Abstract
Right atrial (RA) and right ventricular (RV) parameters assessed by traditional echocardiography lack sensitivity to identify pulmonary embolism (PE). We sought to determine if alterations in RV free wall longitudinal strain (FWS) would be present in PE patients and improve evaluation. This retrospective study comprised of 84 consecutive PE patients from 2 centres, with adequate transthoracic echocardiography (TTE) images for RV FWS analysis. PE patients were compared to 66 healthy controls. Compared to controls, PE patients had increased RV parasternal long-axis diameter (RVPLAX) (33.4 ± 5.8 mm vs 39.9 ± 4.1 mm) and RA area (17.4 ± 5.6 cm2 vs 14.5 ± 3.1 cm2) (p < 0.001 for both). RV function was reduced in PE patients (RV fractional area change 31.1 ± 13.2% vs 41.7 ± 9.1%, TAPSE 17.0 ± 4.5 vs 21.3 ± 2.2 mm; p < 0.001 for both). RV FWS was reduced in PE patients (−14.4 ± 7.2% vs − 26.0 ± 4.4%, p < 0.001). RV FWS was the best discriminator for PE (AUC 0.912). In comparative multiple logistic regression models for PE, the model which included traditional measures of RV size and function and RV FWS, produced a powerful classifier (AUC 0.966, SE 0.013) with significantly better performance (p < 0.022) than the model without RV FWS (AUC 0.921, SE 0.024). RV FWS is a discriminator of PE patients; addition of RV FWS to existing parameters of RV size and function, significantly improves sensitivity and specificity for diagnosis of PE, and may play a role in diagnosis and guiding therapy. Validation in other PE groups is required to confirm these observations and its prognostic value needs evaluation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary embolism (PE) is the third most common cardiovascular cause of death after myocardial infarction and stroke [1]. The incidence of PE has been increasing [2], with significant associated mortality; a recent registry of PE patients reported a mortality rate > 10% at 30 days [3].
There are a number of emerging contemporary PE therapies, including thrombolysis, catheter intervention and surgery [4]. Early and accurate identification of PE in patients is therefore critical. A transthoracic echocardiogram (TTE) may help when there is a clinical suspicion for PE, and its use in confirmed PE is widely recommended [5, 6]. A number of right atrial (RA) and right ventricular (RV) parameters have been shown to be abnormal in patients with PE [7]. In addition, RA and RV enlargement portends a worse prognosis in these patients [7,8,9].
Despite this, traditional TTE markers of RA and RV function lack sensitivity to rule out PE [10]. Speckle tracking strain echocardiography (STE) is a novel echocardiographic technique that quantifies myocardial deformation [11], and incorporation of strain imaging parameters is increasingly used in routine clinical practice [12]. RV free wall strain (FWS) has been demonstrated to be an adverse prognostic factor in PE patients [13, 14]. However, the role of RV FWS in the diagnosis of PE has not been completely elucidated.
In this study, we sought to determine the clinical utility of traditional TTE parameters and RV FWS in diagnosis of PE.
Materials and methods
Study population
Our study included 84 consecutive PE patients, identified from a larger PE database, from two tertiary institutions (Liverpool Hospital and Concord Hospital, Sydney, Australia from 2001 to 2010). The methods used to identify the index PE cohort have been described previously [7]. In brief, patients were identified retrospectively using medical records, requiring a documented clinical diagnosis of acute PE and an imaging study consistent with the diagnosis (either an intermediate-high probability ventilation-perfusion scintigraphy or computed-tomography pulmonary angiogram (CTPA) confirming PE). Consecutive patients who had a TTE performed during their index admission, with a sufficiently high frame rate and image quality permitting RV speckle tracking analysis comprised the study group. A group of healthy subjects with no significant history of cardiovascular or respiratory illness served as controls. Informed consent was obtained from all individual participants included in the study. The study protocol conformed to the ethical guidelines of the 1964 Declaration of Helsinki and was approved by the Human Research Ethics Committee of both institutions.
Data collection and study outcomes
This study focussed on echocardiographic parameters in the subgroup of PE patients who underwent a TTE during their index admission with PE. Detailed patient demographics and clinical characteristics were collected from hospital medical records as previously described [15], including the imaging modality used to diagnose the PE, risk factors for PE, and in-hospital outcomes. A history of cardiovascular disease (ischaemic heart disease, prior coronary artery bypass surgery, heart failure, valvular heart disease, prosthetic heart valves, atrial fibrillation/flutter, peripheral vascular disease, stroke), cardiac risk factors (hypertension, hyperlipidaemia, diabetes, current or ex-smoker), malignancy, pulmonary disease (asthma and/or emphysema), neurodegenerative disease (dementia and/or Parkinson’s disease), and chronic renal disease, based on the International Classification of Disease, Tenth Revision (ICD-10), were recorded for each patient.
Echocardiography-derived parameters of right sided chamber size and function
All patients underwent a comprehensive echocardiogram based on departmental protocol at both centres. All TTEs were independently reviewed by an experienced cardiologist, blinded to patient details, for inclusion in this study.
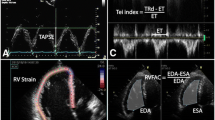
Echocardiographic images were analysed offline using GE EchoPac 3.1.3, Milwaukee, Wisconsin. Established guideline-based criteria were used for measurement of left and right heart chambers [16]. The proximal RV outflow diameter was measured in the parasternal long axis view (RVPLAX), and RV basal, mid diameters and length were measured from the RV focussed apical 4 chamber view [16]. LV and RV end-diastolic and end-systolic area were measured by tracing the endocardial border as previously described [16], and the RV fractional area change (RVFAC) was calculated.
Right atrial (RA) area was measured by tracing the RA from the apical 4-chamber view. RA pressure was estimated from the inferior vena cava (IVC) diameter and collapsibility with respiration from subcostal views [16]. Tricuspid annular plane systolic excursion (TAPSE) was assessed via M-mode by placing a cursor in the tricuspid annulus and measuring the amount of longitudinal motion of the annulus at peak systole [17].
RV free wall longitudinal strain
Two-dimensional speckle tracking strain analysis was performed from the apical RV focussed view as previously reported [18]. Aortic valve closure from the Doppler profile identified systole. The RV endocardium was traced along the free wall, apex and the interventricular septum, and the region of interest was manually adjusted to the RV wall thickness (Fig. 1). The endocardial border of the entire RV was traced (free wall and septum); RV FWS was the arithmetic average of the longitudinal strain of the three free wall segments—basal, mid, and apical free wall [19] (Fig. 1). Septal segments were not included to eliminate any LV interaction; additionally RV FWS has previously demonstrated the greatest dysfunction in acute PE [20].
Statistical analysis
Categorical variables are expressed as frequencies/percentages, and continuous variables as mean ± standard deviation. Student’s t test was used to compute p values comparing the continuous variables between PE and control groups. The area under the receiver operating characteristic curve was used to determine the optimal echocardiographic parameters for discriminating between PE patients and controls. DeLong’s method was used for pairwise comparisons of the ROC curves of each of the existing parameter compared to that of RV FWS [21]. Using traditional TTE parameters and RV FWS, backward stepwise variable selection was used to identify the best fitting multiple logistic regression model for PE. Box plots were created to illustrate the differences between the 2 models (with and without RV FWS included). Analyses were performed using IBM SPSS version 25 (IBM Inc., New York, NY). A p value of < 0.05 was considered statistically significant.
Results
A total of 84 consecutive PE patients with adequate echocardiographic images for RV FWS analysis, and 66 healthy controls were included in the study.
Demographic and clinical characteristics
Table 1 shows the clinical and demographic characteristics of PE patients; mean age of PE patients was 67 ± 17 years and 61% (n = 51) were females while that of controls was 56 ± 10 years, with 64% (n = 42) being females. Presence of PE was confirmed by CTPA in 42%, by ventilation-perfusion scintigraphy in 48%, and in 10% with both imaging studies performed. The mean length of hospital stay was 9 ± 8 days. Forty percent of patients had documented deep vein thrombosis during the admission, and 9.5% had an associated malignancy. Associated cardiac co-morbidities included atrial arrhythmias (14.3%), cardiac failure (9.5%), and ischaemic heart disease (6%). Only a relatively small percentage of patients had lung disease that can affect RV function, including 2.4% with previous pulmonary hypertension and 6% with chronic pulmonary disease.
Echocardiographic characteristics
Echocardiographic parameters in PE patients and controls are shown in Table 2. PE patients were more likely to have enlarged right heart chambers (RV diameter, RA and RV area) with reduced RV function (RVFAC). TAPSE was reduced in PE patients. A significant difference in the RA/LA ratio was observed between groups (Table 2). RV FWS was significantly reduced in the PE group, in the basal, mid, and apical segments. The estimated pulmonary artery systolic pressure was significantly higher in the PE group.
There was no significant difference in the LA area or LV ejection fraction (EF) between the groups (Table 2).
Echocardiographic predictors of pulmonary embolism
We evaluated the association between parameters of RV size (RVPLAX diameter and RV area) and function (TAPSE, RVFAC and RV FWS); receiver operating characteristic curve for RV parameters is shown in Fig. 2 with area under the curves (AUC) in Table 3. RV FWS was the best discriminator between PE patients and controls (AUC 0.91). There was a statistically significant difference between RV FWS and each of the other traditional RV parameters [21] (Table 3).
Multiple logistic regression model for PE
The candidate variables with significant differences compared to controls were age, RVFAC, RV end diastolic area, RVPLAX, TAPSE together with RV FWS. Backward stepwise variable selection was used to identify the best fitting multiple logistic regression model for diagnosis of PE (Table 4).
The AUC associated with the predicted probability from this best fitting logistic regression model was compared to that based on the model in which RV FWS was omitted (Fig. 3).
The model with inclusion of RV FWS produced an almost perfect classifier (AUC 0.966, SE 0.013) with significantly better performance (p < 0.022) [21] than that which omitted RV FWS (AUC 0.921, SE 0.024).
Box plots presented in Fig. 4 illustrate the superior performance of the model with RV FWS included. Considering the seriousness of a diagnosis of PE, if 95% sensitivity is sought, the model including RV FWS provided 75% specificity, while the model excluding RV FWS provided only 65% specificity.
Discussion
The salient findings of this study were: (1) RV FWS was the best discriminator between PE patients and controls, and (2) addition of RV FWS to existing parameters of RV size and function significantly improves sensitivity and specificity in the diagnosis of PE.
Role of TTE in acute PE
While guidelines recommend the performance of a TTE in PE patients [5], in routine clinical practice, a TTE is often not performed. Our group has previously reported that only ~ 30% of PE patients had a TTE; moreover TTE was less likely performed in patients with malignancy and more likely performed in older patients with associated cardiac failure, atrial fibrillation and diabetes [22]. While previous studies have validated qualitative assessments of RV function in acute PE [8], subjective approaches rely on individual expertise [23]. While CTPA/VQ scan is necessary for PE diagnosis, the use of RV FWS offers supporting evidence in borderline cases. In regional centres lacking CT or nuclear imaging the use of strain echocardiography may help identify patients who require transfer for advanced imaging and therapy.
In our study, several traditional echocardiographic parameters of RV size and function were altered in acute PE, consistent with previous literature [18, 24, 25]. An enlarged RA, particularly when utilised as a ratio to the LA, has been described and validated as a sensitive marker, particularly in the early phase of PE [26].
RV FWS: utility in PE
2D speckle tracking strain is a semiautomated technology that tracks naturally occurring speckles to determine myocardial deformation, with several advantages as it is angle independent [27], quantitative and generally reproducible [28]. RV FWS measures free wall RV function which has the theoretical benefit in avoiding potential sampling of only basal segmental function as does measurements such as TAPSE and RV S’ velocity.
RV FWS was the best discriminator for PE, suggesting that it may provide incremental benefit beyond traditional measures of RV function. Indeed, in the present study, multiple logistic regression models demonstrated that the addition of RV FWS resulted in a near perfect classifier and significantly better performance than if RV FWS is omitted.
RV FWS may be useful in a point of care setting or in diagnostic algorithms where PE is suspected. The AUC for RV FWS of 0.91 compares favourably to TAPSE and RVSP and is similar to 3D RVEF [24]. All three RV free wall segments demonstrated significantly impaired strain with no discernible gradient or regional pattern, which is in keeping with previous studies investigating regional strain in PE [2, 29]. A uniform pattern of reduction of RV FWS seems at odds with the clinically observed McConnell sign. However, it has been previously postulated that myocardial deformation may be uniformly impaired throughout the RV, while apical excursion is preserved by tethering of the RV apex to a normally functioning left ventricle [24].
In a recent systematic review and meta-analysis, 22 studies were identified that investigated test performance characteristic for TTE in suspected PE [10]. The authors concluded that traditional TTE parameters of RV size and function lack sensitivity to rule out PE [10]. Our study, albeit in a relatively small group of PE patients, demonstrates the clinical utility of RV FWS in the diagnosis of PE. In addition, only a relatively small percentage of patients in our study had chronic lung disease that may potentially influence RV function.
RV FWS has also been validated in other patient populations with pulmonary pathology. In patients with pulmonary hypertension, RV FWS has been shown to be an independent predictor of future right-sided heart failure, clinical deterioration, and mortality [30, 31].
Study limitations
The retrospective nature of the study did not allow for comprehensive vital sign collection or specifics regarding administered therapies. Therefore, inconsistencies in blood pressure values and oxygen saturation precluded the calculation of the pulmonary embolus severity index (PESI) score. Use of anticoagulants and thrombolytic therapies could not be ascertained; however, given the time of presentation, these therapies were uncommon.
Cardiac biomarkers were not routinely evaluated in PE patients, although they have utility in PE [6].
Due to the relatively small sample size of PE patients, our results need further validation in larger, multi-centre studies.
Conclusions
PE is a common cardiovascular problem and TTE is recommended for evaluation. Our study suggests that RV FWS is the best discriminator between PE patients and controls, and addition of RV FWS to existing parameters of RV size and function significantly improves sensitivity and specificity for diagnosis of PE. Application of RV FWS in identifying PE patients may play a major role in its diagnosis, and therefore the selection of specific treatment strategies, monitoring and follow up of PE patients.
References
Wendelboe AM, Raskob GE (2016) Global burden of thrombosis: epidemiologic aspects. Circ Res 118(9):1340–1347
Mediratta A, Addetia K, Medvedofsky D, Gomberg-Maitland M, Mor‐Avi V, Lang RM (2016) Echocardiographic diagnosis of acute pulmonary embolism in patients with McConnell's sign. Echocardiography 33(5):696–702
Stergiopoulos K, Bahrainy S, Strachan P, Kort S (2011) Right ventricular strain rate predicts clinical outcomes in patients with acute pulmonary embolism. Acute Card Care 13(3):181–188
Kabrhel C, Rosovsky R, Channick R, Jaff MR, Weinberg I, Sundt T et al (2016) A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest 150(2):384–393
Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P et al (2008) Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 29(18):2276–2315
Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ et al (2011) Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 123(16):1788–1830
Terluk AD, Trivedi SJ, Kritharides L, Chow V, Chia EM, Byth K et al (2019) Echocardiographic predictors of long-term mortality in patients presenting with acute pulmonary embolism. Am J Cardiol 124(2):285–291
McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT (1996) Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol 78(4):469–473
Becattini C, Agnelli G, Lankeit M, Masotti L, Pruszczyk P, Casazza F et al (2016) Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J 48(3):780–786
Fields JM, Davis J, Girson L, Au A, Potts J, Morgan CJ et al (2017) Transthoracic echocardiography for diagnosing pulmonary embolism: a systematic review and meta-analysis. J Am Soc Echocardiogr 30(7):714–23.e4
Trivedi SJ, Altman M, Stanton T, Thomas L (2019) Echocardiographic strain in clinical practice. Heart Lung Circ 28(9):1320–1330
Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G et al (2017) Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 18(12):1301–1310
Kanar BG, Gol G, Ogur E, Kavas M, Atas H, Mutlu B (2019) Assessment of right ventricular function and relation to mortality after acute pulmonary embolism: a speckle tracking echocardiography-based study. Echocardiography 36(7):1298–1305
Lee K, Kwon O, Lee EJ, Sin MJ, Lee JS, Lee S et al (2019) Prognostic value of echocardiographic parameters for right ventricular function in patients with acute non-massive pulmonary embolism. Heart Vessel 34(7):1187–1195
Ng ACC, Chung T, Yong ASC, Wong HSP, Chow V, Celermajer DS et al (2011) Long-term cardiovascular and noncardiovascular mortality of 1023 patients with confirmed acute pulmonary embolism. Circulation 4(1):122–128
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J 16(3):233–271
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713 (quiz 86–8)
Dahhan T, Alenezi F, Samad Z, Rajagopal S (eds) (2017) Echocardiography in the risk assessment of acute pulmonary embolism. Seminars in respiratory and critical care medicine. Thieme Medical Publishers, New York
Muraru D, Onciul S, Peluso D, Soriani N, Cucchini U, Aruta P et al (2016) Sex- and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking echocardiography. Circulation 9(2):e003866
Descotes-Genon V, Chopard R, Morel M, Meneveau N, Schiele F, Bernard Y (2013) Comparison of right ventricular systolic function in patients with low risk and intermediate‐to‐high risk pulmonary embolism: a two‐dimensional strain imaging study. Echocardiography 30(3):301–308
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Bing R, Chow V, Lau JK, Thomas L, Kritharides L, Ng ACC (2016) Prevalence of echocardiography use in patients hospitalized with confirmed acute pulmonary embolism: a real-world observational multicenter study. PLoS ONE 11(12):e0168554
Taylor RA, Moore CL (2014) Accuracy of emergency physician-performed limited echocardiography for right ventricular strain. Am J Emerg Med 32(4):371–374
Vitarelli A, Barillà F, Capotosto L, D'angeli I, Truscelli G, De Maio M et al (2014) Right ventricular function in acute pulmonary embolism: a combined assessment by three-dimensional and speckle-tracking echocardiography. J Am Soc Echocardiogr 27(3):329–338
Dahhan T, Siddiqui I, Tapson VF, Velazquez EJ, Sun S, Davenport CA et al (2016) Clinical and echocardiographic predictors of mortality in acute pulmonary embolism. Cardiovasc Ultrasound 14(1):44
Chow V, Ng ACC, Chung T, Thomas L, Kritharides L (2013) Right atrial to left atrial area ratio on early echocardiography predicts long-term survival after acute pulmonary embolism. Cardiovasc Ultrasound 11(1):17
Forsha D, Risum N, Rajagopal S, Dolgner S, Hornik C, Barnhart H et al (2015) The influence of angle of insonation and target depth on speckle-tracking strain. J Am Soc Echocardiogr 28(5):580–586
Khemasuwan D, Yingchoncharoen T, Tunsupon P, Kusunose K, Moghekar A, Klein A et al (2015) Right ventricular echocardiographic parameters are associated with mortality after acute pulmonary embolism. J Am Soc Echocardiogr 28(3):355–362
Sugiura E, Dohi K, Onishi K, Takamura T, Tsuji A, Ota S et al (2009) Reversible right ventricular regional non-uniformity quantified by speckle-tracking strain imaging in patients with acute pulmonary thromboembolism. J Am Soc Echocardiogr 22(12):1353–1359
Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF et al (2011) Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest 139(6):1299–1309
Fine Nowell M, Chen L, Bastiansen Paul M, Frantz Robert P, Pellikka Patricia A, Oh Jae K et al (2013) Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circulation 6(5):711–721
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trivedi, S.J., Terluk, A.D., Kritharides, L. et al. Right ventricular speckle tracking strain echocardiography in patients with acute pulmonary embolism. Int J Cardiovasc Imaging 36, 865–872 (2020). https://doi.org/10.1007/s10554-020-01779-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-01779-8