:
-
serial analysis of gene expression
- SBT:
-
serous borderline ovarian tumour
References
Wada, T., & Penninger, J. M. (2004). Mitogen-activated protein kinases in apoptosis regulation. Oncogene, 23(16), 2838–2849.
Davis, R. J. (2000). Signal transduction by the JNK group of MAP kinases. Cell, 103(2), 239–252.
Chang, L., & Karin, M. (2001). Mammalian MAP kinase signalling cascades. Nature, 410(6824), 37–40.
Johnson, G. L., & Lapadat, R. (2002). Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science, 298(5600), 1911–1912.
Pearson, G., Robinson, F., Beers Gibson, T., Xu, B. E., Karandikar, M., Berman, K., et al. (2001). Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine Reviews, 22(2), 153–183.
Marshall, C. J. (1994). MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Current Opinion in Genetics & Development, 4(1), 82–89.
Cohen, P. (1997). The search for physiological substrates of the MAP and SAP kinases in mammalian cells. Trends in Cell Biology, 7, 353–361.
Dhillon, A. S., Hagan, S., Rath, O., & Kolch, W. (2007). MAP kinase signalling pathways in cancer. Oncogene, 26(22), 3279–3290.
Kennedy, N. J., & Davis, R. J. (2003). Role of JNK in tumor development. Cell Cycle, 2(3), 199–201.
Kennedy, N. J., Sluss, H. K., Jones, S. N., Bar-Sagi, D., Flavell, R. A., & Davis, R. J. (2003). Suppression of Ras-stimulated transformation by the JNK signal transduction pathway. Genes & Development, 17(5), 629–637.
Dolado, I., Swat, A., Ajenjo, N., De Vita, G., Cuadrado, A., & Nebreda, A. R. (2007). p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell, 11(2), 191–205.
Ventura, J. J., Tenbaum, S., Perdiguero, E., Huth, M., Guerra, C., Barbacid, M., et al. (2007). p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nature Genetics, 39(6), 750–758.
Sun, P., Yoshizuka, N., New, L., Moser, B. A., Li, Y., Liao, R., et al. (2007). PRAK is essential for ras-induced senescence and tumor suppression. Cell, 128(2), 295–308.
Han, J., & Sun, P. (2007). The pathways to tumor suppression via route p38. Trends in Biochemical Sciences, 32(8), 364–371.
Marshall, C. J. (1995). Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell, 80(2), 179–185.
Keyse, S. M. (2000). Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Current Opinion in Cell Biology, 12(2), 186–192.
Saxena, M., & Mustelin, T. (2000). Extracellular signals and scores of phosphatases: All roads lead to MAP kinase. Seminars in Immunology, 12(4), 387–396.
Theodosiou, A., & Ashworth, A. (2002). MAP kinase phosphatases. Genome Biol, 3(7), REVIEWS3009.
Kondoh, K., & Nishida, E. (2007). Regulation of MAP kinases by MAP kinase phosphatases. Biochimica Et Biophysica Acta, 1773(8), 1227–1237.
Owens, D. M., & Keyse, S. M. (2007). Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene, 26(22), 3203–3213.
Farooq, A., & Zhou, M. M. (2004). Structure and regulation of MAPK phosphatases. Cell Signal, 16(7), 769–779.
Dickinson, R. J., & Keyse, S. M. (2006). Diverse physiological functions for dual-specificity MAP kinase phosphatases. Journal of Cell Science, 119(Pt 22), 4607–4615.
Chi, H., Barry, S. P., Roth, R., Wu, J. J., Jones, E. A., Bennett, A. M., et al. (2006). Dynamic regulation of pro-and anti-inflammatory cytokines by MKP-1 in innate immune responses. Proceedings of the National Academy of Sciences of the United States of America, 103, 2274–2279.
Hammer, M., Mages, J., Dietrich, H., Servatius, A., Howells, N., Cato, A. C., et al. (2006). Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. Journal of Experimental Medicine, 203(1), 15–20.
Abraham, S. M., Lawrence, T., Kleiman, A., Warden, P., Medghalchi, M., Tuckermann, J., et al. (2006). Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. Journal of Experimental Medicine, 203(8), 1883–1889.
Zhao, Q., Wang, X., Nelin, L. D., Yao, Y., Matta, R., Manson, M. E., et al. (2006). MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. Journal of Experimental Medicine, 203(1), 131–140.
Wu, J. J., Roth, R. J., Anderson, E. J., Hong, E. G., Lee, M. K., Choi, C. S., et al. (2006). Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metabolism, 4(1), 61–73.
Zhang, Y., Blattman, J. N., Kennedy, N. J., Duong, J., Nguyen, T., Wang, Y., et al. (2004). Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature, 430(7001), 793–797.
Jeffrey, K. L., Brummer, T., Rolph, M. S., Liu, S. M., Callejas, N. A., Grumont, R. J., et al. (2006). Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nature Immunology, 7(3), 274–283.
Li, C., Scott, D. A., Hatch, E., Tian, X., & Mansour, S. L. (2007). Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development, 134(1), 167–176.
Eblaghie, M. C., Lunn, J. S., Dickinson, R. J., Munsterberg, A. E., Sanz-Ezquerro, J. J., Farrell, E. R., et al. (2003). Negative feedback regulation of FGF signaling levels by Pyst1/MKP3 in chick embryos. Current Biology, 13(12), 1009–1018.
Christie, G. R., Williams, D. J., Macisaac, F., Dickinson, R. J., Rosewell, I., & Keyse, S. M. (2005). The dual-specificity protein phosphatase DUSP9/MKP-4 is essential for placental function but is not required for normal embryonic development. Molecular and Cellular Biology, 25(18), 8323–8333.
Loda, M., Capodieci, P., Mishra, R., Yao, H., Corless, C., Grigioni, W., et al. (1996). Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. American Journal of Pathology, 149(5), 1553–1564.
Zhang, L., Zhou, W., Velculescu, V. E., Kern, S. E., Hruban, R. H., Hamilton, S. R., et al. (1997). Gene expression profiles in normal and cancer cells. Science, 276(5316), 1268–1272.
Emslie, E. A., Jones, T. A., Sheer, D., & Keyse, S. M. (1994). The CL100 gene, which encodes a dual specificity (Tyr/Thr) MAP kinase phosphatase, is highly conserved and maps to human chromosome 5q34. Human Genetics, 93(5), 513–516.
Li, M., Zhou, J. Y., Ge, Y., Matherly, L. H., & Wu, G. S. (2003). The phosphatase MKP1 is a transcriptional target of p53 involved in cell cycle regulation. Journal of Biological Chemistry, 278(42), 41059–41068.
Keyse, S. M., & Emslie, E. A. (1992). Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature, 359(6396), 644–647.
Laderoute, K. R., Mendonca, H. L., Calaoagan, J. M., Knapp, A. M., Giaccia, A. J., & Stork, P. J. (1999). Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is induced by low oxygen conditions found in solid tumor microenvironments. A candidate MKP for the inactivation of hypoxia-inducible stress-activated protein kinase/c-Jun N-terminal protein kinase activity. Journal of Biological Chemistry, 274(18), 12890–12897.
Leav, I., Galluzzi, C. M., Ziar, J., Stork, P. J., Ho, S. M., & Loda, M. (1996). Mitogen-activated protein kinase and mitogen-activated kinase phosphatase-1 expression in the Noble rat model of sex hormone-induced prostatic dysplasia and carcinoma. Laboratory Investigation, 75(3), 361–370.
Magi-Galluzzi, C., Montironi, R., Cangi, M. G., Wishnow, K., & Loda, M. (1998). Mitogen-activated protein kinases and apoptosis in PIN. Virchows Archiv, 432(5), 407–413.
Magi-Galluzzi, C., Mishra, R., Fiorentino, M., Montironi, R., Yao, H., Capodieci, P., et al. (1997). Mitogen-activated protein kinase phosphatase 1 is overexpressed in prostate cancers and is inversely related to apoptosis. Laboratory Investigation, 76(1), 37–51.
Srikanth, S., Franklin, C. C., Duke, R. C., & Kraft, R. S. (1999). Human DU145 prostate cancer cells overexpressing mitogen-activated protein kinase phosphatase-1 are resistant to Fas ligand-induced mitochondrial perturbations and cellular apoptosis. Molecular and Cellular Biochemistry, 199(1–2), 169–178.
Denkert, C., Schmitt, W. D., Berger, S., Reles, A., Pest, S., Siegert, A., et al. (2002). Expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) in primary human ovarian carcinoma. International Journal of Cancer, 102(5), 507–513.
Wang, H. Y., Cheng, Z., & Malbon, C. C. (2003). Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Letters, 191(2), 229–237.
Small, G. W., Shi, Y. Y., Higgins, L. S., & Orlowski, R. Z. (2007). Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Research, 67(9), 4459–4466.
Sanchez-Perez, I., Martinez-Gomariz, M., Williams, D., Keyse, S. M., & Perona, R. (2000). CL100/MKP-1 modulates JNK activation and apoptosis in response to cisplatin. Oncogene, 19(45), 5142–5152.
Wang, Z., Xu, J., Zhou, J. Y., Liu, Y., & Wu, G. S. (2006). Mitogen-activated protein kinase phosphatase-1 is required for cisplatin resistance. Cancer Research, 66(17), 8870–8877.
Vicent, S., Garayoa, M., Lopez-Picazo, J. M., Lozano, M. D., Toledo, G., Thunnissen, F. B., et al. (2004). Mitogen-activated protein kinase phosphatase-1 is overexpressed in non-small cell lung cancer and is an independent predictor of outcome in patients. Clinical Cancer Research, 10(11), 3639–3649.
Lim, E. H., Aggarwal, A., Agasthian, T., Wong, P. S., Tan, C., Sim, E., et al. (2003). Feasibility of using low-volume tissue samples for gene expression profiling of advanced non-small cell lung cancers. Clinical Cancer Research, 9(16 Pt 1), 5980–5987.
Chattopadhyay, S., Machado-Pinilla, R., Manguan-Garcia, C., Belda-Iniesta, C., Moratilla, C., Cejas, P., et al. (2006). MKP1/CL100 controls tumor growth and sensitivity to cisplatin in non-small-cell lung cancer. Oncogene, 25(23), 3335–3345.
Small, G. W., Shi, Y. Y., Edmund, N. A., Somasundaram, S., Moore, D. T., & Orlowski, R. Z. (2004). Evidence that mitogen-activated protein kinase phosphatase-1 induction by proteasome inhibitors plays an antiapoptotic role. Molecular Pharmacology, 66(6), 1478–1490.
Sieben, N. L., Oosting, J., Flanagan, A. M., Prat, J., Roemen, G. M., Kolkman-Uljee, S. M., et al. (2005). Differential gene expression in ovarian tumors reveals Dusp 4 and Serpina 5 as key regulators for benign behavior of serous borderline tumors. Journal of Clinical Oncology, 23(29), 7257–7264.
Yip-Schneider, M. T., Lin, A., & Marshall, M. S. (2001). Pancreatic tumor cells with mutant K-ras suppress ERK activity by MEK-dependent induction of MAP kinase phosphatase-2. Biochemical and Biophysical Research Communications, 280(4), 992–997.
Givant-Horwitz, V., Davidson, B., Goderstad, J. M., Nesland, J. M., Trope, C. G., & Reich, R. (2004). The PAC-1 dual specificity phosphatase predicts poor outcome in serous ovarian carcinoma. Gynecologic Oncology, 93(2), 517–523.
Kim, S. C., Hahn, J. S., Min, Y. H., Yoo, N. C., Ko, Y. W., & Lee, W. J. (1999). Constitutive activation of extracellular signal-regulated kinase in human acute leukemias: Combined role of activation of MEK, hyperexpression of extracellular signal-regulated kinase, and downregulation of a phosphatase, PAC1. Blood, 93(11), 3893–3899.
Furukawa, T., Yatsuoka, T., Youssef, E. M., Abe, T., Yokoyama, T., Fukushige, S., et al. (1998). Genomic analysis of DUSP6, a dual specificity MAP kinase phosphatase, in pancreatic cancer. Cytogenetics and Cell Genetics, 82(3–4), 156–159.
Warmka, J. K., Mauro, L. J., & Wattenberg, E. V. (2004). Mitogen-activated protein kinase phosphatase-3 is a tumor promoter target in initiated cells that express oncogenic Ras. Journal of Biological Chemistry, 279(32), 33085–33092.
Croonquist, P. A., Linden, M. A., Zhao, F., & Van Ness, B. G. (2003). Gene profiling of a myeloma cell line reveals similarities and unique signatures among IL-6 response, N-ras-activating mutations, and coculture with bone marrow stromal cells. Blood, 102(7), 2581–2592.
Bloethner, S., Chen, B., Hemminki, K., Muller-Berghaus, J., Ugurel, S., Schadendorf, D., et al. (2005). Effect of common B-RAF and N-RAS mutations on global gene expression in melanoma cell lines. Carcinogenesis, 26(7), 1224–1232.
Marchetti, S., Gimond, C., Roux, D., Gothie, E., Pouyssegur, J., & Pages, G. (2004). Inducible expression of a MAP kinase phosphatase-3-GFP chimera specifically blunts fibroblast growth and ras-dependent tumor formation in nude mice. Journal of Cellular Physiology, 199(3), 441–450.
Furukawa, T., Sunamura, M., Motoi, F., Matsuno, S., & Horii, A. (2003). Potential tumor suppressive pathway involving DUSP6/MKP-3 in pancreatic cancer. American Journal of Pathology, 162(6), 1807–1815.
Furukawa, T., Fujisaki, R., Yoshida, Y., Kanai, N., Sunamura, M., Abe, T., et al. (2005). Distinct progression pathways involving the dysfunction of DUSP6/MKP-3 in pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms of the pancreas. Modern Pathology, 18(8), 1034–1042.
Xu, S., Furukawa, T., Kanai, N., Sunamura, M., & Horii, A. (2005). Abrogation of DUSP6 by hypermethylation in human pancreatic cancer. Journal of Human Genetics, 50(4), 159–167.
Leach, S. D. (2004). Mouse models of pancreatic cancer: the fur is finally flying!. Cancer Cell, 5(1), 7–11.
Levy-Nissenbaum, O., Sagi-Assif, O., Raanani, P., Avigdor, A., Ben-Bassat, I., & Witz, I. P. (2003). cDNA microarray analysis reveals an overexpression of the dual-specificity MAPK phosphatase PYST2 in acute leukemia. Methods in Enzymology, 366, 103–113.
Levy-Nissenbaum, O., Sagi-Assif, O., Raanani, P., Avigdor, A., Ben-Bassat, I., & Witz, I. P. (2003). Overexpression of the dual-specificity MAPK phosphatase PYST2 in acute leukemia. Cancer Letters, 199(2), 185–192.
Levy-Nissenbaum, O., Sagi-Assif, O., Kapon, D., Hantisteanu, S., Burg, T., Raanani, P., et al. (2003). Dual-specificity phosphatase Pyst2-L is constitutively highly expressed in myeloid leukemia and other malignant cells. Oncogene, 22(48), 7649–7660.
Muda, M., Boschert, U., Smith, A., Antonsson, B., Gillieron, C., Chabert, C., et al. (1997). Molecular cloning and functional characterization of a novel mitogen-activated protein kinase phosphatase, MKP-4. Journal of Biological Chemistry, 272(8), 5141–5151.
Dickinson, R. J., Williams, D. J., Slack, D. N., Williamson, J., Seternes, O. M., & Keyse, S. M. (2002). Characterization of a murine gene encoding a developmentally regulated cytoplasmic dual-specificity mitogen-activated protein kinase phosphatase. Biochemical Journal, 364(Pt 1), 145–155.
Liu, Y., Lagowski, J., Sundholm, A., Sundberg, A., & Kulesz-Martin, M. (2007). Microtubule disruption and tumor suppression by mitogen-activated protein kinase phosphatase 4. Cancer Research, 67(22), 10711–10719.
Acknowledgements
Work in my laboratory is supported by Cancer Research UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keyse, S.M. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev 27, 253–261 (2008). https://doi.org/10.1007/s10555-008-9123-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-008-9123-1