Abstract
To quantify stiffness of the descending aorta (DAo) in stroke patients using 4D flow MRI and compare results with transesophageal echocardiography (TEE). 48 acute stroke patients undergoing 4D flow MRI and TEE were included. Intima-media-thickness (IMT) was measured in the DAo and the aorta was scrutinized for atherosclerotic plaques using TEE. Stiffness of the DAo was determined by (a) 4D flow MRI at 3 T by calculating pulse wave velocity (PWV) and by (b) TEE calculating arterial strain, stiffness index, and distensibility coefficient. Mean IMT was 1.43 ± 1.75. 7 (14.6%) subjects had no sign of atherosclerosis, 10 (20.8%) had IMT-thickening or plaques < 4 mm, and 31 (66.7%) had at least one large and/or complex plaque in the aorta. Increased IMT significantly correlated (p < 0.001) with increased DAo stiffness in MRI (PWV r = 0.66) and in TEE (strain r = 0.57, stiffness index r = 0.64, distensibility coefficient r = 0.57). Patients with at least IMT-thickening had significantly higher stiffness values compared to patients without atherosclerosis. However, no difference was observed between patients with plaques < 4 mm and patients with plaques ≥ 4 mm. PWV and TEE parameters of stiffness correlated significantly [strain (r = − 0.36; p = 0.011), stiffness index (r = 0.51; p = 0.002), and distensibility coefficient (r = − 0.59; p < 0.001)]. 4D flow MRI and TEE-based parameters of aortic stiffness were associated with markers of atherosclerosis such as IMT-thickness and presence of plaques. We believe that 4D flow MRI is a promising tool for future studies of aortic atherosclerosis, due to its longer coverage of the aorta and non-invasiveness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is the leading cause of cardiovascular disease and the most important driver of morbidity and mortality in the Western world [1]. Beginning in childhood it remains subclinical for decades [2] until symptomatic cardiovascular disease (i.e. coronary heart disease, stroke, peripheral artery disease) develops in mid-age. Detection of disease in its early stages of development would allow initiation of profound lifestyle changes and/or medical therapy in order to hinder progression into a symptomatic disease. It has been shown that increased stiffness is a strong predictor of future cardiovascular events and all-cause mortality [3]. In this regard, quantification of arterial stiffness could be used as a marker of subclinical atherosclerotic disease, as stiffness increases long before wall thickening occurs. Furthermore, increased aortic stiffness was shown to be associated with reduced heart function [4] and presence of left ventricular hypertrophy [5] and may serve as a target parameter for antihypertensive treatment [6]. The current diagnostic standard of noninvasive arterial stiffness quantification is carotid-to-femoral pulse wave velocity (PWV) [7], which only measures systemic stiffness. However, regional stiffness assessment may also be of clinical importance when screening for early stages of atherosclerotic disease; for example in the aorta, where atherosclerosis occurs earlier than in the carotid arteries [8]. Regional arterial stiffness can be assessed using local PWV quantification based on 4D flow MRI [9]. It can also be measured using routine transesophageal echocardiography (TEE) through quantification of stiffness indicators such as arterial strain, arterial stiffness index, and distensibility coefficient in circumscribed sections of the aorta. However, such measurements require additional parameters such as vessel diameter change in systole and diastole and values of systolic and diastolic blood pressure which are not needed for PWV based on MRI [10]. So far, a comparison between MRI- and TEE-based parameters of aortic stiffness has not been undertaken. Moreover, it is unclear if all parameters are equally suitable to predict the presence of early atherosclerosis. Therefore in order to provide an insight on relevant parameters, in this work, we calculated arterial stiffness in the descending aorta of 48 acute stroke patients, who underwent both 4D flow MRI and transesophageal echocardiography.
Methods
Study cohort
Forty-eight patients undergoing TEE as part of the diagnostic workup of acute ischemic stroke were included in this study. All patients underwent additional 4D flow MRI of the aorta. Cardiovascular risk factors were prospectively documented. The study was approved by the University of Freiburg ethics committee (IRB number 227/14) and informed consent was obtained from all participants.
MRI of the aorta
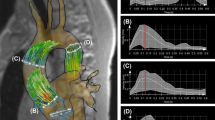
All MRI examinations were conducted using a 3-Tesla MRI system (TIM Trio, Siemens Healthcare AG, Erlangen, Germany). 4D flow MRI (echo time/repetition time (TE/TR) = 2.52/40 ms, flip angle = 7°, temporal resolution = 40 ms, matrix size = 320 × 240 × 58, bandwidth = 450 Hz/pixel, spatial resolution = 2.0 × 1.7 × 2.2 mm3, velocity sensitivity along all three directions = 150 cm/s, and parallel imaging (GRAPPA) along the phase encoding direction (y) with a reduction factor of R = 2 (24 reference lines)) was performed in order to acquire time-resolved and three-dimensional blood flow parameters with a velocity sensitivity (venc) of 150 cm/s. Experiments were ECG-synchronized and respiration-controlled using navigator-gating. 4D flow MRI datasets were further analyzed using the MEVISFlow software (Fraunhofer MEVIS, Bremen, Germany) which comprised correction for velocity aliasing and eddy-currents and calculation of a time-averaged 3D PC MR angiography (for technical details see [11]). For quantification of PWV, a series of 25 virtual 2D emitter planes were positioned along the entire descending aorta. The number of emitter planes was a compromise between increasing accuracy with more sampling points at the expense of lower temporal resolution [12]. To guarantee comparability of the results between different subjects, an initial plane #0 was positioned directly distal to the outlet of the left subclavian artery. Subsequently, all other 2D analysis planes were automatically positioned downstream in 10 mm intervals (see Fig. 1) ending at the level of the diaphragm. The temporal evolution of blood flow for each analysis plane was calculated by multiplying the lumen area with the mean absolute velocity inside the lumen for each time frame in the cardiac cycle. PWV was derived from the data by automatically identifying the time to peak (TTP) and time to foot (TTF) of the flow waveform. TTF was extracted from the flow waveforms by fitting a line to the upslope portion of the waveform (between 20 and 80% of the peak flow) and was defined as the intersection of the fitted line with the zero line. PWV was then determined by plotting the resulting TTP or TTF of all evaluated analysis planes as a function of location along the aortic lumen. In addition, XCor was used to estimate the temporal differences between the velocity–time curves in different analysis planes. Briefly, the waveform at each location was compared to the waveform of the first analysis plane. A cross-correlation function was used to apply a time shift to the more distal waveforms until the highest correlation value between the two waveforms was obtained, returning a time shift for each analysis plane relative to the first location. The local PWV of the DAo was calculated as the inverse of the slope of a linear fit to the data (see Fig. 1). For the following analysis we used only the TTF values, as an earlier study proved them do be the most reliable measure of PWV [13].
Stiffness quantification in the aorta. Analysis comprised (I) TEE based quantification of stiffness and atherosclerotic burden (i.e. quantification of intima-media-thickness and detection of plaques) and (II) 4D flow MRI based pulse wave velocity quantification by assessing the temporal evolution of blood flow for each of 25 analysis planes positioned in the descending aorta using dedicated software
TEE of the aorta
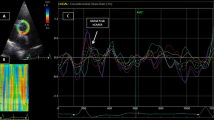
Median time between 4D flow MRI and TEE was 2 days (inter quartile range 1–3). TEE examinations of the aorta were performed by one experienced cardiologist using a Philips iU22 (Philips Healthcare, Best, The Netherlands) ultrasonic device and a 2–7 MHz ultrasound probe (S7-2t Omni Sector transesophageal phased array; 180° electronic rotation, field of view: 90°, electrocautery suppression). Blood pressure was measured every 5 min at the left upper arm with the patient in a supine position. After routine TEE examination (i.e. evaluation of the heart), the complete aorta was scrutinized for atherosclerotic plaques. Maximum plaque thickness was documented for each plaque and plaques were defined as complex plaques if they were ≥ 4 mm thick, ulcerated, and/or had thrombi attached. The transducer was then rotated in order to visualize the descending aorta (DAo) in transverse (0°) section. The transition zone between the aortic arch curvature and the straight DAo was located by retracting the probe in cross-sectional view until the round lumen of the straight DAo became oval. Intima-media-thickness (IMT) and lumen area was assessed in cross-section (Fig. 1). Parameters of arterial stiffness were calculated in the same plane. The following definitions were used [10]: Arterial strain = [(maximum systolic area − maximum diastolic area)/maximum diastolic area], stiffness index = natural logarithm [(systolic blood pressure/diastolic blood pressure)/strain], and distensibility coefficient = [(2 × strain)/(systolic blood pressure − diastolic blood pressure)]. Area change was used instead of diameter change for strain calculation, as area change was shown to correlate better with global PWV in an earlier study [14]. Measurements were recorded and quantifications were made offline on the ultrasound platform.
Statistical analysis
Data are presented as mean (± standard deviations) or median (interquartile range) for continuous, absolute, and relative frequencies for categorical variables. Departures from normality were detected with the Shapiro–Wilk statistic. Differences between parameters were evaluated using Chi square tests, Fishers exact test was used for qualitative and independent samples, and t-tests was applied for quantitative parameters. The degree of individual atherosclerotic burden was classified as follows: (1) no atherosclerosis (IMT < 1.1 mm), (2) presence of atherosclerosis (IMT ≥ 1.1 mm and/or presence of atherosclerotic plaques any thickness), (3) advanced atherosclerosis (i.e. presence of complex plaques). Bivariate correlation analyses were performed to quantify the association of arterial stiffness with IMT. The quantity of stiffness in patients with different degrees of atherosclerotic burden was studied using analysis of variance (ANOVA) with the Tukey post-hoc test. Furthermore, association between TEE-based parameters of stiffness and MRI-based PWV was studied using Pearson correlation analysis. A receiver operator characteristic (ROC) curve was performed to calculate the threshold value of stiffness index and PWV in differentiating subjects with and without aortic atherosclerosis. The optimal cutpoint was determined by maximizing the Youden-index. To quantify variability of the stiffness measurements we repeated stiffness quantification in all 48 datasets using TEE and in 10 randomly selected datasets using MRI in a blinded fashion by a second rater (inter-rater agreement), and the first rater after a time interval of 3 months (intra-rater agreement). 0.05 was used as the level of statistical significance. Statistical analyses were performed using IBM-SPSS Statistics version 19.0.1.
Results
Baseline characteristics
Demographics, cardiovascular risk factors, and markers of atherosclerotic burden in the 48 stroke patients are given in Table 1. Median stroke severity according to the National Institute of Health Stroke Scale (NIHSS) was 2 (interquartile range 0–4).
Parameters of aortic stiffness
Intima-media-thickness correlated with increased pulse-wave-velocity (r = 0.66; p < 0.001), decreased strain (r = 0.57; p < 0.001), increased stiffness index (r = 0.64; p < 0.001), and decreased distensibility coefficient (r = 0.57, p < 0.001) (see Fig. 2). When comparing PWV with TEE-based measures, we detected significant correlations for PWV and decreased distensibility coefficient (r = 0.59; p < 0.001), increased stiffness index (r = 0.51; p = 0.002) and decreased strain (r = 0.36; p = 0.011) (see Fig. 3). Significant correlations in the subgroup analysis were not observed between IMT and stiffness parameters and PWV and TEE-based measures, which was probably due to the limited sample size in this group (N = 7). In the group of patients with IMT-thickening and/or small plaques (N = 10) we detected significant correlations for IMT and PWV (r = 0.76; p = 0.011), and between PWV and distensibility coefficient (r = − 0.79; p = 0.007) and strain (r = − 0.73; p = 0.016). For the largest group (N = 31) of patients with plaques ≥ 4 mm we detected significant correlations between IMT and distensibility coefficient (r = − 0.50; p = 0.005), stiffness index (r = 0.625; p = 0.002), strain (r = − 0.499, p = 0.005), and PWV (r = 0.487, p = 0.006). We furthermore observed significant correlations between PWV and distensibility coefficient (r = − 0.39; p = 0.031).
Parameters of aortic stiffness are presented in Table 2. When comparing parameters of stiffness between patients with different degrees of atherosclerosis we detected a significant difference between patients with no sign of atherosclerosis and patients with presence of atherosclerosis. However, there was no difference between patients with IMT-thickening and/or plaques < 4 mm and patients with plaques ≥ 4 mm. This was true for distensibility coefficient, stiffness index and PWV, but not for strain where there was no difference between patients without atherosclerosis and IMT-thickening and/or plaques < 4 mm, but between IMT-thickening and/or plaques < 4 mm and patients with plaques ≥ 4 mm.
Determining cutoff values for stiffness index and PWV
A receiver-operator-characteristic (ROC) (Fig. 4) curve analysis was performed using stiffness index and PWV values and revealed an area under the curve of 0.89 (p < 0.001) and 0.91 (p < 0.001), respectively. When choosing a stiffness index cutoff value of 6.58, the sensitivity was 0.83 with a specificity of 0.80. The positive predictive value was 0.83 and the negative predictive value was 0.80. For PWV, we chose a cutoff value of 7.09 which resulted in a sensitivity of 0.90 and a specificity of 0.80. The positive predictive value was 0.94 and the negative predictive value was 0.71.
Intra- and inter-observer agreement of stiffness quantification
Stiffness quantification using the TEE-based parameter strain in the DAo in 48 patients showed significant and high correlations for both inter-observer analysis (r = 0.71, p < 0.001) and intra-observer analysis (r = 0.88 p < 0.001). The same was true for the MRI-based parameter PWV which we studied in 10 datasets (inter-observer r = 0.94, p < 0.001; intra-observer r = 0.96, p < 0.001).
Discussion
This is the first study to compare parameters of arterial stiffness of the descending aorta between 4D flow MRI and TEE and to investigate the correlation of such functional parameter with morphological parameters (IMT and presence of plaques) of the aorta.
Previous studies have reported a high reliability of MRI and TEE regarding assessment of arterial stiffness [10, 15, 16]. Earlier studies demonstrated a strong correlation between invasive measurements of arterial stiffness and noninvasively calculated aortic stiffness index [17], strain, and distensibility [18] by echocardiography. However, no studies have directly compared 4D flow MRI based PWV calculation with invasive stiffness quantification. Hence, the high correlations of regional aortic stiffness measures between MRI and TEE observed in our study increase the reliability of MRI based stiffness quantification. Inter- and intra-observer variability regarding 4D flow MRI based PWV quantification proved to be low in an earlier study [15]. When compared with reference data from the literature, individual PWV values were higher in our study with a mean PWV of 8.3 m/s compared to a mean PWV of 3.6 m/s (n = 71; age 16.4 ± 7.6) which is probably due to the older age of our stroke patients and the higher prevalence of atherosclerosis in more than 80% of subjects in our study [19].
When testing whether presence of atherosclerotic lesions may be predicted using parameters of stiffness, we observed high associations between stiffness-parameters and IMT and presence of plaques (any thickness). We did not detect a difference in arterial stiffness between patients with small plaques or IMT-thickening and patients with large plaques. Furthermore, correlations were not higher when performing correlation analysis in the subgroup analysis. However, this comparison was limited by the lack of further subclassification of plaques based on plaque composition. Yet, this may be of importance as calcified and stable plaques are associated with higher stiffness [20] as compared to lipid-rich and unstable plaques, and probably independent of plaque size. Hence, we are currently working on characterizing the association of plaque composition assessed using 3D multi contrast MRI in the aorta [21] and stiffness assessed using 4D flow MRI. This may help in discriminating patients with critical or vulnerable atherosclerotic lesions in the future.
We were able to determine cutoff values for stiffness index and PWV with high sensitivity and specificity. Accordingly, we believe that both semi-invasive TEE and non-invasive 4D flow MRI can be used to assess arterial stiffening in the aorta. However, 4D flow MRI is a cost-intensive technique and TEE is invasive which is why both techniques have limited value as a screening test for subclinical atherosclerosis. Nevertheless, PWV may serve as a target parameter in future trials on prevention of atherosclerosis through interventions such as antihypertensive medication [6], statins [22], or physical activity [23,24,25]. 4D flow MRI based regional stiffness quantification using PWV would be ideally suited for such studies as it allows comprehensive assessment of the vasculature, offline analysis and retrospective comparisons of datasets, and is not invasive.
Limitations of our study were the relative high amount of patients with atherosclerotic lesions in the aorta in comparison with patients without any atherosclerotic lesion. This was mainly due to the inclusion of acute stroke patients who have a higher prevalence of systemic atherosclerotic disease compared to the general population. However, inclusion of healthy individuals as reference is ethically not possible as study participants had to undergo semi-invasive TEE.
Conclusions
We have shown that parameters of aortic stiffness can be equally assessed using non-invasive 4D flow MRI and semi-invasive TEE. Both, 4D flow MRI- and TEE-based parameters showed high correlations with presence of atherosclerosis but not presence of complex plaques. Hence, 4D flow MRI based stiffness quantification using PWV may be ideally suited for interventional studies using regional stiffness as a surrogate for subclinical atherosclerosis.
References
Mozaffarian D, Benjamin EJ, Go AS et al (2016) Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 133:447–454
Strong JP, Malcom GT, McMahan CA et al (1999) Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA 281:727–735
Vlachopoulos C, Aznaouridis K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55:1318–1327
Ohyama Y, Ambale-Venkatesh B, Noda C et al (2016) Association of aortic stiffness with left ventricular remodeling and reduced left ventricular function measured by magnetic resonance imaging: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.115.004426
Yambe M, Tomiyama H, Koji Y et al (2006) B-type natriuretic peptide and arterial stiffness in healthy Japanese men. Am J Hypertens 19:443–447
Rodilla E, Millasseau S, Costa JA, Pascual JM (2017) Arterial destiffening in previously untreated mild hypertensives after 1 year of routine clinical management. Am J Hypertens 30:510–517
Safar ME (2010) Arterial aging—hemodynamic changes and therapeutic options. Nat Rev Cardiol 7:442–449
Dawson JD, Sonka M, Blecha MB et al (2009) Risk factors associated with aortic and carotid intimal medial thickness in adolescents and young adults: the Muscatine Offspring Study. J Am Coll Cardiol 53:2273–2279
Wentland AL, Grist TM, Wieben O (2014) Review of MRI-based measurements of pulse wave velocity: a biomarker of arterial stiffness. Cardiovasc Diagn Ther 4:193–206
Cho JY, Kim KH (2016) Evaluation of arterial stiffness by echocardiography: methodological aspects. Chonnam Med J 52:101–106
Wehrum T, Kams M, Schroeder L et al (2014) Accelerated analysis of three-dimensional blood flow of the thoracic aorta in stroke patients. Int J Cardiovasc Imaging 30:1571–1577
Kröner ESJ, van der Geest RJ, Scholte AJHA. et al (2012) Evaluation of sampling density on the accuracy of aortic pulse wave velocity from velocity-encoded MRI in patients with Marfan syndrome. J Magn Reson Imaging 36:1470–1476
Ibrahim E-SH, Johnson KR, Miller AB et al (2010) Measuring aortic pulse wave velocity using high-field cardiovascular magnetic resonance: comparison of techniques. J Cardiovasc Magn Reson 12:26
Kim KH, Park JC, Yoon HJ et al (2009) Usefulness of aortic strain analysis by velocity vector imaging as a new echocardiographic measure of arterial stiffness. J Am Soc Echocardiogr 22:1382–1388
Markl M, Wallis W, Strecker C et al (2012) Analysis of pulse wave velocity in the thoracic aorta by flow-sensitive four-dimensional MRI: reproducibility and correlation with characteristics in patients with aortic atherosclerosis. J Magn Reson Imaging 35:1162–1168
Kaneko K, Takahashi T, Saito H et al (2014) Assessment of aortic arch stiffness using pulse-wave tissue Doppler imaging: a transesophageal echocardiographic comparison study of acute ischemic stroke patients and stroke-free patients. Echocardiography 31:1113–1121
Stefanadis C, Stratos C, Boudoulas H et al (1990) Distensibility of the ascending aorta: comparison of invasive and non-invasive techniques in healthy men and in men with coronary artery disease. Eur Heart J 11:990–996
Yoon HJ, Kim KH, Lee SH et al (2013) Differences of aortic stiffness and aortic intima-media thickness according to the type of initial presentation in patients with ischemic stroke. J Cardiovasc Ultrasound 21:12–17
Voges I, Jerosch-Herold M, Hedderich J et al (2012) Normal values of aortic dimensions, distensibility, and pulse wave velocity in children and young adults: a cross-sectional study. J Cardiovasc Magn Reson 14:77
Kondo K, Nemoto M, Harada N et al (2017) Comparison between quantitative stiffness measurements and ultrasonographic findings of fresh carotid plaques. Ultrasound Med Biol 43:138–144
Wehrum T, Dragonu I, Strecker C et al (2017) Multi-contrast and three-dimensional assessment of the aortic wall using 3 T MRI. Eur J Radiol 91:148–154
Mitsiou E, Boutari C, Kotsis V et al (2017) Effect of low (5 mg) vs high (20–40 mg) rosuvastatin dose on 24 h arterial stiffness, central haemodynamics, and non-alcoholic fatty liver disease in patients with optimally controlled arterial hypertension. Curr Vasc Pharmacol. https://doi.org/10.2174/1570161115666170630122833
Ahmadi-Abhari S, Sabia S, Shipley MJ et al (2017) Physical activity, sedentary behavior, and long-term changes in aortic stiffness: the Whitehall II Study. J Am Heart Assoc. https://doi.org/10.1161/JAHA.117.005974
Kobayashi R, Hashimoto Y, Hatakeyama H, Okamoto T (2016) Acute effects of aerobic exercise intensity on arterial stiffness after glucose ingestion in young men. Clin Physiol Funct Imaging. https://doi.org/10.1111/cpf.12395
Kang SJ, Kim EH, Ko KJ (2016) Effects of aerobic exercise on the resting heart rate, physical fitness, and arterial stiffness of female patients with metabolic syndrome. J Phys Ther Sci 28:1764–1768
Funding
This study was funded by Deutsche Forschungsgemeinschaft (DFG) (Grant #HA5399/3-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has financial disclosures related to the performance or content of this study and to the submission of this paper.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Wehrum, T., Günther, F., Kams, M. et al. Quantification of aortic stiffness in stroke patients using 4D flow MRI in comparison with transesophageal echocardiography. Int J Cardiovasc Imaging 34, 1629–1636 (2018). https://doi.org/10.1007/s10554-018-1369-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1369-2