Abstract
We compared first-generation and second-generation drug-eluting stent (DES) with respect to neoatherosclerosis using optical coherence tomography or optical frequency domain imaging. In-stent restenoses in 102 first-generation and 114 second-generation DES were retrospectively assessed. Neoatherosclerosis, which was defined as the presence of lipid-laden neointima or calcification inside a stent, was observed in 33 (27.2%) and 31 (32.4%) lesions in the first-generation and second-generation DES respectively. In the first-generation DES group, the lipid length was significantly longer (5.5 ± 3.8 vs. 3.1 ± 2.1 mm, P = 0.0007), the lipid arc was significantly larger (324 ± 70° vs. 250 ± 94°, P = 0.002), the prevalence of a 360° lipid arc was significantly greater (58 vs. 31%, P = 0.03), and the fibrous cap was significantly thinner (153 ± 85 vs. 211 ± 95 µm, P = 0.02) compared with those in the second-generation DES group. These differences remained significant after adjusting for the age of the stent (lipid length: P < 0.001; lipid arc: P = 0.019; and fibrous cap thickness: P < 0.001). The proliferation course and stability of neoatherosclerosis over time might be superior in second-generation DES.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although first-generation drug-eluting stent (DES) dramatically reduced the in-stent restenosis (ISR) and repeat revascularization rates compared with those associated with bare metal stents, [1] the cumulative incidence of late and very late stent thrombosis beyond 1 year was more widely acknowledged during the first-generation DES era [2, 3]. Many causes of stent thrombosis have been described, [4,5,6] and neoatherosclerosis is one trigger that may be associated with late stent failure after DES implantation [7,8,9,10]. Nakazawa et al. undertook a histopathology study and reported that while neoatherosclerosis occurred in bare metal stent and first-generation DES, it occurred more rapidly and more frequently in first-generation DES than that observed in bare metal stent [11]. In addition, the findings from recent studies have demonstrated that the frequency of neoatherosclerosis in second-generation DES was not lower than its frequency in first-generation DES [12, 13]. However, it is well established that second-generation DES are associated with fewer late stent failures, including stent thrombosis, compared with first-generation DES [14]. We aimed to evaluate the differences between first-generation and second-generation DES with respect to the morphology of neoatherosclerosis plaques using optical coherence tomography (OCT) or optical frequency domain imaging (OFDI).
Methods
Study design and patients
ISR (n = 297) that had undergone repeat percutaneous coronary interventions under OCT or OFDI guidance from October 2011 until December 2016, were retrospectively identified in our institute’s database. ISR was defined as a stenosis that occupied > 50% of the angiographic diameter with ischemic symptoms or a positive stress test, which was determined using quantitative coronary angiographic analysis, or a stenosis that occupied > 70% of the angiographic diameter without ischemic symptoms or a positive stress test. Of the 297 ISR, 81 were excluded, because they were bare metal stent restenoses (n = 35), ISR with poor OCT or OFDI results (n = 18), and they were not first-time ISR (n = 28). Finally, 102 ISR in first-generation DES and 114 ISR in second-generation DES were enrolled in the present study. The first-generation and second-generation DES were compared in relation to the incidence and characteristics of neoatherosclerosis. The first-generation DES included sirolimus-eluting stent (Cypher®; Cordis Corporation/Johnson & Johnson Inc, Warren, NJ, USA) and paclitaxel-eluting stent (Taxus®; Boston Scientific Corporation, Natick, MA, USA), and the second-generation DES included everolimus-eluting stent (Xience V®/Xience Prime®; Abbot Vascular, Santa Clara, CA, USA, and Promus®/Promus Element®; Boston Scientific Corporation), zotarolimus-eluting stent (Endeavor® and Resolute Integrity®; Medtronic Inc., Minneapolis, MI, USA), and biolimus A9-eluting stent (Nobori®; Terumo Medical Corporation, Tokyo, Japan).
The study’s protocol complied with the principles of the Declaration of Helsinki and it was approved by the hospital’s institutional review board. Written informed consent to participate in the study was obtained from all patients.
Optical coherence tomography and optical frequency domain image acquisition
All of the ISR were evaluated using a frequency-domain OCT system (C7-XR™, Ilumien™, or Ilumien Optis™; St. Jude Medical, St. Paul, MN, USA) or an OFDI system (Lunawave™; Terumo Corporation, Tokyo, Japan). After manual calibration, the OCT catheter (C7 Dragonfly™ or Dragonfly Duo™; St. Jude Medical) or the OFDI catheter (Fast View™; Terumo Corporation, Tokyo, Japan) was advanced distally towards the treated lesion over a 0.014-inch conventional guidewire. Contrast medium was continuously injected at 2–4 mL/s using an injection pump to obtain a blood-free image. The acquisition of the OCT and OFDI images was automatically pulled-back at a rate of 20 mm/s.
Optical coherence tomography and optical frequency domain image analysis
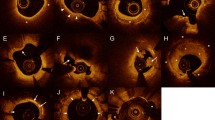
Two independent investigators who were blinded to the patients’ information, analyzed the images using an offline review workstation (LightLab Imaging, Inc, St. Jude Medical) or offline OFDI analysis software (Lunawave Offline Viewer; Terumo Corporation). Neoatherosclerosis was defined as an ISR that had a lipid-laden neointima or a neointima with calcification (Fig. 1) [15]. A lipid-laden neointima was defined as a signal-poor, high-attenuation lesion with a diffuse border [16]. To distinguish between a layered neointima and a lipid-laden neointima, we postulated that a signal-poor region that completely masked the stent struts behind the neointima should be considered a lipid-laden neointima. Calcification was defined as a clearly delineated signal-poor region with a low level of backscatter [17]. For a lipid-laden neointima, we measured the angle of the lipid arc at the widest part of the lipid core and the length of the lipid on the frames that showed the lipid core. Qualitative analyses were performed on the cross-sectional area of the entire stented segment. A thin-cap fibroatheroma was defined as a lipid-rich neointima with a fibrous cap of ≤ 65 µm and a lipid tissue angle of ≥ 180° [18]. We also compared the groups with respect to the presence of peri-strut low-intensity areas, peri-strut ulcer-like appearances, neovascularization, intraluminal material, and the presence of neointimal ruptures in the neoatherosclerotic plaques between the peri-strut low-intensity areas, which were defined as regions around the stent struts that had homogenous lower intensities than the surrounding tissue without signal attenuation on the OCT images [19]. A peri-strut ulcer-like appearance was defined as a hollow shape (> 90°/frame) in the vessel wall that was adjacent to the stent struts [20]. Neovascularization was defined as a small vesicular or tubular structure with a diameter of < 200 µm [21]. Intraluminal material was defined as visible material inside the vessel’s lumen [22]. A neointimal rupture was defined as fibrous cap discontinuity with cavity formation [16]. When there was discord between the investigators, a consensus assessment was obtained from a third independent investigator. We randomly selected 30 lesions to assess the two independent investigators’ interobserver reproducibility and the intraobserver reproducibility at two separate time points. We calculated the κ coefficients and the intraclass correlation coefficients for the qualitative and quantitative analyses of the OCT and OFDI images, respectively.
Statistical analysis
The categorical variables are presented as counts and percentages, and were compared using Fisher’s exact test or the Chi square test. The continuous variables are presented as the means and the standard deviations, and they were compared using unpaired t tests. Multivariable linear regression analyses were used to adjust for the baseline differences, in which non-normally distributed data were log-transformed. A two-sided P value of < 0.05 was considered to indicate a statistically significant difference. All of the statistical analyses were performed using PASW software, version 19 (IBM Corporation, Armonk, NY, USA).
Results
Baseline and angiographic characteristics
This study included 102 ISR associated with first-generation DES and 114 ISR associated with second-generation DES. The incidence of neoatherosclerosis was 32.4% (33 lesions) in the restenoses associated with the first-generation DES and 27.2% (31 lesions) in the restenoses associated with the second-generation DES. Table 1 shows the patients’ baseline characteristics. The patients in the second-generation DES group were younger (69 ± 11 years) than those in the first-generation DES group (75 ± 9 years) (P = 0.03). The groups did not differ with respect to the laboratory data and the medications used, and they were similar in relation to the clinical presentation at the initial percutaneous coronary intervention. Table 2 presents the patients’ angiographic characteristics. There were no significant differences between the groups in relation to the target coronary arteries or the findings from the quantitative coronary angiographic assessments undertaken before and after the initial percutaneous coronary interventions. The first-generation DES comprised sirolimus—(64%) and paclitaxel—(36%) eluting stents, and the second-generation DES comprised zotarolimus—(6%), everolimus- (50%), and biolimus A9—(44%) eluting stents. The groups were similar with respect to the stent diameters, total stent lengths, and the number of stents. The age of the stents was significantly higher in first-generation DES group (55 ± 27 months) compared with that in the second-generation DES group (32 ± 19 months) (P = 0.001). The groups did not differ regarding the angiographic restenosis patterns.
Optical coherence tomography and optical frequency domain imaging
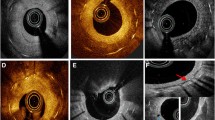
In the first-generation DES group, the lipid length was significantly longer (5.5 ± 3.8 vs. 3.1 ± 2.1 mm, P = 0.0007), the lipid arc was significantly larger (324 ± 70° vs. 250 ± 94°, P = 0.002), the prevalence of a 360° lipid arc was significantly higher (58 vs. 31%, P = 0.03), and the fibrous cap was significantly thinner (153 ± 85 vs. 211 ± 95 µm, P = 0.02) compared with those in the second-generation DES group (Fig. 2). These differences remained significant after adjusting for the age of the stent (lipid length: P < 0.001; lipid arc: P = 0.019; and fibrous cap thickness: P < 0.001). The thin-cap fibroatheroma and neointimal rupture rates tended to be higher in the first-generation DES group compared with those in the second-generation DES group, but these differences were not significant (Table 3).
Comparisons of the first-generation and second-generation DES using OCT and OFDI. a Total lipid lengths in the first-generation and second-generation DES. b Lipid arc angles in the first-generation and second-generation DES. c Prevalence of 360° lipid arc in the first-generation and second-generation DES. d Fibrous cap thicknesses in the first-generation and second-generation DES
Reproducibility of the optical coherence tomography and optical frequency domain image analyses
The interobserver reproducibility for the qualitative OCT and OFDI analyses for 360° lipid arcs, thin-cap fibroatheromas, the presence of intraluminal material, the presence of neointimal ruptures, the presence of neovascularization, the presence of peri-strut low-intensity areas, and peri-strut ulcer-like appearances were κ = 0.93 and 0.80, 0.84 and 0.82, 0.87 and 0.81, 0.86 and 0.85, 0.92 and 0.88, 0.88 and 0.88, and 0.84 and 0.82, respectively. The intraclass correlation coefficients for the measurements of the lipid lengths, lipid arcs, and fibrous cap thicknesses by the same observer were [1,1] = 0.84, [1,1] = 0.82, and [1,1] = 0.84, respectively. The intraclass correlation coefficients for the measurements of the lipid lengths, lipid arcs, and fibrous cap thicknesses by two different observers were [2,1] = 0.82, [2,1] = 0.81, and [2,1] = 0.81, respectively.
Discussion
This study’s findings show that the total lipid length of the neoatherosclerosis was significantly longer, the lipid arc of the neoatherosclerosis was significantly larger, the frequency of a 360° lipid arc was significantly higher, and that the fibrous cap was significantly thinner in the first-generation DES group compared with those in the second-generation DES group.
Stent thrombosis is a serious complication that follows coronary stenting, and it is caused by strut malapposition, uncovered struts, underexpansion, stent fractures, and neoatherosclerosis [7, 8, 10, 23]. Stent thrombosis rates have improved in association with the use of second-generation DES compared with those associated with the use of first-generation DES, and the findings from many large-scale studies have demonstrated the superiority of second-generation DES at reducing stent thrombosis. While several OCT studies’ findings have shown improvements in strut coverage and apposition at follow-up in association with the use of second-generation DES, [24] the findings from autopsy studies demonstrated that the use of second-generation DES did not reduce neoatherosclerosis and that the prevalence of neoatherosclerosis increased with longer follow-up times of up to 7 years in first-generation and second-generation DES [11,12,13, 25]. More importantly, Adriaenssens et al. recently reported the findings from an OCT study of stent thrombosis in 215 cases, comprising 62 early stent thromboses and 155 late or very late stent thromboses, and they showed that neoatherosclerosis was associated with 31.3% of the very late stent thromboses, but it was not observed in acute, subacute, and late stent thromboses [23]. Lee et al. also reported that patients with delayed very late stent thromboses (onset time > 55.1 months) had a significantly higher frequency of neoatherosclerosis compared with patients who had earlier very late stent thromboses (onset time > 12 months and < 55.1 months) [10]. On the other hand, Kang et al. suggested that the unstable features associated with neoatherosclerosis increased as the follow-up time increased. They showed that the fibrous cap thickness was negatively correlated with the follow-up time (r = − 0.318, P = 0.024) and that thin-cap fibroatheroma-containing neointima increased over time following drug-eluting stent implantation [26]. As the findings from these studies suggest, the incidence of neoatherosclerosis continues to increase and the neoatherosclerosis becomes more unstable as the age of the drug-eluting stent increases; however, all of these studies included both first-generation and second-generation DES, and the reports do not discuss differences in the progression of neoatherosclerosis in the context of the stent generation. Most of the previous studies lacked adequate sample sizes to enable the investigators to examine neoatherosclerosis in second-generation drug-eluting stents; therefore, the differences between first-generation and second-generation DES in relation to the time-dependent characteristics of neoatherosclerosis remain unclear.
To the best of our knowledge, this is the first study to evaluate the differences between first-generation and second-generation DES in relation to the characteristics of neoatherosclerosis. The proliferation of neoatherosclerosis with respect to its length and arc was significantly greater in the first-generation drug-eluting stents compared with that in the second-generation drug-eluting stents. In addition, the fibrous cap was significantly thinner in the first-generation DES. Hence, these differences might contribute to the reduced frequency of late stent thrombosis in second-generation DES. Indeed, Otsuka et al. reported from their histopathological study that the unstable features associated with neoatherosclerosis, including thin-cap fibroatheromas and plaque ruptures, were not observed following the implantation of cobalt-chromium everolimus-eluting stents, which were representative of second-generation drug-eluting stents [12]. The first-generation drug-eluting stents strongly inhibit endothelialization and delay vascular healing. In addition, allergic reactions to the polymer and persistent inflammation seem to be stronger in first-generation DES. These are considered to be associated with the occurrence and strong proliferation of neoatherosclerosis. On the other hand, second-generation DES provided a better vascular response and less inflammation, caused by antiproliferative drugs and polymers. Therefore, we believe that the proliferation and stability of neoatherosclerosis associated with second-generation DES over time might be more acceptable compared with those associated with first-generation DES, but we do not consider that second-generation DES can reduce the frequency of neoatherosclerosis. This study had several limitations. First, this was a retrospective observational study, and the study population was relatively small. Therefore, the characteristics with low rates, including thin-cap fibroatheromas, tended not to show statistically significant differences between the groups. To confirm this study’s findings, a study with a large sample size should be undertaken. Second, this study only included patients who had undergone repeat interventions under optical coherence tomography or optical frequency domain imaging guidance; therefore, a patient selection bias must be considered. Third, although there was no statistical significance, LDL level tended to be higher in the first-generation DES group, which may affect lipid accumulation in the NA in first-generation DES. Unfortunately, this study was retrospectively performed with a small sample size, due to which we could not adjust the LDL level. Finally, an absolute consensus does not exist regarding the OCT criteria for in-stent neoatherosclerosis, so the assessment of this study’s results may change. Comparisons of the histopathology features and the OCT findings must continue.
Conclusions
The current study’s findings suggest that compared with second-generation DES, the proliferation of neoatherosclerosis in terms of its length and arc was significantly greater and the fibrous cap was significantly thinner in first-generation DES. Even if we adjusted stent age using multivariable linear regression analyses, our study sample size is very small; more importantly, the adjustment could be insufficient. Longer stent age is an important factor influencing the growth of in-stent neoatherosclerosis. Therefore, the possibility that our results may be explained by the differences in stent age cannot be ruled out. Future large sample studies adjusting stent age and dealing with newer generation DES with biodegradable polymer will be required to investigate this issue.
References
Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE (2003) Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349(14):1315–1323. https://doi.org/10.1056/NEJMoa035071
Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Juni P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW (2007) Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369(9562):667–678. https://doi.org/10.1016/s0140-6736(07)60314-6
Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Juni P, Vaina S, Hellige G, Tsuchida K, Morger C, Boersma E, Kukreja N, Meier B, Serruys PW, Windecker S (2008) Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J Am Coll Cardiol 52(14):1134–1140. https://doi.org/10.1016/j.jacc.2008.07.006
Fujii K, Carlier SG, Mintz GS, Yang YM, Moussa I, Weisz G, Dangas G, Mehran R, Lansky AJ, Kreps EM, Collins M, Stone GW, Moses JW, Leon MB (2005) Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol 45(7):995–998. https://doi.org/10.1016/j.jacc.2004.12.066
Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R (2007) Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115(18):2435–2441. https://doi.org/10.1161/CIRCULATIONAHA.107.693739
Kosonen P, Vikman S, Jensen LO, Lassen JF, Harnek J, Olivecrona GK, Erglis A, Fossum E, Niemela M, Kervinen K, Ylitalo A, Pietila M, Aaroe J, Kellerth T, Saunamaki K, Thayssen P, Hellsten L, Thuesen L, Niemela K (2013) Intravascular ultrasound assessed incomplete stent apposition and stent fracture in stent thrombosis after bare metal versus drug-eluting stent treatment the Nordic Intravascular Ultrasound Study (NIVUS). Int J Cardiol 168(2):1010–1016. https://doi.org/10.1016/j.ijcard.2012.10.033
Kang SJ, Lee CW, Song H, Ahn JM, Kim WJ, Lee JY, Park DW, Lee SW, Kim YH, Mintz GS, Park SW, Park SJ (2013) OCT analysis in patients with very late stent thrombosis. JACC Cardiovasc Imaging 6(6):695–703. https://doi.org/10.1016/j.jcmg.2013.02.006
Taniwaki M, Radu MD, Zaugg S, Amabile N, Garcia-Garcia HM, Yamaji K, Jorgensen E, Kelbaek H, Pilgrim T, Caussin C, Zanchin T, Veugeois A, Abildgaard U, Juni P, Cook S, Koskinas KC, Windecker S, Raber L (2016) Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation 133(7):650–660. https://doi.org/10.1161/circulationaha.115.019071
Nakamura D, Attizzani GF, Toma C, Sheth T, Wang W, Soud M, Aoun R, Tummala R, Leygerman M, Fares A, Mehanna E, Nishino S, Fung A, Costa MA, Bezerra HG (2016) Failure mechanisms and neoatherosclerosis patterns in very late drug-eluting and bare-metal stent thrombosis. Circ Cardiovasc Interv. https://doi.org/10.1161/circinterventions.116.003785
Lee SY, Ahn JM, Mintz GS, Hur SH, Choi SY, Kim SW, Cho JM, Hong SJ, Kim JW, Hong YJ, Lee SG, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Park SJ, Hong MK (2017) Characteristics of earlier versus delayed presentation of very late drug-eluting stent thrombosis: an optical coherence tomographic study. J Am Heart Assoc. https://doi.org/10.1161/jaha.116.005386
Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R (2011) The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol 57(11):1314–1322. https://doi.org/10.1016/j.jacc.2011.01.011
Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, Kutys R, Ladich E, Finn AV, Kolodgie FD, Virmani R (2014) Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 129(2):211–223. https://doi.org/10.1161/circulationaha.113.001790
Lee SY, Hur SH, Lee SG, Kim SW, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Hong MK (2015) Optical coherence tomographic observation of in-stent neoatherosclerosis in lesions with more than 50% neointimal area stenosis after second-generation drug-eluting stent implantation. Circ Cardiovasc Interv 8(2):e001878. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001878
Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, Doostzadeh J, Cao S, Simonton CA, Sudhir K, Lansky AJ, Cutlip DE, Kereiakes DJ (2010) Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med 362(18):1663–1674. https://doi.org/10.1056/NEJMoa0910496
Lee SY, Shin DH, Mintz GS, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Hong MK (2013) Optical coherence tomography-based evaluation of in-stent neoatherosclerosis in lesions with more than 50% neointimal cross-sectional area stenosis. EuroIntervention 9(8):945–951. https://doi.org/10.4244/eijv9i8a158
Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudek D, Falk E, Feldman MD, Fitzgerald P, Garcia-Garcia HM, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CC, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Raber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PW, Shimada K, Shinke T, Shite J, Siegel E, Sonoda S, Suter M, Takarada S, Tanaka A, Terashima M, Thim T, Uemura S, Ughi GJ, van Beusekom HM, van der Steen AF, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G (2012) Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 59(12):1058–1072. https://doi.org/10.1016/j.jacc.2011.09.079
Kume T, Okura H, Kawamoto T, Yamada R, Miyamoto Y, Hayashida A, Watanabe N, Neishi Y, Sadahira Y, Akasaka T, Yoshida K (2011) Assessment of the coronary calcification by optical coherence tomography. EuroIntervention 6(6):768–772. https://doi.org/10.4244/eijv6i6a130
Regar E, van Beusekom HM, van der Giessen WJ, Serruys PW (2005) Images in cardiovascular medicine. Optical coherence tomography findings at 5-year follow-up after coronary stent implantation. Circulation 112(23):e345–e346. https://doi.org/10.1161/circulationaha.104.531897
Teramoto T, Ikeno F, Otake H, Lyons JK, van Beusekom HM, Fearon WF, Yeung AC (2010) Intriguing peri-strut low-intensity area detected by optical coherence tomography after coronary stent deployment. Circ J 74(6):1257–1259
Goto I, Itoh T, Kimura T, Fusazaki T, Matsui H, Sugawara S, Komuro K, Nakamura M (2011) Morphological and quantitative analysis of vascular wall and neointimal hyperplasia after coronary stenting: comparison of bare-metal and sirolimus-eluting stents using optical coherence tomography. Circ J 75(7):1633–1640
Takano M, Yamamoto M, Inami S, Murakami D, Ohba T, Seino Y, Mizuno K (2009) Appearance of lipid-laden intima and neovascularization after implantation of bare-metal stents extended late-phase observation by intracoronary optical coherence tomography. J Am Coll Cardiol 55(1):26–32. https://doi.org/10.1016/j.jacc.2009.08.032
Gonzalo N, Serruys PW, Okamura T, van Beusekom HM, Garcia-Garcia HM, van Soest G, van der Giessen W, Regar E (2009) Optical coherence tomography patterns of stent restenosis. Am Heart J 158(2):284–293. https://doi.org/10.1016/j.ahj.2009.06.004
Adriaenssens T, Joner M, Godschalk TC, Malik N, Alfonso F, Xhepa E, De Cock D, Komukai K, Tada T, Cuesta J, Sirbu V, Feldman LJ, Neumann FJ, Goodall AH, Heestermans T, Buysschaert I, Hlinomaz O, Belmans A, Desmet W, Ten Berg JM, Gershlick AH, Massberg S, Kastrati A, Guagliumi G, Byrne RA (2017) Optical coherence tomography findings in patients with coronary stent thrombosis: a report of the PRESTIGE consortium (prevention of late stent thrombosis by an Interdisciplinary Global European effort). Circulation 136(11):1007–1021. https://doi.org/10.1161/circulationaha.117.026788
Kim JS, Jang IK, Kim JS, Kim TH, Takano M, Kume T, Hur NW, Ko YG, Choi D, Hong MK, Jang Y (2009) Optical coherence tomography evaluation of zotarolimus-eluting stents at 9-month follow-up: comparison with sirolimus-eluting stents. Heart 95(23):1907–1912. https://doi.org/10.1136/hrt.2009.167759
Otsuka F, Byrne RA, Yahagi K, Mori H, Ladich E, Fowler DR, Kutys R, Xhepa E, Kastrati A, Virmani R, Joner M (2015) Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J 36(32):2147–2159. https://doi.org/10.1093/eurheartj/ehv205
Kang SJ, Mintz GS, Akasaka T, Park DW, Lee JY, Kim WJ, Lee SW, Kim YH, Whan Lee C, Park SW, Park SJ (2011) Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation 123(25):2954–2963. https://doi.org/10.1161/circulationaha.110.988436
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial relationships or other conflicts of interest relevant to the contents of this paper.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kobayashi, N., Ito, Y., Yamawaki, M. et al. Differences between first-generation and second-generation drug-eluting stent regarding in-stent neoatherosclerosis characteristics: an optical coherence tomography analysis. Int J Cardiovasc Imaging 34, 1521–1528 (2018). https://doi.org/10.1007/s10554-018-1375-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1375-4