Abstract
In patients with heart failure with preserved ejection fraction (HFpEF), diabetes mellitus (DM) and obesity are important comorbidities as well as major risk factors. Their conjoint impact on the myocardium provides insight into the HFpEF aetiology. We sought to investigate the association between obesity, DM, and their combined effect on alterations in the myocardial tissue in HFpEF patients. One hundred and sixty-two HFpEF patients (55 ± 12 years, 95 men) and 45 healthy subjects (53 ± 12 years, 27 men) were included. Patients were classified according to comorbidity prevalence (36 obese patients without DM, 53 diabetic patients without obesity, and 73 patients with both). Myocardial remodeling, fibrosis, and longitudinal contractility were quantified with cardiovascular magnetic resonance imaging using cine and myocardial native T1 images. Patients with DM and obesity had impaired global longitudinal strain (GLS) and increased myocardial native T1 compared to patients with only one comorbidity (DM + Obesity vs. DM and Obesity; GLS, − 15 ± 2.1 vs − 16.5 ± 2.4 and − 16.7 ± 2.2%; native T1, 1162 ± 37 vs 1129 ± 25 and 1069 ± 29 ms; P < 0.0001 for all). A negative synergistic effect of combined obesity and DM prevalence was observed for native T1 (np2 = 0.273, p = 0.002) and GLS (np2 = 0.288, p < 0.0001). Additionally, severity of insulin resistance was associated with GLS (R = 0.590, P < 0.0001), and native T1 (R = 0.349, P < 0.0001). The conjoint effect of obesity and DM in HFpEF patients is associated with diffuse myocardial fibrosis and deterioration in GLS. The negative synergistic effects observed on the myocardium may be related to severity of insulin resistance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heart failure with preserved ejection fraction (HFpEF) affects approximately half of all clinical HF patients and leads to greater morbidity and mortality than HF with reduced ejection fraction (HFrEF) [1, 2]. Coupled with its complex pathopysiology, HFpEF is considered a heterogeneous syndrome linked to several comorbidities, such as diabetes mellitus (DM) and obesity [1,2,3,4,5,6,7].
Obesity is a growing worldwide health burden and strongly associated with HFpEF [8,9,10,11]. Its effects on the cardiovascular system are multifaceted. Specifically, changes in volume status, altered cardiac loading, reduced energy substrate utilization, abnormal tissue metabolism, and systemic inflammation are all factors considered to promote HFpEF progression [8,9,10,11,12]. Additionally, increased body mass is independently associated with symptom severity in HFpEF patients [9,10,11,12,13,14]. Similarly, diabetes mellitus (DM) is present in ~ 40% of HFpEF patients, and its prevalence is increasing in patients with new-onset HFpEF [5, 15, 16]. DM is generally associated with left ventricular (LV) function decline through increased myocardial stiffness, fibrosis, and deterioration in longitudinal contractility. Prognostically, DM is associated with a nearly twofold increase in morbidity and mortality [4, 5], with insulin resistance being a main contributor to myocardial damage [4, 5, 15,16,17].
Multi-morbidity, or the presence of two or more chronic diseases in an individual, is highly prevalent in HFpEF patients [3,4,5,6,7]. Obesity is present in most patients with type 2 DM [4,5,6]. The presence of obesity in DM may exacerbate cardiovascular risk factors, as the added presence of obesity raises the mortality risk from five to eightfold compared to DM alone [4,5,6, 18], likely due to detoriated insulin resistance [12, 19,20,21,22]. Insulin resistance is shared among both diseases and represents a critical initial step in myocardial alteration [12, 19,20,21,22]. Currently, the association between obesity and cardiovascular morbidity and mortality in diabetic patients is inconclusive [12, 19,20,21,22,23]. Therefore, studies investigating the link between myocardial structure/function and weight in DM patients may provide further insight into underlying pathophysiological processes.
Cardiovascular magnetic resonance imaging (CMR) offers a promising lens for assessing HFpEF-specific cardiac remodelling. Cardiac morphology and function are crucial steps in the diagnosis and management of HFpEF patients and can be accurately analyzed by CMR [24,25,26,27]. Moreover, native T1 mapping allows quantification of interstitial myocardial fibrosis [28, 29] and CMR features tracking (CMR-FT) techniques based on cine sequences allow assessment of myocardial longitudinal contractility indices such as global longitudinal strain (GLS) which has prognostic value in HFpEF patients [30]. In multimorbid HFpEF patients, significant deterioration of the myocardial structure and function can occur, beyond the independent effects of diabetes and obesity.
In this study, we hypothesized that the presence of both obsesity and DM in HFpEF patients is associated with increased subclinical myocardial damage, potentially due to increased insulin resistance. We therefore sought to investigate the impact of obesity, DM, and their combination in HFpEF patients by myocardial fibrosis and longitudinal contractility assessment using CMR.
Methods
Study population
One hundred and sixty-two patients with HFpEF (55 ± 12 years, 95 men) and 45 healthy controls (53 ± 12 years, 27 men) were retrospectively enrolled in an observational study. Patients were categorized into three groups according to their DM and obesity status [(36 in the obese group defined as obese patients without DM], [53 in the DM group defined as diabetic patients without obesity], and [73 in the Diabesity group defined as patients with both obesity and DM)]. The DM diagnosis was based on medical history and/or documented HbA1c values > 6.5%. Obesity was defined using a body mass index (BMI) threshold of 30 kg/m2. Coronary artery disease (CAD) was defined based on a history of coronary angiography with reported obstructive coronary lesions. Exclusion criteria included severe valvular disease, renal impairment (eGFR < 30 ml/min/m2), specific causes of HF (i.e. pericardial disease, hypertrophic, dilated and amyloid cardiomyopathies, and congenital diseases), isolated right sided HF, and severe pulmonary disease. Control subjects were selected among those with normal cardiac dimensions/volumes, normal cardiac function on CMR and echocardiography exams (if applicable), no clinical symptoms/cardiac history, BMI values < 30 kg/m2, and H2FPEF scores ≤ 1. The study protocol was approved by the Beth Israel Deaconess Medical Center Institutional Review Board, and a written informed consent was obtained from each study participant.
Demographic and laboratory analyses
Clinical assessments and biochemical tests such as fasting plasma glucose, HbA1c, cholesterol panels, C-reactive protein, and creatinine levels were obtained from medical records. All biochemical blood tests were performed within a median of 37 days [interquartile range (IQR) 1–82 days] before or after CMR imaging. Vital signs such as blood pressure measurements, pulse, and BMI and current medication history were collected on the day of the CMR scan.
Heart failure with preserved ejection fraction diagnosis
In accordance with contemporary guidelines [31] and publications [2, 14, 15, 32, 33], HFpEF diagnosis was based on three main criteria: (1) clinical symptoms consistent with HF (New York Heart Association (NYHA) status ≥ II), (2) preserved systolic function (LVEF ≥ 50%), and (3) evidence of cardiac dysfunction. Cardiac dysfunction was defined as diastolic dysfunction assessed by tissue Doppler echocardiography in accordance with guidelines and publications [31,32,33,34], and a H2FPEF score ≥ 6 points [range 0 to 9] [32]. According to Reddy et al. [32], a score of 6 or above indicates > 90% probability of HFpEF prevalence in patients with unexplained dyspnea. For this purpose, New York Heart Association (NYHA) classification was applied and this data were acquired through patient medical records accordingly.
Diastolic dysfunction assessment
Transthoracic echocardiography (TTE) was conducted using conventional two-dimensional guided tissue and pulse-wave Doppler imaging in accordance to the American Society of Echocardiography guidelines [31, 34]. Images were interpreted by a level 2 or 3 trained reader certified by the National Board of Echocardiography Special Competence in Echocardiography examination. The rationale for inclusion of Doppler echocardiography data was improved diagnostic accuracy for HFpEF by the H2FPEF score system. Peak early (E) and atrial (A) velocities of mitral inflow, early mitral inflow deceleration time (DT), septal and lateral mitral annular E′ velocities were measured and averaged. The E/A ratio and trans-mitral early diastolic velocity ratios (E/E′) were calculated. Furthermore, pulmonary systolic pressure (PASP) was obtained for use in the H2FPEF score calculation, and PASP was computed as the sum of the trans-tricuspid gradient and the estimated right atrial pressure. All diastolic function measurements by TTE were performed either prior to CMR scanning or after 3 months at the latest.
Cardiovascular MR protocol
CMR was performed on a 1.5-T scanner (Phillips Achieva, Best, Netherlands), using a 5-element or 32-element cardiac coil. Images were analyzed using commercial workstations (Extend MR WorkSpace, version 2.3.6.3, Philips Healthcare). To determine LV myocardial function, volumes, and mass, 10 to 12 short-axis stack cine images and 4 and 2-chamber long axis images were acquired using a breath-hold balanced steady-state free precession cine sequence with ECG gating (slice thickness, 8-mm; in-plane spatial resolution 2 × 2 mm, 30 ms temporal resolution). At end-diastole and end-systole, endocardial and epicardial contours were manually delineated on contiguous short-axis cine images covering the LV apex to the mitral valve plane for calculation of end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume, and LVEF. Papillary muscles were excluded from the myocardial mass, and included when measuring LV volumes. LV mass (LVM) was calculated as the sum of the myocardial volume multiplied by the specific gravity (1.05 g/mL) of the myocardial tissue, and LV mass index (LVMI) was calculated according to indexed body surface area (BSA). To quantify myocardial remodeling, the LV remodeling index (LVRI) was calculated as the ratio between LVM and LVEDV [35], and the relative wall thickness (RWT) was defined as (2 × diastolic posterior wall thickness)/LV diastolic diameter [36]. All volume measurements were indexed to BSA. LVRI, LVMI, and RWT were used as myocardial remodeling parameters [37].
Native T1 mapping was performed using an ECG-triggered free-breathing slice-interleaved T1 (STONE) sequence with the following parameters: 5 slices, in-plane resolution = 2.1 × 2.1 mm2, slice thickness = 8 mm, slice gap = 4 mm, field-of-view = 360 × 351 mm2, TR/TE/flip angle = 2.8 ms/1.4 ms/70°, TFE factor = 86, a sensitivity encoding (SENSE) rate = 2, linear ordering, 10 linear ramp-up pulses and bandwidth = 1894 Hz, acquisition duration = 1 min 38 s. Eleven inversion images were acquired with inversion times of ∞, 130, 1030, 1930, 2830, 3730, 350, 1250, 2150, 3050, and 3950 ms [38, 39]. The inversion preparation pulse was an adiabatic hyperbolic secant pulse with an 11 ms pulse duration. The radiofrequency (RF) excitation pulse was a slice-selective Sinc-Gauss pulse with a duration of 0.43 ms. Images were acquired without prospective slice tracking, and the slice order was 1-4-2-5-3. STONE T1 maps were generated by performing voxel-wise curve fitting of the signal with a 2P model to the image intensities of all four T1-weighted imaging volumes [38,39,40]. The crosses on the inversion recovery curve correspond to different images acquired along the recovery curve with pre-defined inversion times. Short-axis slices of native T1 mapping images were analyzed using custom software (MedIACare, Boston, Massachusetts) on MATLAB (MathWorks Inc., Natick, Massachusetts, USA). To calculate the global native T1 of the LV, 5 short-axis LV slices were divided into 6 segments for basal and mid-ventricular slices, and into 4 segments for the apical slice using the anterior right ventricular-LV insertion point as the reference (Fig. 1).
Native T1 maps from base to apex slices for each group. Native T1 maps were performed using STONE sequence from basal (top) to apex (bottom). Four different individuals represent each study subgroup (left to right): healthy subjects (global native T1 time, 1074 ms), obese group (global native T1 time, 1085 ms), DM group (global native T1 time, 1125 ms),and Diabesity group (global native T1, 1190 ms). Bulls-eye representations in the bottom row show per segment native T1 values and corresponding average values in the center. DM diabetes mellitus
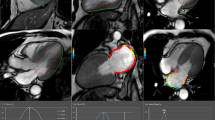
CMR-FT analysis was performed using CVi42 (Circle Cardiovascular Imaging Inc, Calgary, Alberta, Canada). Corresponding two- and four-chamber LV images were uploaded into the software. Endo- and epicardial borders on the end-diastolic frame of each slice were manually traced (excluding papillary muscles and trabeculae). Tracking was visually reviewed and manually corrected by border adjustment with consecutive reapplication of the algorithm as necessary. GLS, 2-chamber strain, and 4-chamber strain were automatically calculated and displayed for further analysis (Fig. 2).
Global longitudinal strain measurements in two and -four chamber for each group. Peak (blue) and rest (green) global longitudinal strain (GLS) measurements superimposed on cine images (top row) are depicted for the four study groups: healthy subjects (GLS, − 20.15%), obese group (GLS, − 16.92%), DM group (GLS, − 17.70%), Diabesity group (GLS, − 15.50%). The bottom row covers GLS over one heart cycle for each subject. DM indicates diabetes mellitus, A2C apical two chambers, A4C apical four chambers, L longitudinal
Triglyceride-glucose (TyG)-BMI index as an insulin resistance biomarker
The metabolic biomarker TyG-index was calculated as Ln [triglyceride level (mg/dL) × fasting plasma glucose level (mg/dL)/2] to estimate insulin resistance in individuals with DM [41, 42]. The product of TyG-index and BMI (TyG-BMI index) has previously proven to be a clinically useful surrogate marker for early identification of insulin resistance and cardiovascular events, particularly in obese individuals [43,44,45].
Data analysis and statistics
Statistical analyses were performed using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as mean ± standard deviations (SD), while categorical variables are expressed as a frequency (%). Differences in baseline characteristics according to diabetes status were evaluated by one-way analysis of variance. Scheffe and Games–Howell post hoc analyses were performed for equal and unequal variances, respectively. Chi square tests were used to compare categorical data. The Bonferroni correction adjusted for multiple comparisons. Univariate linear regression analyses were used to test confounding variables for GLS and native T1. Parameters significantly associated with GLS and native T1 were included in the analysis of covariance models. To test for synergism between two entities, the interaction value, such as partial eta squared (eta2), and its significance were calculated via linear regression analysis.
Results
Clinical and demographic characteristics of 162 patients were separated into 3 groups and 45 healthy controls (Table 1). Hypertension and ACEI/ARB usage were more prevalent in the DM and Diabesity groups (P < 0.01, for both), while hyperlipidemia and statin usage were more frequent in obese patients (P < 0.01, for obese and Diabesity groups). Consistent with HFpEF disease, diuretic usage and heart failure symptoms were most prevalent in the Diabesity group (P < 0.0001, for all). Additionally, both the TyG index (8.7 ± 0.3 for obese, 9.1 ± 0.5 for DM and 9.3 ± 0.3 in patients\ group compared to 8.4 ± 0.2 for control group) and the TyG-BMI index (258 ± 32 for obese, 266 ± 18 for DM and 302 ± 23 for Diabesity groups compared to 208 ± 19 for control group) were greater in patients than in control subjects (P < 0.01).
TTE and CMR findings for patients and control subjects are summarized in Table 2. Myocardial remodeling parameters including RWT (0.52 ± 0.04, p < 0.0001), LVMI (66 ± 13, p = 0.002) and LVRI (0.84 ± 0.19, p = 0.004) were significantly elevated in the Diabesity group relative to the other three groups. Native T1 was significantly altered in DM (1129 ± 25 ms) and Diabesity groups (1162 ± 37 ms) compared to the obese (1069 ± 29 ms) and control groups (1071 ± 27 ms) (P < 0.01 for all). Comparisons among the DM and Diabesity groups showed a significant increase in native T1 in the Diabesity group (1162 ± 37 ms) (P < 0.0001; Fig. 1). GLS was significantly impaired in the Diabesity group (− 15 ± 2%, P < 0.01, Fig. 2), while no statistically significant difference was detected among the remaining groups (P > 0.01, Fig. 3). Imaging markers of remodeling, myocardial fibrosis, and longitudinal contractility were similar between the control and obese groups (RWT: 0.39 ± 0.04 vs 0.42 ± 0.06; LVMI: 46 ± 11 g/m2 vs 47 ± 9 g/m2; LVRI: 0.52 ± 0.12 vs 0.55 ± 0.32; native T1: 1071 ± 27 ms vs 1069 ± 29 ms; GLS: − 17.2 ± 1% vs − 16.6 ± 2%, respectively) (P > 0.01 for all) (Table 2). To illustrate potential correlation between insulin resistance and myocardial structure and function, the relationship between TyG-BMI index vs. GLS (R = 0.590, P < 0.0001) and TyG-BMI index vs. native T1 (R = 0.349, P < 0.001) was depicted (Fig. 4). TyG-BMI index was significantly correlated with mean E′ (R = − 0.552, P < 0.0001), and the mean E/E′ ratio (R = 0.576, P < 0.0001) emphasized the association between insulin resistance and LV diastolic function (Fig. 5).
Boxplots illustrating mean ± SD value of GLS and native T1 for each group. Association between the groups in terms of GLS and native T1. Box plots are consistent to the visual assessment. DM diabetes mellitus, GLS global longitudinal strain, TyG_BMI index triglyceride–glucose-Body mass index, NS statistically non-significant
Association between GLS, native T1, and TyG-BMI index. Scatterplots show the relationship between GLS, native T1, and the TyG-BMI index. Linear regression correlation coefficients (R) corresponding p values, and linear regression equations are listed. Each study subgroup is color and shape coded (controls = green squares; non-diabetic obese = yellow triangles; lean diabetics = orange ovals; obese diabetics = red circles). DM diabetes mellitus, GLS global longitudinal strain, TyG_BMI index triglyceride–glucose-Body mass index
Association between mean mitral annular E′ velocity, mean trans-mitral early diastolic velocity ratio (E/E′), and the TyG-BMI index. Scatterplots show the relationship between mean E′, mean E/E′, and the TyG-BMI index. Linear regression correlation coefficients (R) corresponding p values, and linear regression equations are listed. Each study subgroup is color and shape coded (controls = green squares; non-diabetic obese = yellow triangles; lean diabetics = orange ovals; obese diabetics = red circles). DM diabetes mellitus, TyG_BMI index triglyceride–glucose-Body mass index
In univariate linear regression analyses, GLS was associated with gender (β = 0.81, R2 = 0.03, p = 0.03), ACE/ARB usage (β = 0.86, R2 = 0.03, p = 0.012), and TyG-BMI index (β = 0.02, R2 = 0.35, p < 0.0001) (Table 3). Native T1 was associated with age (β = 0.79, R2 = 0.03, p = 0.01), hypertension (β = 17.74, R2 = 0.1, p = 0.01), and TyG-BMI index (β = 0.6, R2 = 0.16, p < 0.001) (Table 3). Consequently, analysis of covariance controlled for age, gender, ACEI/ARB usage, hypertension, and TyG-BMI index. Table 4 lists the fibrosis and longitudinal contractility markers and the corresponding predicted means for each subgroup after adjustment for confounding variables. A statistically significant difference was observed between the Diabesity group and all other groups regardless of adjusting for confounders (P < 0.01), with the notable exception of GLS. There was no difference in GLS between the Diabesity group vs. obese and DM groups after adjustment for TyG-BMI and Bonferroni correction (P = 0.03; Table 4).
Significant synergistic effects between obesity and DM (f = 49.89, p < 0.0001, η2ρ = 0.288 on GLS, and f = 118.92, p = 0.002, η2ρ = 0.273 on native T1) were more powerful than the effect of each individual comorbidity [(f = 19.29, p = 0.003, η2ρ = 0.095 for DM and f = 9.5, p = 0.058, η2ρ = 0.048 for obesity on GLS) vs. (f = 87.11, p = 0.015, η2ρ = 0.182 for DM and f = 14.6, p = 0.14, η2ρ = 0.068 for obesity on native T1)] (Table 5).
Discussion
This retrospective study investigates the interaction between obesity and DM on the myocardium in HFpEF patients by assessment of LV remodeling, fibrosis, and longitudinal contractility. The findings make several contributions to current literature on HFpEF related processes: (1) Obesity and DM have a negative synergistic relationship. Co-occurring DM and obesity show high native T1, deterioration in global longitudinal strain, and increased LV remodeling; (2) DM is associated with structural myocardial changes, whereas obesity alone is not; and (3) there is a well-recognized association between insulin resistance and GLS, native T1, and LV diastolic function parameters.
CMR parameters and the synergism of comorbidities
Structural myocardial changes in HFpEF patients, such as diffuse fibrosis, LV remodeling, and impairment in longitudinal contractility, are well documented [5, 27, 46] and the influence of isolated obesity or DM on these changes has been reported [12, 14, 15, 21]. Lindman et al. observed an elevation in LV filling pressures in obese and diabetic HFpEF patients compared to those without DM in their randomized multicenter RELAX study [4]. In a report from the I-Preserve study [16], presence of diabetes in obese HFpEF patients is not only associated with cardiac changes in structure and function, but also correspond to symptom severity. HFpEF patients with obesity and DM are generally more susceptible to clinical signs of cardiac congestion, worse quality of life, and poorer prognosis with an increased risk for cardiovascular mortality and HF hospitalization. Our study results extend these findings to non-obese diabetic patients. Moreover, our study shows that myocardial fibrosis calculated by native T1, myocardial longitudinal contractility measured by GLS, and LV remodeling markers such as LVMI, LVRI, and RWT can differentiate between obese and diabesity patients.
Preclinical studies in HFpEF rats have shown synergism between obesity and hypertension in the development of diastolic dysfunction [47]. Similar to Brandt et al., animals in our study were divided into four groups. The group characterized by obesity and hypertension showed a high remodeling ratio, increased inflammation, expansion of the extracellular spaces, and accumulation of collagen tissue—all biomarkers of myocardium injury and interstitial fibrosis. In our clinical study, tissue characterization by CMR replicates some of these histological findings. In particular, we found that combinations of comorbidities had different effects on native T1 time, a surrogate marker of myocardial fibrosis. Our detailed CMR phenotyping was therefore able to non-invasively confirm the synergistic effect between obesity and DM on the myocardium.
Cardiac alterations in patients with obesity or DM
Although the ASIAN-HF [7] registry compared isolated obesity with isolated DM in HFpEF patients, our direct comparisons verify the importance of DM prevalence in myocardial deterioration. In its study of 6,480 enrolled patients with HF and multi comorbidities from various Asian countries, isolated diabetics had the highest incidence of all-cause mortality and HF related hospitalization. In subgroup analyses for HFpEF patients, isolated diabetics had increased LVMI and RWT, LV filling pressures, and left atrial volume indexes by TTE indicating a higher degree of subclinical damage, LV remodeling and diastolic dysfunction than their metabolic or diabetic obese counterparts. In our study, DM patients had increased LVMI and LV remodeling indexes relative to healthy controls; furthermore, CMR imaging demonstrated myocardial fibrosis and impairment in LV longitudinal contractility despite maintained LV function.
Insulin resistance and heart failure
Insulin resistance not only has a strong association with HF, but also predicts the development of HF in cohorts with various risk factors [17, 20, 22, 48]. In a mice study, cardiomyocyte-specific deletion of insulin receptors led to decreased insulin-stimulated glucose uptake and a decline in cardiac function [48]. Insulin resistance is a characteristic feature of HF pathophysiology that affects symptoms and mortality [17].
Abdominal obesity is closely associated with insulin resistance and diabetes manifestation [12, 23, 49]. In this context, DM and obesity are associated with myocardial disease, even after adjustment for traditional risk factors. In a community-dwelling cohort of 22,681 participants, Savji et al. [11] showed the effects of obesity and cardiometabolic traits on incident of HFpEF and HFrEF. They observed that greater BMI portends higher HFpEF risk, and insulin resistance is associated with HFpEF with a hazard ratio of 1.20. Their findings are consistent with the results of our study, in which we observed that a high BMI and high insulin resistance tend to deteriorate myocardial structure in HFpEF patients. In another study, increased insulin resistance was independently associated with LVMI, LV filling pressures, and GLS [20]. Similarly, we show the association of insulin resistance with LVMI and GLS and observed moderate correlation between insulin resistance and LV filling pressures parameters. Furthermore, elevation of Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), a common marker for insulin resistance, was associated with the onset of HFpEF [17]. TyG-BMI index, our approach to insulin resistance quantification based on several contemporary research studies [41,42,43,44], showed a strong correlation with HOMA-IR and basal insulin level, as well as high sensitivity and specificity for recognition of insulin resistance [41,42,43,44]. The observed association between insulin resistance and myocardial alterations warrant further investigation on reversibility.
Our study has some limitations. With late gadolinium enhancement and extracellular volume (ECV) quantification, CMR provides further myocardial characterization techniques that are not consistently available in our study cohort. Contrary to native T1 time, an established marker of myocardial fibrosis [29, 50, 51], both LGE and ECV require gadolinium administration and are therefore not routinely performed at our institution. Given the context of our retrospective observational study, HOMA-IR, which currently uses insulin resistance quantification in clinical practice, was also unavailable. However, its surrogate, the TyG-BMI index, provided results consistent with the contemporary literature [41,42,43,44]. Lastly, echocardiography data was lacking in 17% of our cohort. Considering its contribution to the H2FPEF score system, the HFpEF diagnosis may be underrepresented in a small number of patients.
Conclusions
We demonstrate that DM is associated with myocardial fibrosis and impairment in longitudinal contraction independent of confounding factors, whereas no such relationship is observed in obese patients without DM. However, presence of obesity and DM in the same HFpEF patients is associated with a negative synergistic effect. Furthermore, the close correlation of CMR biomarkers with TyG-BMI index indicates that insulin resistance contributes to cardiac function and structure deterioration in HFpEF.
References
TE Owan HD, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355:251–259
Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Lewis EF, Markov V, O’Meara E, Kobulia B, Shaburishvili T, Solomon SD, Pitt B, Pfeffer MA, Li R (2013) Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail 6(2):184–192. https://doi.org/10.1161/CIRCHEARTFAILURE.112.972794
Chirinos JA, Bhattacharya P, Kumar A, Proto E, Konda P, Segers P, Akers SR, Townsend RR, Zamani P (2019) Impact of diabetes mellitus on ventricular structure, arterial stiffness, and pulsatile hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc 8(4):e011457. https://doi.org/10.1161/JAHA.118.011457
Lindman BR, Davila-Roman VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, de las Fuentes L, Joseph SM, Vader J, Hernandez AF, Redfield MM (2014) Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol 64(6):541–549. https://doi.org/10.1016/j.jacc.2014.05.030
McHugh K, DeVore AD, Wu J, Matsouaka RA, Fonarow GC, Heidenreich PA, Yancy CW, Green JB, Altman N, Hernandez AF (2019) Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J Am Coll Cardiol 73(5):602–611. https://doi.org/10.1016/j.jacc.2018.11.033
Samson R, Jaiswal A, Ennezat PV, Cassidy M, Le Jemtel TH (2016) Clinical phenotypes in heart failure with preserved ejection fraction. J Am Heart Assoc 5(1):e002154. https://doi.org/10.1161/JAHA.115.002477
Tromp J, Tay WT, Ouwerkerk W, Teng TK, Yap J, MacDonald MR, Leineweber K, McMurray JJV, Zile MR, Anand IS, Richards AMR, Lam CSP, Authors AH (2018) Multimorbidity in patients with heart failure from 11 Asian regions: a prospective cohort study using the ASIAN-HF registry. PLoS Med 15(3):e1002541. https://doi.org/10.1371/journal.pmed.1002541
Tsujimoto T, Kajio H (2017) Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol 70(22):2739–2749. https://doi.org/10.1016/j.jacc.2017.09.1111
Packer M, Kitzman DW (2018) Obesity-related heart failure with a preserved ejection fraction: the mechanistic rationale for combining inhibitors of aldosterone, neprilysin, and sodium-glucose cotransporter-2. JACC Heart Fail 6(8):633–639. https://doi.org/10.1016/j.jchf.2018.01.009
Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD, Lavie CJ (2018) Physical activity, fitness, and obesity in heart failure with preserved ejection fraction. JACC Heart Fail 6(12):975–982. https://doi.org/10.1016/j.jchf.2018.09.006
Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS, Ho JE (2018) The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail 6(8):701–709. https://doi.org/10.1016/j.jchf.2018.05.018
Altara R, Giordano M, Norden ES, Cataliotti A, Kurdi M, Bajestani SN, Booz GW (2017) Targeting obesity and diabetes to treat heart failure with preserved ejection fraction. Front Endocrinol 8:160. https://doi.org/10.3389/fendo.2017.00160
Dalos D, Mascherbauer J, Zotter-Tufaro C, Duca F, Kammerlander AA, Aschauer S, Bonderman D (2016) Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J Am Coll Cardiol 68(2):189–199. https://doi.org/10.1016/j.jacc.2016.04.052
Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA (2017) Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 136(1):6–19. https://doi.org/10.1161/CIRCULATIONAHA.116.026807
Gulsin GS, Kanagala P, Chan DCS, Cheng ASH, Athithan L, Graham-Brown MPM, Singh A, Yang J, Li Z, Khunti K, Davies MJ, Arnold JR, Squire IB, Ng LL, McCann GP (2019) Differential left ventricular and left atrial remodelling in heart failure with preserved ejection fraction patients with and without diabetes. Ther Adv Endocrinol Metab 10:2042018819861593. https://doi.org/10.1177/2042018819861593
Kristensen SL, Mogensen UM, Jhund PS, Petrie MC, Preiss D, Win S, Kober L, McKelvie RS, Zile MR, Anand IS, Komajda M, Gottdiener JS, Carson PE, McMurray JJ (2017) Clinical and echocardiographic characteristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction: a report from the i-preserve trial (irbesartan in heart failure with preserved ejection fraction). Circulation 135(8):724–735. https://doi.org/10.1161/CIRCULATIONAHA.116.024593
Scherbakov N, Bauer M, Sandek A, Szabo T, Topper A, Jankowska EA, Springer J, von Haehling S, Anker SD, Lainscak M, Engeli S, Dungen HD, Doehner W (2015) Insulin resistance in heart failure: differences between patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail 17(10):1015–1021. https://doi.org/10.1002/ejhf.317
Lindman BR (2017) The diabetic heart failure with preserved ejection fraction phenotype: is it real and is it worth targeting therapeutically? Circulation 135(8):736–740. https://doi.org/10.1161/CIRCULATIONAHA.116.025957
Ashrafian H, Frenneaux MP, Opie LH (2007) Metabolic mechanisms in heart failure. Circulation 116(4):434–448. https://doi.org/10.1161/circulationaha.107.702795
Cauwenberghs N, Knez J, Thijs L, Haddad F, Vanassche T, Yang WY, Wei FF, Staessen JA, Kuznetsova T (2018) Relation of insulin resistance to longitudinal changes in left ventricular structure and function in a general population. J Am Heart Assoc 7(7):e008315. https://doi.org/10.1161/JAHA.117.008315
Haykowsky MJ, Nicklas BJ, Brubaker PH, Hundley WG, Brinkley TE, Upadhya B, Becton JT, Nelson MD, Chen H, Kitzman DW (2018) Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. JACC Heart Fail 6(8):640–649. https://doi.org/10.1016/j.jchf.2018.06.002
Ingelsson ESJ, Arnlöv J, Zethelius B, Lind L (2005) Insulin resistance and risk of congestive heart failure. JAMA 294(3):334–341
Steinberger J, Daniels SR, American Heart Association Atherosclerosis H, Obesity in the Young C, American Heart Association Diabetes C (2003) Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation 107(10):1448–1453. https://doi.org/10.1161/01.cir.0000060923.07573.f2
Kanagala P, Cheng ASH, Singh A, Khan JN, Gulsin GS, Patel P, Gupta P, Arnold JR, Squire IB, Ng LL, McCann GP (2019) Relationship between focal and diffuse fibrosis assessed by CMR and clinical outcomes in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2018.11.031
Kanagala P, Cheng ASH, Singh A, McAdam J, Marsh AM, Arnold JR, Squire IB, Ng LL, McCann GP (2018) Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in heart failure with preserved ejection fraction—implications for clinical trials. J Cardiovasc Magn Reson 20(1):4. https://doi.org/10.1186/s12968-017-0424-9
Su MY, Lin LY, Tseng YH, Chang CC, Wu CK, Lin JL, Tseng WY (2014) CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging 7(10):991–997. https://doi.org/10.1016/j.jcmg.2014.04.022
Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Taguri M, Kimura K, Umemura S (2015) Prognostic significance of quantitative assessment of focal myocardial fibrosis in patients with heart failure with preserved ejection fraction. Int J Cardiol 191:314–319. https://doi.org/10.1016/j.ijcard.2015.05.048
Pathan F, Puntmann VO, Nagel E (2018) Role of cardiac magnetic resonance in heart failure with preserved ejection fraction. Curr Cardiovasc Imaging Rep 11(5):8. https://doi.org/10.1007/s12410-018-9450-8
Nakamori S, Dohi K, Ishida M, Goto Y, Imanaka-Yoshida K, Omori T, Goto I, Kumagai N, Fujimoto N, Ichikawa Y, Kitagawa K, Yamada N, Sakuma H, Ito M (2018) Native T1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc Imaging 11(1):48–59. https://doi.org/10.1016/j.jcmg.2017.04.006
Kammerlander AA, Kraiger JA, Nitsche C, Dona C, Duca F, Zotter-Tufaro C, Binder C, Aschauer S, Loewe C, Hengstenberg C, Bonderman D, Mascherbauer J (2019) Global longitudinal strain by CMR feature tracking is associated with outcome in HFPEF. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2019.02.016
Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28(20):2539–2550. https://doi.org/10.1093/eurheartj/ehm037
Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA (2018) A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 138(9):861–870. https://doi.org/10.1161/CIRCULATIONAHA.118.034646
Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Ljung Faxen U, Fermer ML, Broberg MA, Gan LM, Lund LH (2018) Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 39(37):3439–3450. https://doi.org/10.1093/eurheartj/ehy531
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22(2):107–133. https://doi.org/10.1016/j.echo.2008.11.023
De Castro S, Caselli S, Maron M, Pelliccia A, Cavarretta E, Maddukuri P, Cartoni D, Di Angelantonio E, Kuvin JT, Patel AR, Pandian NG (2007) Left ventricular remodelling index (LVRI) in various pathophysiological conditions: a real-time three-dimensional echocardiographic study. Heart 93(2):205–209. https://doi.org/10.1136/hrt.2006.093997
Sundström JLL, Nyström N, Zethelius B, Andrén B, Hales CN, Lithell HO (2000) Left ventricular concentric remodeling rather than left ventricular hypertrophy is related to the insulin resistance syndrome in elderly men. Circulation 101(22):2595–2600
Shah RV, Abbasi SA, Neilan TG, Hulten E, Coelho-Filho O, Hoppin A, Levitsky L, de Ferranti S, Rhodes ET, Traum A, Goodman E, Feng H, Heydari B, Harris WS, Hoefner DM, McConnell JP, Seethamraju R, Rickers C, Kwong RY, Jerosch-Herold M (2013) Myocardial tissue remodeling in adolescent obesity. J Am Heart Assoc 2(4):e000279. https://doi.org/10.1161/JAHA.113.000279
Weingartner S, Roujol S, Akcakaya M, Basha TA, Nezafat R (2015) Free-breathing multislice native myocardial T1 mapping using the slice-interleaved T1 (STONE) sequence. Magn Reson Med 74(1):115–124. https://doi.org/10.1002/mrm.25387
Roujol S, Foppa M, Weingartner S, Manning WJ, Nezafat R (2015) Adaptive registration of varying contrast-weighted images for improved tissue characterization (ARCTIC): application to T1 mapping. Magn Reson Med 73(4):1469–1482. https://doi.org/10.1002/mrm.25270
Zhu Y, Kang J, Duan C, Nezafat M, Neisius U, Jang J, Nezafat R (2019) Integrated motion correction and dictionary learning for free-breathing myocardial T1 mapping. Magn Reson Med 81(4):2644–2654. https://doi.org/10.1002/mrm.27579
Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F (2018) Metabolic clustering of risk factors: evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr 10:74. https://doi.org/10.1186/s13098-018-0376-8
Won KB, Park GM, Lee SE, Cho IJ, Kim HC, Lee BK, Chang HJ (2018) Relationship of insulin resistance estimated by triglyceride glucose index to arterial stiffness. Lipids Health Dis 17(1):268. https://doi.org/10.1186/s12944-018-0914-2
Lim J, Kim J, Koo SH, Kwon GC (2019) Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007-2010 Korean National Health and Nutrition Examination Survey. PLoS ONE 14(3):e0212963. https://doi.org/10.1371/journal.pone.0212963
Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL (2016) Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE 11(3):e0149731. https://doi.org/10.1371/journal.pone.0149731
Li S, Guo B, Chen H, Shi Z, Li Y, Tian Q, Shi S (2019) The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: a retrospective cohort analysis. Sci Rep 9(1):7320. https://doi.org/10.1038/s41598-019-43776-5
Paulus WJ, Tschope C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62(4):263–271. https://doi.org/10.1016/j.jacc.2013.02.092
Brandt MM, Nguyen ITN, Krebber MM, van de Wouw J, Mokry M, Cramer MJ, Duncker DJ, Verhaar MC, Joles JA, Cheng C (2019) Limited synergy of obesity and hypertension, prevalent risk factors in onset and progression of heart failure with preserved ejection fraction. J Cell Mol Med 23(10):6666–6678. https://doi.org/10.1111/jcmm.14542
McQueen AP, Zhang D, Hu P, Swenson L, Yang Y, Zaha VG, Hoffman JL, Yun UJ, Chakrabarti G, Wang Z, Albertine KH, Abel ED, Litwin SE (2005) Contractile dysfunction in hypertrophied hearts with deficient insulin receptor signaling: possible role of reduced capillary density. J Mol Cell Cardiol 39(6):882–892. https://doi.org/10.1016/j.yjmcc.2005.07.017
Horwich TB, Fonarow GC (2010) Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. J Am Coll Cardiol 55(4):283–293. https://doi.org/10.1016/j.jacc.2009.07.029
Ferreira VMPS, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG, Neubauer S (2014) Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson 23(16):36
Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S (2016) Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 18(1):89. https://doi.org/10.1186/s12968-016-0308-4
Funding
Dr. Nezafat receives grant funding from the National Institutes of Health 1R01HL129185-01, 1R01HL129157, and 1R01HL127015 (Bethesda, MD, USA); and the American Heart Association (AHA) 15EIA22710040 (Waltham, MA, USA). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Ethical approval
The study protocol was approved by the Beth Israel Deaconess Medical Center Institutional Review Board.
Informed Consent
A written informed consent was obtained from each study participant.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kucukseymen, S., Neisius, U., Rodriguez, J. et al. Negative synergism of diabetes mellitus and obesity in patients with heart failure with preserved ejection fraction: a cardiovascular magnetic resonance study. Int J Cardiovasc Imaging 36, 2027–2038 (2020). https://doi.org/10.1007/s10554-020-01915-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-01915-4